The Risk of Breast Cancer between Western and Mediterranean Dietary Patterns
Abstract
1. Introduction
2. Methods
3. Results
3.1. Western Dietary Pattern and Breast Cancer
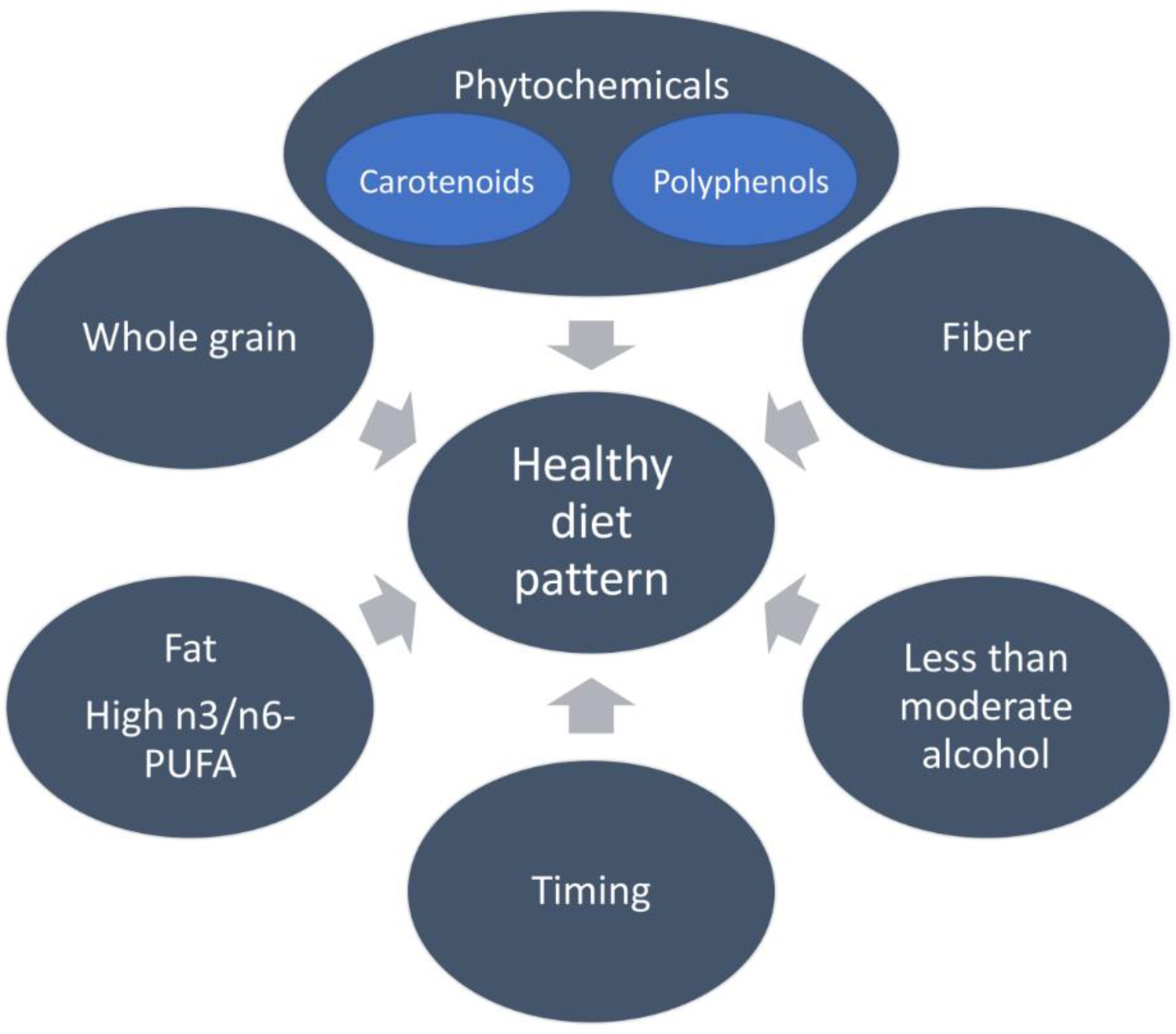
3.2. Mediterranean Dietary Pattern and Breast Cancer
3.2.1. Fruits, Vegetables, and Plant-Based Foods
3.2.2. Carotenoids
3.2.3. Polyphenols
3.2.4. Digestive Fiber
3.2.5. Olive Oil
3.2.6. Fish
3.2.7. Alcohol Consumption
4. Dietary Pattern and Histological and Molecular Classification of Breast Cancer
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 1130. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.S.; Roder, D.M.; Yu, X.Q.; Egger, S.; O’connell, D.L. Estimating prevalence of distant metastatic breast cancer: A means of filling a data gap. Cancer Causes Control 2012, 23, 1625–1634. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; Alo, R.A.; Payton, M.; Tchounwou, P.B. Health and Racial Disparity in Breast Cancer. Adv. Exp. Med. Biol. 2019, 1152, 31–49. [Google Scholar] [CrossRef]
- Reid, S.; Spalluto, L.B.; Lang, K.; Weidner, A.; Pal, T. An overview of genetic services delivery for hereditary breast cancer. Breast Cancer Res. Treat. 2022, 191, 491–500. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ritte, R.; Tikk, K.; Lukanova, A.; Tjønneland, A.; Olsen, A.; Overvad, K.; Dossus, L.; Fournier, A.; Clavel-Chapelon, F.; Grote, V.; et al. Reproductive factors and risk of hormone receptor positive and negative breast cancer: A cohort study. BMC Cancer 2013, 13, 584. [Google Scholar] [CrossRef]
- Gao, Y.-T.; Shu, X.-O.; Dai, Q.; Potter, J.; Brinton, L.A.; Wen, W.; Sellers, T.A.; Kushi, L.; Ruan, Z.; Bostick, R.M.; et al. Association of menstrual and reproductive factors with breast cancer risk: Results from the Shanghai breast cancer study. Int. J. Cancer 2000, 87, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Stubert, J.; Reimer, T.; Erickson, N.; Berling, A. Influence of Lifestyle Factors on Breast Cancer Risk. Breast Care 2014, 9, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Golubicic, I.; Borojevic, N.; Pavlovic, T. Risk factors for breast cancer: Is ionizing radiation among them? J. Buon. 2008, 13, 487–494. [Google Scholar]
- Rocha, P.R.S.; Oliveira, V.D.; Vasques, C.I.; dos Reis, P.E.D.; Amato, A.A. Exposure to endocrine disruptors and risk of breast cancer: A systematic review. Crit. Rev. Oncol. 2021, 161, 103330. [Google Scholar] [CrossRef]
- Macon, M.B.; Fenton, S.E. Endocrine Disruptors and the Breast: Early Life Effects and Later Life Disease. J. Mammary Gland. Biol. Neoplasia 2013, 18, 43–61. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endo-crine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western Diet: How We Got Here. Mo. Med. 2010, 117, 536–538. [Google Scholar]
- Martínez Steele, E.; Baraldi, L.G.; da Costa Louzada, M.L.; Moubarac, J.-C.; Mozaffarian, D.; Monteiro, C.A. Ultra-Processed Foods and Added Sugars in the US Diet: Evidence from a Nationally Representative Cross-Sectional Study. BMJ Open 2016, 6, e009892. [Google Scholar] [CrossRef]
- Oikonomou, E.; Psaltopoulou, T.; Georgiopoulos, G.; Siasos, G.; Kokkou, E.; Antonopoulos, A.; Vogiatzi, G.; Tsalamandris, S.; Gennimata, V.; Papanikolaou, A.; et al. Western Dietary Pattern Is Associated With Severe Coronary Artery Disease. Angiology 2017, 69, 339–346. [Google Scholar] [CrossRef]
- Mey, J.T.; Godin, J.-P.; Scelsi, A.R.; Kullman, E.L.; Malin, S.K.; Yang, S.; Floyd, Z.E.; Poulev, A.; Fielding, R.; Ross, A.B.; et al. A Whole-Grain Diet Increases Whole-Body Protein Balance Compared with a Macronutrient-Matched Refined-Grain Diet. Curr. Dev. Nutr. 2021, 5, nzab121. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Malin, S.K.; Scelsi, A.R.; Kullman, E.L.; Navaneethan, S.D.; Pagadala, M.R.; Haus, J.M.; Filion, J.; Godin, J.P.; Kochhar, S.; et al. A Whole-Grain Diet Reduces Cardiovascular Risk Factors in Overweight and Obese Adults: A Ran-domized Controlled Trial. J. Nutr. 2016, 146, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hoang, T.; Bu, S.Y.; Kim, J.-M.; Choi, J.-H.; Park, E.; Lee, S.-M.; Park, E.; Min, J.Y.; Lee, I.S.; et al. Associations of Dietary Intake with Cardiovascular Disease, Blood Pressure, and Lipid Profile in the Korean Population: A Systematic Review and Meta-Analysis. J. Lipid Atheroscler. 2020, 9, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Godin, J.-P.; Ross, A.B.; Kirwan, J.P. A Whole-Grain Diet Increases Glucose-Stimulated Insulin Secretion Independent of Gut Hormones in Adults at Risk for Type 2 Diabetes. Mol. Nutr. Food Res. 2019, 63, e1800967. [Google Scholar] [CrossRef]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Haus, J.M.; Filion, J.; Pagadala, M.R.; Godin, J.-P.; Kochhar, S.; Ross, A.B.; Kirwan, J.P. A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: A randomized-controlled trial. Metabolism 2018, 82, 111–117. [Google Scholar] [CrossRef]
- Gaesser, G.A. Refined Grain Intake and Risk of Type 2 Diabetes. Mayo. Clin. Proc. 2022, 97, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Basiak-Rasała, A.; Różańska, D.; Zatońska, K. Food groups in dietary prevention of type 2 diabetes. Rocz. Panstw. Zakl. Hig. 2019, 70, 347–357. [Google Scholar] [CrossRef]
- Sievenpiper, J.L. Low-carbohydrate diets and cardiometabolic health: The importance of carbohydrate quality over quantity. Nutr. Rev. 2020, 78, 69–77. [Google Scholar] [CrossRef]
- Xiao, Y.; Ke, Y.; Wu, S.; Huang, S.; Li, S.; Lv, Z.; Yeoh, E.-K.; Lao, X.; Wong, S.; Kim, J.H.; et al. Association between whole grain intake and breast cancer risk: A systematic review and meta-analysis of observational studies. Nutr. J. 2018, 17, 87. [Google Scholar] [CrossRef]
- Mourouti, N.; Kontogianni, M.; Papavagelis, C.; Psaltopoulou, T.; Kapetanstrataki, M.; Plytzanopoulou, P.; Vassilakou, T.; Malamos, N.; Linos, A.; Panagiotakos, D.B. Whole Grain Consumption and Breast Cancer: A Case-Control Study in Women. J. Am. Coll. Nutr. 2015, 35, 143–149. [Google Scholar] [CrossRef]
- Wartella, E.A.; Lichtenstein, A.H.; Boon, C.S. (Eds.) Institute of Medicine Committee on Examination of Front-of-Package Nutrition Rating, S. and Symbols, in Front-of-Package Nutrition Rating Systems and Symbols: Phase I Report; National Academies Press (USA): Washington, DC, USA, 2010. [Google Scholar]
- Bradshaw, P.T.; Sagiv, S.K.; Kabat, G.C.; Satia, J.A.; Britton, J.A.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Consumption of sweet foods and breast cancer risk: A case-control study of women on Long Island, New York. Cancer Causes Control 2009, 20, 1509–1515. [Google Scholar] [CrossRef]
- Paglia, L. The sweet danger of added sugars. Eur. J. Paediatr. Dent. 2019, 20, 89. [Google Scholar] [CrossRef]
- Paglia, L.; Scaglioni, S.; Torchia, V.; De Cosmi, V.; Moretti, M.; Marzo, G.; Giuca, M.R. Familial and dietary risk factors in Early Childhood Caries. Eur. J. Paediatr. Dent. 2016, 17, 93–99. [Google Scholar] [PubMed]
- Arroyo-Quiroz, C.; Brunauer, R.; Alavez, S. Sugar-Sweetened Beverages and Cancer Risk: A Narrative Review. Nutr. Cancer 2022, 74, 3077–3095. [Google Scholar] [CrossRef] [PubMed]
- Epner, M.; Yang, P.; Wagner, R.W.; Cohen, L. Understanding the Link between Sugar and Cancer: An Examination of the Preclinical and Clinical Evidence. Cancers 2022, 14, 6042. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.P.; Aggarwal, K.K.; Zhang, P.Y. Omega-3 fatty acids and cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 441–445. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Sousa, T.M.; Santos, L.C.D. Dietary fatty acids, omega-6/omega-3 ratio and cholesterol intake associated with depressive symptoms in low-risk pregnancy. Nutr. Neurosci. 2022, 25, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Wang, W.; Wang, J.; Zhang, Y.; Jiao, J. Polyunsaturated fatty acids intake, omega-6/omega-3 ratio and mortality: Findings from two independent nationwide cohorts. Clin. Nutr. 2019, 38, 848–855. [Google Scholar] [CrossRef]
- Ruuth, M.; Lahelma, M.; Luukkonen, P.K.; Lorey, M.B.; Qadri, S.; Sädevirta, S.; Hyötyläinen, T.; Kovanen, P.T.; Hodson, L.; Yki-Järvinen, H.; et al. Overfeeding Saturated Fat Increases LDL (Low-Density Lipoprotein) Aggregation Susceptibility While Overfeeding Unsaturated Fat Decreases Proteoglycan-Binding of Lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2823–2836. [Google Scholar] [CrossRef]
- Chiu, S.; Williams, P.T.; Krauss, R.M. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS ONE 2017, 12, e0170664. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qi, X.; Li, N.; Kaifi, J.T.; Chen, S.; Wheeler, A.A.; Kimchi, E.T.; Ericsson, A.C.; Rector, R.S.; Staveley-O’carroll, K.F.; et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 2023, 14, 228. [Google Scholar] [CrossRef]
- Shen, Q.; Chen, Y.; Shi, J.; Pei, C.; Chen, S.; Huang, S.; Li, W.; Shi, X.; Liang, J.; Hou, S. Asperuloside alleviates lipid accumulation and inflammation in HFD-induced NAFLD via AMPK signaling pathway and NLRP3 inflammasome. Eur. J. Pharmacol. 2023, 942, 175504. [Google Scholar] [CrossRef]
- Hases, L.; Stepanauskaite, L.; Birgersson, M.; Brusselaers, N.; Schuppe-Koistinen, I.; Archer, A.; Engstrand, L.; Williams, C. High-fat diet and estrogen modulate the gut microbiota in a sex-dependent manner in mice. Commun. Biol. 2023, 6, 20. [Google Scholar] [CrossRef]
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans Fat Intake and Its Dietary Sources in General Populations Worldwide: A Systematic Review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef]
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. Int. Rev. J. 2019, 11, 697–708. [Google Scholar] [CrossRef]
- Matta, M.; Huybrechts, I.; Biessy, C.; Casagrande, C.; Yammine, S.; Fournier, A.; Olsen, K.S.; Lukic, M.; Gram, I.T.; Ardanaz, E.; et al. Dietary intake of trans fatty acids and breast cancer risk in 9 European countries. BMC Med. 2021, 19, 81. [Google Scholar] [CrossRef]
- Napier, B.A.; Andres-Terre, M.; Massis, L.M.; Hryckowian, A.J.; Higginbottom, S.K.; Cumnock, K.; Casey, K.M.; Haileselassie, B.; Lugo, K.A.; Schneider, D.S.; et al. Western diet regulates immune status and the response to LPS-driven sepsis independent of diet-associated microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 3688–3694. [Google Scholar] [CrossRef] [PubMed]
- Protani, M.; Coory, M.; Martin, J. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Field, R.; Pourkazemi, F.; Turton, J.; Rooney, K. Dietary Interventions Are Beneficial for Patients with Chronic Pain: A Systematic Review with Meta-Analysis. Pain Med. 2020, 22, 694–714. [Google Scholar] [CrossRef]
- Dianatinasab, M.; Rezaian, M.; HaghighatNezad, E.; Bagheri-Hosseinabadi, Z.; Amanat, S.; Rezaeian, S.; Masoudi, A.; Ghiasvand, R. Dietary Patterns and Risk of Invasive Ductal and Lobular Breast Carcinomas: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2020, 20, e516–e528. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, Cd009825. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Pastore, E.; Caini, S.; Bendinelli, B.; Palli, D.; Ermini, I.; Cavalcabo’, N.D.B.; Assedi, M.; Ambrogetti, D.; Fontana, M.; Masala, G. Dietary Patterns, Dietary Interventions, and Mammographic Breast Density: A Systematic Literature Review. Nutrients 2022, 14, 5312. [Google Scholar] [CrossRef]
- Mantzorou, M.; Tolia, M.; Poultsidi, A.; Vasios, G.K.; Papandreou, D.; Theocharis, S.; Kavantzas, N.; Troumbis, A.Y.; Giaginis, C. Adherence to Mediterranean Diet and Nutritional Status in Women with Breast Cancer: What Is Their Impact on Disease Progression and Recurrence-Free Patients’ Survival? Curr. Oncol. 2022, 29, 7482–7497. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Pre-vention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean Diet and Breast Cancer Risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Tomato, olives, chili pepper, wheat flour and wheat germ. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E4–E11. [Google Scholar] [PubMed]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control 1991, 2, 325–357. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.-H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. et Biophys. Acta (BBA)-Rev. Cancer 2014, 1846, 405–424. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Dutta, S.; Akter, R.; Rahman, H.; Karthika, C.; Nagaswarupa, H.P.; Murthy, H.C.A.; Fratila, O.; Brata, R.; Bungau, S. Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer. Biomolecules 2021, 11, 1176. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. Natural Compounds in Prostate Cancer Prevention and Treatment: Mechanisms of Action and Molecular Targets. Cells 2020, 9, 460. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Shang, A.; Gan, R.Y.; Wu, D.T.; Atanasov, A.G.; Li, H.B. Phytochemicals for the Prevention and Treat-ment of Gastric Cancer: Effects and Mechanisms. Int. J. Mol. Sci. 2020, 21, 570. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.-B. Dietary Natural Products for Prevention and Treatment of Liver Cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.J.; Caan, B.J.; Chen, W.Y.; Lu, W.; Chen, Z.; Kwan, M.L.; Flatt, S.W.; Zheng, Y.; Zheng, W.; Pierce, J.P.; et al. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012, 96, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Agaj, A.; Peršurić, Ž.; Pavelić, S.K. Mediterranean Food Industry By-Products as a Novel Source of Phytochemicals with a Promising Role in Cancer Prevention. Molecules 2022, 27, 8655. [Google Scholar] [CrossRef]
- Alsubhi, N.H.; Al-Quwaie, D.A.; Alrefaei, G.I.; Alharbi, M.; Binothman, N.; Aljadani, M.; Qahl, S.H.; Jaber, F.A.; Huwaikem, M.; Sheikh, H.M.; et al. Pomegranate Pomace Extract with Antioxidant, Anticancer, Antimicrobial, and Antiviral Activity Enhances the Quality of Strawberry-Yogurt Smoothie. Bioengineering 2022, 9, 735. [Google Scholar] [CrossRef]
- Ramlal, A.; Samanta, A. In Silico functional and phylogenetic analyses of fungal immunomodulatory proteins of some edible mushrooms. AMB Express 2022, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-Q.; Feng, X.-L.; Wang, Z.-X.; Xie, T.-C.; Duan, Y.; Liu, C.; Gao, J.-M.; Qi, J. Genomic and Metabolomic Analyses of the Medicinal Fungus Inonotus hispidus for Its Metabolite’s Biosynthesis and Medicinal Application. J. Fungi 2022, 8, 1245. [Google Scholar] [CrossRef]
- Tiasto, V.A.; Goncharov, N.V.; Romanishin, A.O.; Zhidkov, M.E.; Khotimchenko, Y.S. κ- and λ-Carrageenans from Marine Alga Chondrus armatus Exhibit Anticancer In Vitro Activity in Human Gastrointestinal Cancers Models. Mar. Drugs 2022, 20, 741. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Busenbender, T.; Hauschild, J.; Girich, E.V.; Kriegs, M.; Hoffer, K.; Graefen, M.; Yurchenko, A.N.; Bokemeyer, C.; von Amsberg, G. Cytotoxic N-Methylpretrichodermamide B Reveals Anticancer Activity and Inhibits P-Glycoprotein in Drug-Resistant Prostate Cancer Cells. Mar. Drugs 2022, 20, 597. [Google Scholar] [CrossRef]
- Trochopoulos, A.G.X.; Ilieva, Y.; Kroumov, A.D.; Dimitrova, L.L.; Tibi, I.P.-E.; Philipov, S.; Berger, M.R.; Najdenski, H.M.; Yoncheva, K.; Konstantinov, S.M.; et al. Micellar Curcumin Substantially Increases the Antineoplastic Activity of the Alkylphosphocholine Erufosine against TWIST1 Positive Cutaneous T Cell Lymphoma Cell Lines. Pharmaceutics 2022, 14, 2688. [Google Scholar] [CrossRef]
- Flint, A.L.; Hansen, D.W.; Brown, L.D.; Stewart, L.E.; Ortiz, E.; Panda, S.S. Modified Curcumins as Potential Drug Candidates for Breast Cancer: An Overview. Molecules 2022, 27, 8891. [Google Scholar] [CrossRef]
- Kabisch, S.; Weickert, M.O.; Pfeiffer, A.F.H. The role of cereal soluble fiber in the beneficial modulation of glycometabolic gastrointestinal hormones. Crit. Rev. Food Sci. Nutr. 2022, Nov 16, 1–17. [Google Scholar] [CrossRef]
- Markiewicz, L.H.; Ogrodowczyk, A.M.; Wiczkowski, W.; Wróblewska, B. Phytate Hydrolysate Differently Modulates the Immune Response of Human Healthy and Cancer Colonocytes to Intestinal Bacteria. Nutrients 2022, 14, 4234. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Citrus, cucumber and grape. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E21–E27. [Google Scholar] [PubMed]
- Fang, X.; Han, D.; Yang, J.; Li, F.; Sui, X. Citrus Consumption and Risk of Melanoma: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2022, 9, 904957. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M.; Dave, A.; Park, E.J.; Beyoğlu, D.; Idle, J.R. Short-Term Grape Consumption Diminishes UV-Induced Skin Erythema. Antioxidants 2022, 11, 2372. [Google Scholar] [CrossRef]
- Caponio, G.R.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-Proliferative and Pro-Apoptotic Effects of Digested Aglianico Grape Pomace Extract in Human Colorectal Cancer Cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Touihri-Barakati, I.; Kallech-Ziri, O.; Morjen, M.; Marrakchi, N.; Luis, J.; Hosni, K. Inhibitory effect of phenolic extract from squirting cucumber (Ecballium elaterium (L.) A. Rich) seed oil on integrin-mediated cell adhesion, migration and angio-genesis. RSC Adv. 2022, 12, 31747–31756. [Google Scholar] [CrossRef]
- Chen, T.; Ma, B.; Lu, S.; Zeng, L.; Wang, H.; Shi, W.; Zhou, L.; Xia, Y.; Zhang, X.; Zhang, J.; et al. Cucumber-Derived Nan-ovesicles Containing Cucurbitacin B for Non-Small Cell Lung Cancer Therapy. Int. J. Nanomed. 2022, 17, 3583–3599. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Trono, D. Molecular Approaches to Genetically Improve the Accumulation of Health-Promoting Secondary Metabolites in Staple Crops—A Case Study: The Lipoxygenase-B1 Genes and Regulation of the Carotenoid Content in Pasta Products. Int. J. Mol. Sci. 2016, 17, 1177. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sang, S. Phytochemicals in whole grain wheat and their health-promoting effects. Mol. Nutr. Food Res. 2017, 61, 1600852. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Liu, R.H. Effect of selected phytochemicals and apple extracts on NF-kappaB activation in human breast cancer MCF-7 cells. J. Agric. Food Chem. 2007, 55, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Grattan, B.J. Plant Sterols as Anticancer Nutrients: Evidence for Their Role in Breast Cancer. Nutrients 2013, 5, 359–387. [Google Scholar] [CrossRef]
- Bilal, I.; Chowdhury, A.; Davidson, J.; Whitehead, S. Phytoestrogens and prevention of breast cancer: The contentious debate. World J. Clin. Oncol. 2014, 5, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Kang, J.H.; Grodstein, F. Plasma carotenoids and tocopherols and cognitive function: A prospective study. Neurobiol. Aging 2008, 29, 1394–1403. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Mendonca, P.; Elhag, R.; Soliman, K.F.A. Anticancer Effects of Fucoxanthin through Cell Cycle Arrest, Apoptosis Induction, Angiogenesis Inhibition, and Autophagy Modulation. Int. J. Mol. Sci. 2022, 23, 16091. [Google Scholar] [CrossRef]
- Cao, S.; Liu, L.; Zhu, Q.; Zhu, Z.; Zhou, J.; Wei, P.; Wu, M. Adherence to the Vegetable-Fruit-Soy Dietary Pattern, a Reference From Mediterranean Diet, Protects Against Postmenopausal Breast Cancer Among Chinese Women. Front. Nutr. 2022, 9, 800996. [Google Scholar] [CrossRef]
- Markellos, C.; Ourailidou, M.-E.; Gavriatopoulou, M.; Halvatsiotis, P.; Sergentanis, T.N.; Psaltopoulou, T. Olive oil intake and cancer risk: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0261649. [Google Scholar] [CrossRef]
- Giani, M.; Montoyo-Pujol, Y.G.; Peiró, G.; Martínez-Espinosa, R.M. Halophilic Carotenoids and Breast Cancer: From Salt Marshes to Biomedicine. Mar. Drugs 2021, 19, 594. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Peraita-Costa, I.; Garcia, P.C.; Morales-Suárez-Varela, M. Is There an Association between β-Carotene and Breast Cancer? A Systematic Review on Breast Cancer Risk. Nutr. Cancer 2020, 74, 39–54. [Google Scholar] [CrossRef]
- Peng, C.; Gao, C.; Lu, D.; Rosner, B.A.; Zeleznik, O.; Hankinson, S.E.; Kraft, P.; Eliassen, A.H.; Tamimi, R.M. Circulating ca-rotenoids and breast cancer among high-risk individuals. Am. J. Clin. Nutr. 2021, 113, 525–533. [Google Scholar] [CrossRef]
- Peng, C.; Zeleznik, O.A.; Shutta, K.H.; Rosner, B.A.; Kraft, P.; Clish, C.B.; Stampfer, M.J.; Willett, W.C.; Tamimi, R.M.; Eliassen, A.H. A Metabolomics Analysis of Circulating Carotenoids and Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2022, 31, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammation-related diseases. Life Sci. 2020, 255, 117850. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, C.-Y.; Varjani, S.; Chang, J.-S. Producing fucoxanthin from algae—Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2021, 344, 126170. [Google Scholar] [CrossRef]
- Din, N.A.S.; Alayudin, S.M.; Sofian-Seng, N.-S.; Rahman, H.A.; Razali, N.S.M.; Lim, S.J.; Mustapha, W.A.W. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, L.; Wang, L.; Xiao, J.; Sun, J.; Zhou, Y.; Xu, X.; Xu, W.; Spiliopoulou, A.; Timofeeva, M.; et al. Alcohol consumption, blood DNA methylation and breast cancer: A Mendelian randomisation study. Eur. J. Epidemiol. 2022, 37, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Woodward, K.A.; Draijer, R.; Thijssen, D.H.J.; Low, D.A. Polyphenols and Microvascular Function in Humans: A Systematic Review. Curr. Pharm. Des. 2018, 24, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248s–3254s. [Google Scholar] [CrossRef]
- Feng, X.L.; Ho, S.C.; Mo, X.F.; Lin, F.Y.; Zhang, N.Q.; Luo, H.; Zhang, X.; Zhang, C.X. Association between flavonoids, fla-vonoid subclasses intake and breast cancer risk: A case-control study in China. Eur. J. Cancer Prev. 2020, 29, 493–500. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Silveira Rabelo, A.C.; Mertens-Talcott, S.U.; Chew, B.P.; Noratto, G. Dark Sweet Cherry (Prunus avium) Anthocyanins Suppressed ERK1/2-Akt/mTOR Cell Signaling and Oxidative Stress: Implications for TNBC Growth and Invasion. Molecules 2022, 27, 7245. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Fouladdel, S.; Movahhed, T.K.; Modaresi, F.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Atashpour, S. Quercetin Effects on Cell Cycle Arrest and Apoptosis and Doxorubicin Activity in T47D Cancer Stem Cells. Asian Pac. J. Cancer Prev. 2022, 23, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Majidinia, M.; Moliani, A.; Alemi, F.; Asemi, Z.; Yousefi, B.; Naghibi, A.F. The modulatory effects of two bioflavonoids, quercetin and thymoquinone on the expression levels of DNA damage and repair genes in human breast, lung and prostate cancer cell lines. Pathol.-Res. Pr. 2022, 240, 154143. [Google Scholar] [CrossRef]
- Tsai, K.-J.; Tsai, H.-Y.; Tsai, C.-C.; Chen, T.-Y.; Hsieh, T.-H.; Chen, C.-L.; Mbuyisa, L.; Huang, Y.-B.; Lin, M.-W. Luteolin Inhibits Breast Cancer Stemness and Enhances Chemosensitivity through the Nrf2-Mediated Pathway. Molecules 2021, 26, 6452. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Dai, Q.; Si, H.; Zhang, L.; Eltom, S.E.; Si, H. Combined Luteolin and Indole-3-Carbinol Synergistically Constrains ERα-Positive Breast Cancer by Dual Inhibiting Estrogen Receptor Alpha and Cyclin-Dependent Kinase 4/6 Pathway in Cultured Cells and Xenograft Mice. Cancers 2021, 13, 2116. [Google Scholar] [CrossRef] [PubMed]
- Romanos-Nanclares, A.; Sánchez-Quesada, C.; Gardeazábal, I.; Martínez-González, M.; Gea, A.; Toledo, E. Phenolic Acid Subclasses, Individual Compounds, and Breast Cancer Risk in a Mediterranean Cohort: The SUN Project. J. Acad. Nutr. Diet. 2020, 120, 1002–1015.e5. [Google Scholar] [CrossRef]
- Xue, W.; Hao, J.; Zhang, Q.; Jin, R.; Luo, Z.; Yang, X.; Liu, Y.; Lu, Q.; Ouyang, Y.; Guo, H. Chlorogenic acid inhibits epithe-li-al-mesenchymal transition and invasion of breast cancer by down-regulating LRP6. J. Pharmacol. Exp. Ther. 2023, 384, 254–264. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Hung, C.-H.; Chan, S.-H.; Hsieh, P.-L.; Ou, H.-C.; Cheng, Y.-H.; Chu, P.-M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; Britto, J.S.J.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. [Google Scholar] [CrossRef]
- Changizi, Z.; Moslehi, A.; Rohani, A.H.; Eidi, A. Chlorogenic acid induces 4T1 breast cancer tumor’s apoptosis via p53, Bax, Bcl-2, and caspase-3 signaling pathways in BALB/c mice. J. Biochem. Mol. Toxicol. 2021, 35, e22642. [Google Scholar] [CrossRef]
- Changizi, Z.; Moslehi, A.; Rohani, A.H.; Eidi, A. Chlorogenic acid inhibits growth of 4T1 breast cancer cells through in-volvement in Bax/Bcl2 pathway. J. Cancer Res. Ther. 2020, 16, 1435–1442. [Google Scholar]
- Liu, J.-L.; Segovia, I.; Yuan, X.-L.; Gao, Z.-H. Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion. Int. J. Mol. Sci. 2020, 21, 910. [Google Scholar] [CrossRef]
- Sakata, T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br. J. Nutr. 1987, 58, 95–103. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Quigley, C.; Paw, C.; Butala, S.; Schneider, E.; Pandiyan, P. Role of Short Chain Fatty Acids in Controlling T(regs) and Immunopathology During Mucosal Infection. Front. Microbiol. 2018, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.; Jannasch, A.; Cooper, B.; Patterson, J.; Kim, C. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2014, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Bass, J. Circadian topology of metabolism. Nature 2012, 491, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Qiu, Y.; Huang, Y.; Zou, H.; Cheng, X.; Niu, Q.; Luo, A.; Sun, J. Effects of short-chain fatty acids in inhibiting HDAC and activating p38 MAPK are critical for promoting B10 cell generation and function. Cell Death Dis. 2021, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.C.; Kouhfar, F.; Shojaosadati, S.A. Pectin/lignocellulose nanofibers/chitin nanofibers bionanocomposite as an efficient biosorbent of cholesterol and bile salts. Carbohydr. Polym. 2021, 261, 117883. [Google Scholar] [CrossRef] [PubMed]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.; Compari, C.; Fisicaro, E. On the mechanism of the cholesterol lowering ability of soluble dietary fibers: In-teraction of some bile salts with pectin, alginate, and chitosan studied by isothermal titration calorimetry. Front. Nutr. 2022, 9, 968847. [Google Scholar] [CrossRef]
- Bin Emran, T.; Islam, F.; Mitra, S.; Paul, S.; Nath, N.; Khan, Z.; Das, R.; Chandran, D.; Sharma, R.; Lima, C.M.G.; et al. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules 2022, 27, 7405. [Google Scholar] [CrossRef]
- Prado, S.B.R.D.; Shiga, T.M.; Harazono, Y.; Hogan, V.A.; Raz, A.; Carpita, N.C.; Fabi, J.P. Migration and proliferation of cancer cells in culture are differentially affected by molecular size of modified citrus pectin. Carbohydr. Polym. 2019, 211, 141–151. [Google Scholar] [CrossRef]
- Gaikwad, D.; Shewale, R.; Patil, V.; Mali, D.; Gaikwad, U.; Jadhav, N. Enhancement in in vitro anti-angiogenesis activity and cytotoxicity in lung cancer cell by pectin-PVP based curcumin particulates. Int. J. Biol. Macromol. 2017, 104, 656–664. [Google Scholar] [CrossRef]
- Sliva, D. Suppression of Cancer Invasiveness by Dietary Compounds. Mini-Reviews Med. Chem. 2008, 8, 677–688. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, L.; Gong, F.L.; Sun, C.; Du, D.D.; Yang, X.X.; Guo, X.L. Modified citrus pectin inhibits breast cancer devel-opment in mice by targeting tumor-associated macrophage survival and polarization in hypoxic microenvironment. Acta Pharmacol. Sin. 2022, 43, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P.A.V.D.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef]
- Hernández, M.L.; Sicardo, M.D.; Belaj, A.; Martínez-Rivas, J.M. The Oleic/Linoleic Acid Ratio in Olive (Olea europaea L.) Fruit Mesocarp Is Mainly Controlled by OeFAD2-2 and OeFAD2-5 Genes Together With the Different Specificity of Ex-traplastidial Acyltransferase Enzymes. Front. Plant Sci. 2021, 12, 653997. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bermudez, B.; la Paz, S.M.-D.; Jaramillo, S.; Varela, L.M.; Ortega-Gomez, A.; Abia, R.; Muriana, F.J. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim. et Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1638–1656. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S. Role of oleic acid in immune system; mechanism of action; a review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar]
- Li, S.; Zhou, T.; Li, C.; Dai, Z.; Che, D.; Yao, Y.; Li, L.; Ma, J.; Yang, X.; Gao, G. High Metastaticgastric and Breast Cancer Cells Consume Oleic Acid in an AMPK Dependent Manner. PLoS ONE 2014, 9, e97330. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, B.; Zhao, Y.; Tao, Z.; Wang, Y.; Chen, G.; Hu, X. Mammary adipocytes protect triple-negative breast cancer cells from ferroptosis. J. Hematol. Oncol. 2022, 15, 72. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S.R. Antitumor effect of oleic acid; mechanisms of action: A review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar] [PubMed]
- Qian, S.Y.; Xu, Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Porras, B.; Pérez-Burillo, S.; Morales-Pérez, J.; Rufián-Henares, J.; Pastoriza, S. Relationship of quality parameters, antioxidant capacity and total phenolic content of EVOO with ripening state and olive variety. Food Chem. 2020, 325, 126926. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Arnal, C.; Navarro, M.A.; Osada, J. Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Mol. Nutr. Food Res. 2012, 56, 1043–1057. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Y.; Li, X.; Shi, D.; Ma, T.; Zhou, R.; Zhang, C. Association of dietary n—3 polyunsaturated fatty acids with breast cancer risk: Serial mediating roles of erythrocyte n—3 polyunsaturated fatty acids. Front. Nutr. 2022, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nindrea, R.D.; Aryandono, T.; Lazuardi, L.; Dwiprahasto, I. Association of Dietary Intake Ratio of n-3/n-6 Polyunsaturated Fatty Acids with Breast Cancer Risk in Western and Asian Countries: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2019, 20, 1321–1327. [Google Scholar] [CrossRef]
- Costa, V.; Costa, M.; Videira, R.A.; Andrade, P.B.; Paiva-Martins, F. Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines 2022, 10, 2990. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J. N-6 Polyunsaturated Fatty Acids and Risk of Cancer: Accumulating Evidence from Prospective Studies. Nutrients 2020, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.G.; Hoover, R.N.; Pike, M.C.; Hildesheim, A.; Nomura, A.M.Y.; West, D.W.; Wu-Williams, A.H.; Kolonel, L.N.; Horn-Ross, P.L.; Rosenthal, J.F.; et al. Migration Patterns and Breast Cancer Risk in Asian-American Women. Gynecol. Oncol. 1993, 85, 1819–1827. [Google Scholar] [CrossRef]
- Anderson, B.M.; Ma, D.W. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of Phenolic Compounds and Their Contribution to Sensory Properties of Olive Oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero Del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from ex-tra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Esposito Salsano, J.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and miRNA Expression in Adipocytes by Attenuating NF-κB Activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef]
- Rojas Gil, A.P.; Kodonis, I.; Ioannidis, A.; Nomikos, T.; Dimopoulos, I.; Kosmidis, G.; Katsa, M.E.; Melliou, E.; Magiatis, P. The Effect of Dietary Intervention With High-Oleocanthal and Oleacein Olive Oil in Patients With Early-Stage Chronic Lym-phocytic Leukemia: A Pilot Randomized Trial. Front. Oncol. 2021, 11, 810249. [Google Scholar] [CrossRef]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- Lund, E.K. Health benefits of seafood; is it just the fatty acids? Food Chem. 2013, 140, 413–420. [Google Scholar] [CrossRef]
- Azzeh, F.S.; Hasanain, D.M.; Qadhi, A.H.; Ghafouri, K.J.; Azhar, W.F.; Ghaith, M.M.; Aldairi, A.F.; Almasmoum, H.A.; As-saggaf, H.M.; Alhussain, M.H.; et al. Consumption of Food Components of the Mediterranean Diet Decreases the Risk of Breast Cancer in the Makkah Region, Saudi Arabia: A Case-Control Study. Front. Nutr. 2022, 9, 863029. [Google Scholar] [CrossRef]
- Engeset, D.; Alsaker, E.; Lund, E.; Welch, A.; Khaw, K.T.; Clavel-Chapelon, F.; Thiébaut, A.; Chajès, V.; Key, T.J.; Allen, N.E.; et al. Fish consumption and breast cancer risk. The European Prospective Investigation into Cancer and Nutrition (EPIC). Int. J. Cancer 2006, 119, 175–182. [Google Scholar] [CrossRef]
- Nindrea, R.D.; Aryandono, T.; Lazuardi, L.; Dwiprahasto, I. Protective Effect of Omega-3 Fatty Acids in Fish Consumption Against Breast Cancer in Asian Patients: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2019, 20, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Fodil, M.; Blanckaert, V.; Ulmann, L.; Mimouni, V.; Chénais, B. Contribution of n-3 Long-Chain Polyunsaturated Fatty Acids to the Prevention of Breast Cancer Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 7936. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Liddle, D.M.; Hutchinson, A.L.; Burns, J.L.; Wellings, H.; Cartwright, N.M.; Muller, W.J.; Power, K.A.; Robinson, L.E.; Ma, W.L.D. Fish oil supplementation increases expression of mammary tumor apoptosis mediators and reduces inflam-mation in an obesity-associated HER-2 breast cancer model. J. Nutr. Biochem. 2021, 95, 108763. [Google Scholar] [CrossRef]
- Liu, J.; Abdelmagid, S.A.; Pinelli, C.J.; Monk, J.M.; Liddle, D.M.; Hillyer, L.M.; Hucik, B.; Silva, A.; Subedi, S.; Wood, G.A.; et al. Marine fish oil is more potent than plant-based n-3 polyunsaturated fatty acids in the prevention of mammary tumors. J. Nutr. Biochem. 2018, 55, 41–52. [Google Scholar] [CrossRef]
- Xu, M.; Li, H.; Chen, D.; Wu, H.; Wen, W.; Xu, H.; Frank, J.; Chen, G.; Luo, J. Adolescent-and adult-initiated alcohol exposure in mice differentially promotes tumorigenesis and metastasis of breast cancer. Alcohol. Clin. Exp. Res. 2022, 47, 251–262. [Google Scholar] [CrossRef]
- Stott, D.J. Alcohol and mortality in older people: Understanding the J-shaped curve. Age Ageing 2020, 49, 332–333. [Google Scholar] [CrossRef] [PubMed]
- de Gaetano, G.; Costanzo, S. Alcohol and Health: Praise of the J Curves. J. Am. Coll. Cardiol. 2017, 70, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56, 635–640. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Speizer, F.E.; Hennekens, C.H. A prospective study of moderate alcohol con-sumption and the risk of coronary disease and stroke in women. N. Engl. J. Med. 1988, 319, 267–273. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef]
- Ambra, R.; Pastore, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; de la Torre, R. Beer Phenolic Composition of Simple Phenols, Prenylated Flavonoids and Alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef]
- Castro, M.C.; Bortoletto, A.M.; Silvello, G.C.; Alcarde, A.R. Lignin-derived phenolic compounds in cachaça aged in new barrels made from two oak species. Heliyon 2020, 6, e05586. [Google Scholar] [CrossRef]
- Gadrat, M.; Emo, C.; Lavergne, J.; Teissèdre, P.-L.; Chira, K. Impact of Barrel Toasting on Ellagitannin Composition of Aged Cognac Eaux-de-Vie. Molecules 2022, 27, 2531. [Google Scholar] [CrossRef] [PubMed]
- Krnic, M.; Modun, D.; Budimir, D.; Gunjaca, G.; Jajic, I.; Vukovic, J.; Salamunic, I.; Sutlovic, D.; Kozina, B.; Boban, M. Comparison of acute effects of red wine, beer and vodka against hyperoxia-induced oxidative stress and increase in arterial stiffness in healthy humans. Atherosclerosis 2011, 218, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Foroozani, E.; Akbari, A.; Amanat, S.; Rashidi, N.; Bastam, D.; Ataee, S.; Sharifnia, G.; Faraouei, M.; Dianatinasab, M.; Safdari, H. Ad-herence to a western dietary pattern and risk of invasive ductal and lobular breast carcinomas: A case-control study. Sci Rep. 2022, 12, 5859. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Boldo, E.; Pérez-Gómez, B.; Lope, V.; Altzibar, J.M.; Martín, V.; Castaño-Vinyals, G.; Guevara, M.; Dierssen-Sotos, T.; Tardón, A.; et al. Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 2017, 103, 8–15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-H.; Yu, J.-C.; Hsu, H.-M.; Chu, C.-H.; Chang, T.-M.; Hong, Z.-J.; Feng, A.-C.; Fu, C.-Y.; Hsu, K.-F.; Dai, M.-S.; et al. The Risk of Breast Cancer between Western and Mediterranean Dietary Patterns. Nutrients 2023, 15, 2057. https://doi.org/10.3390/nu15092057
Tsai H-H, Yu J-C, Hsu H-M, Chu C-H, Chang T-M, Hong Z-J, Feng A-C, Fu C-Y, Hsu K-F, Dai M-S, et al. The Risk of Breast Cancer between Western and Mediterranean Dietary Patterns. Nutrients. 2023; 15(9):2057. https://doi.org/10.3390/nu15092057
Chicago/Turabian StyleTsai, Hsueh-Han, Jyh-Cherng Yu, Huan-Ming Hsu, Chi-Hong Chu, Tzu-Ming Chang, Zhi-Jie Hong, An-Chieh Feng, Chun-Yu Fu, Kuo-Feng Hsu, Ming-Shen Dai, and et al. 2023. "The Risk of Breast Cancer between Western and Mediterranean Dietary Patterns" Nutrients 15, no. 9: 2057. https://doi.org/10.3390/nu15092057
APA StyleTsai, H.-H., Yu, J.-C., Hsu, H.-M., Chu, C.-H., Chang, T.-M., Hong, Z.-J., Feng, A.-C., Fu, C.-Y., Hsu, K.-F., Dai, M.-S., & Liao, G.-S. (2023). The Risk of Breast Cancer between Western and Mediterranean Dietary Patterns. Nutrients, 15(9), 2057. https://doi.org/10.3390/nu15092057





