Human Milk Oligosaccharides Are Associated with Lactation Stage and Lewis Phenotype in a Chinese Population
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Human Milk Sample Collection
2.3. Analysis of Human Milk Oligosaccharides
2.4. Maternal Secretor Status and Geographical Factors
2.5. Statistical Analysis
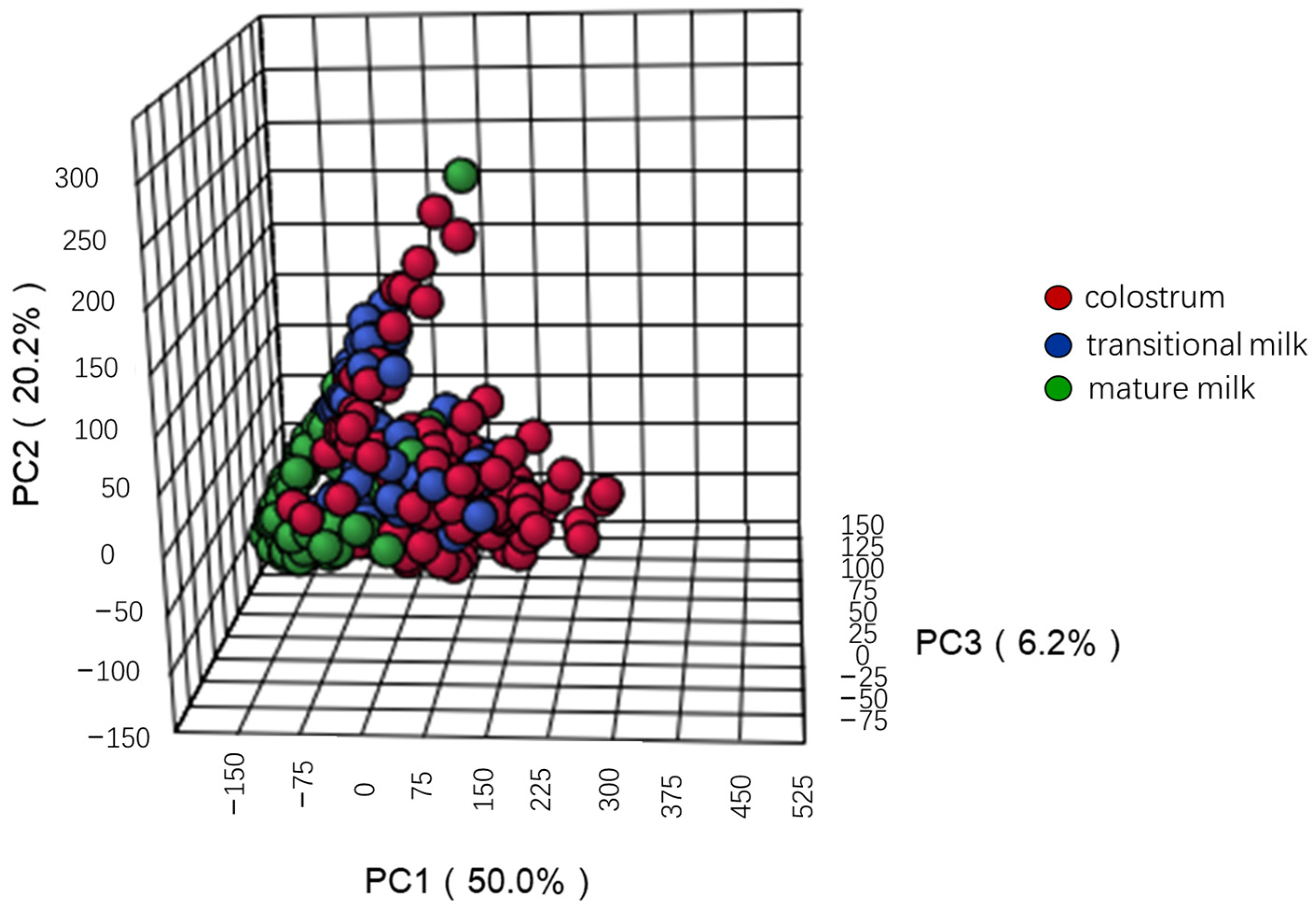
3. Results
3.1. Characteristics of Study Subjects
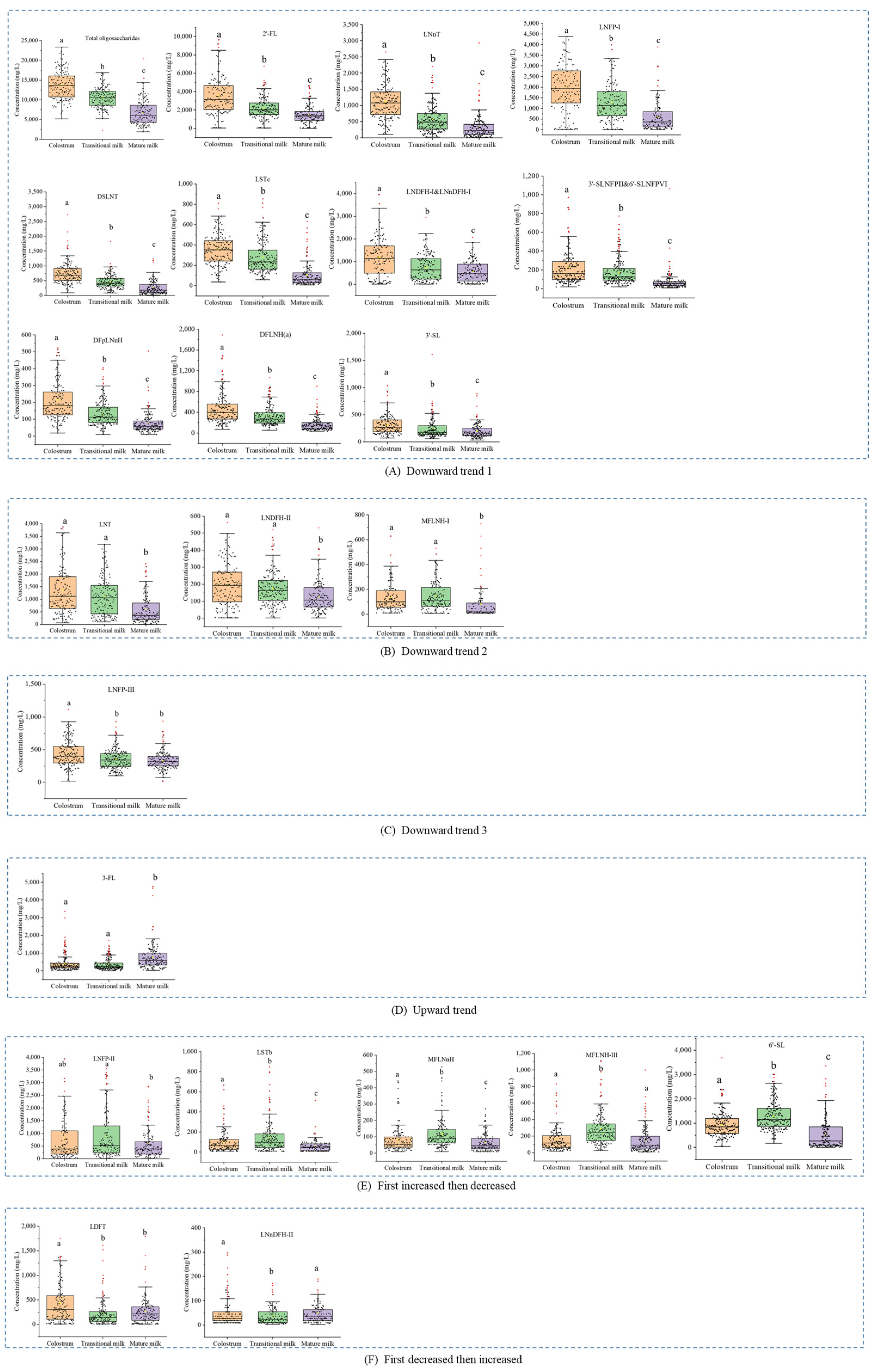
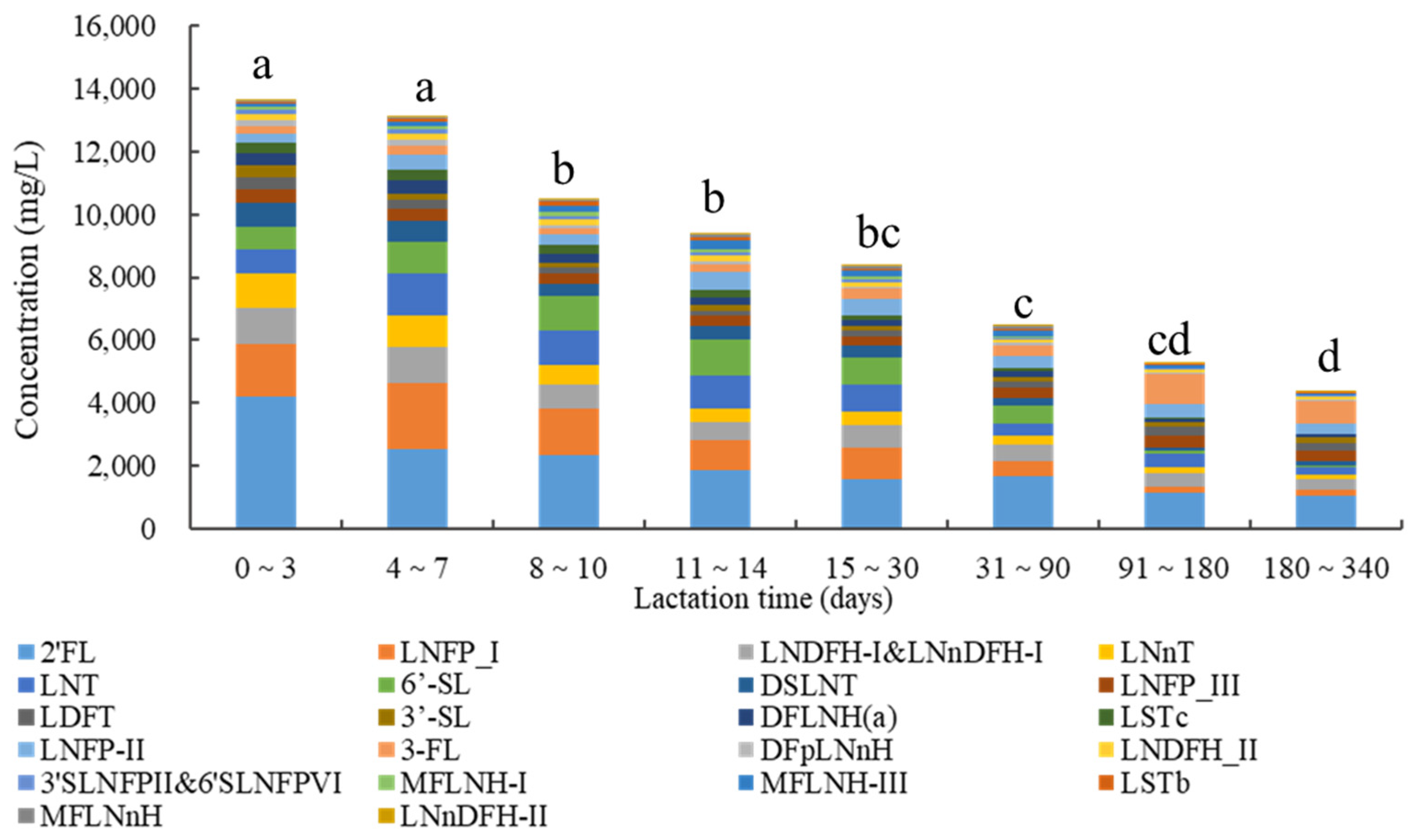
3.2. Concentrations of Human Milk Oligosaccharides during Different Lactation Periods
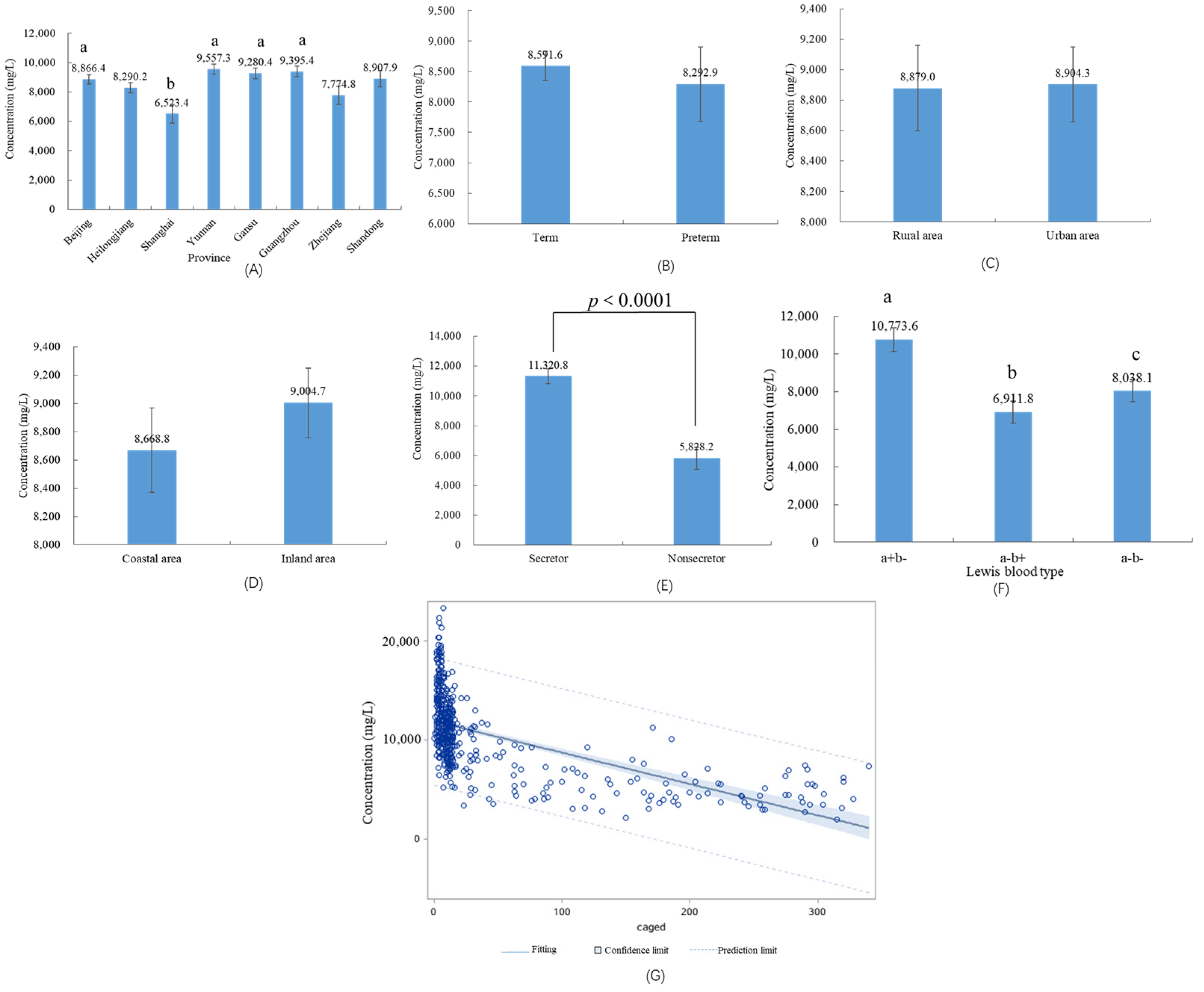
3.3. HMOs in Different Geographical Factors and Preterm Infants
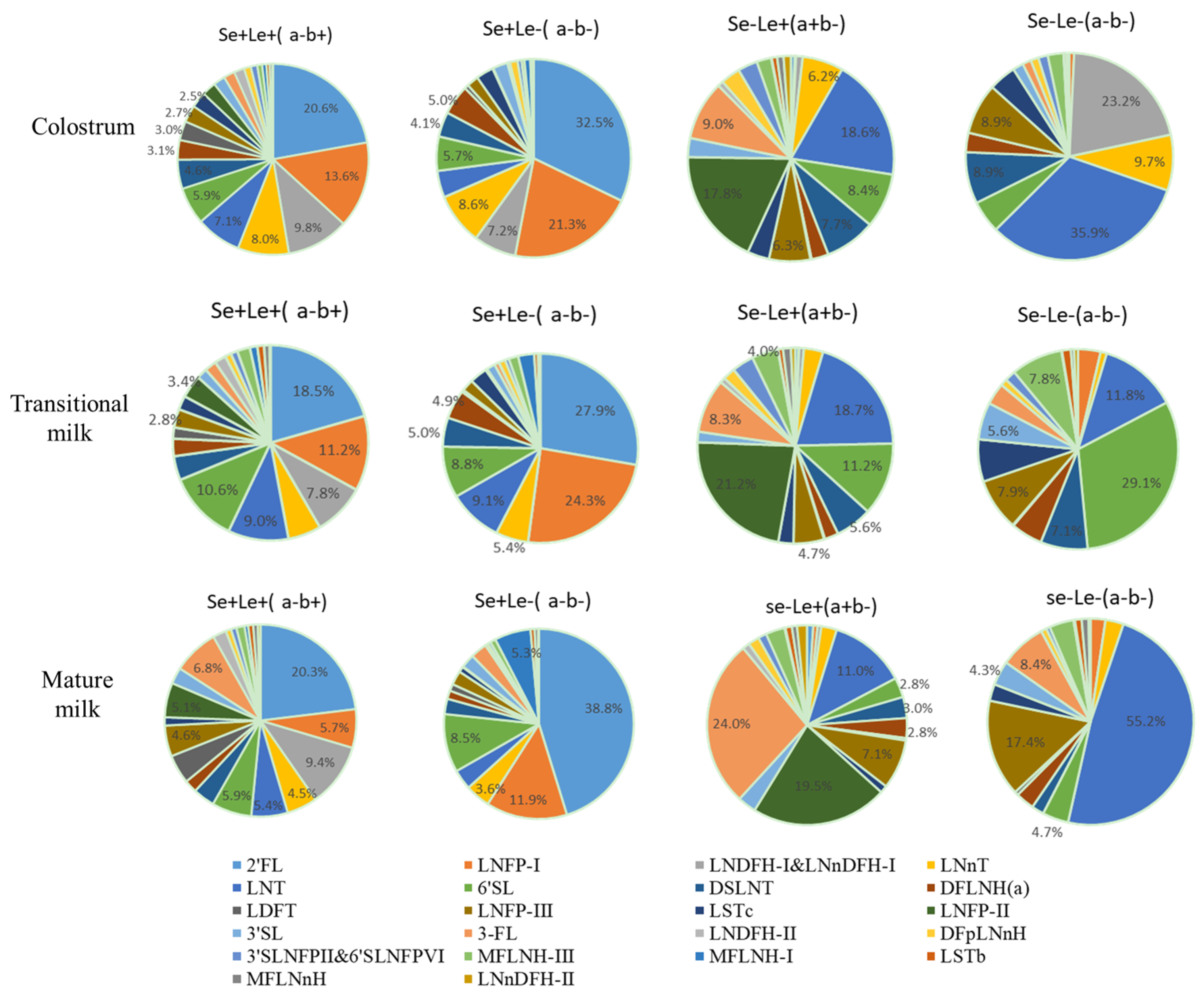
3.4. HMOs in Different Maternal Secretor Status and Lewis Blood Type
3.5. Influencing Factors on the Concentrations of HMOs
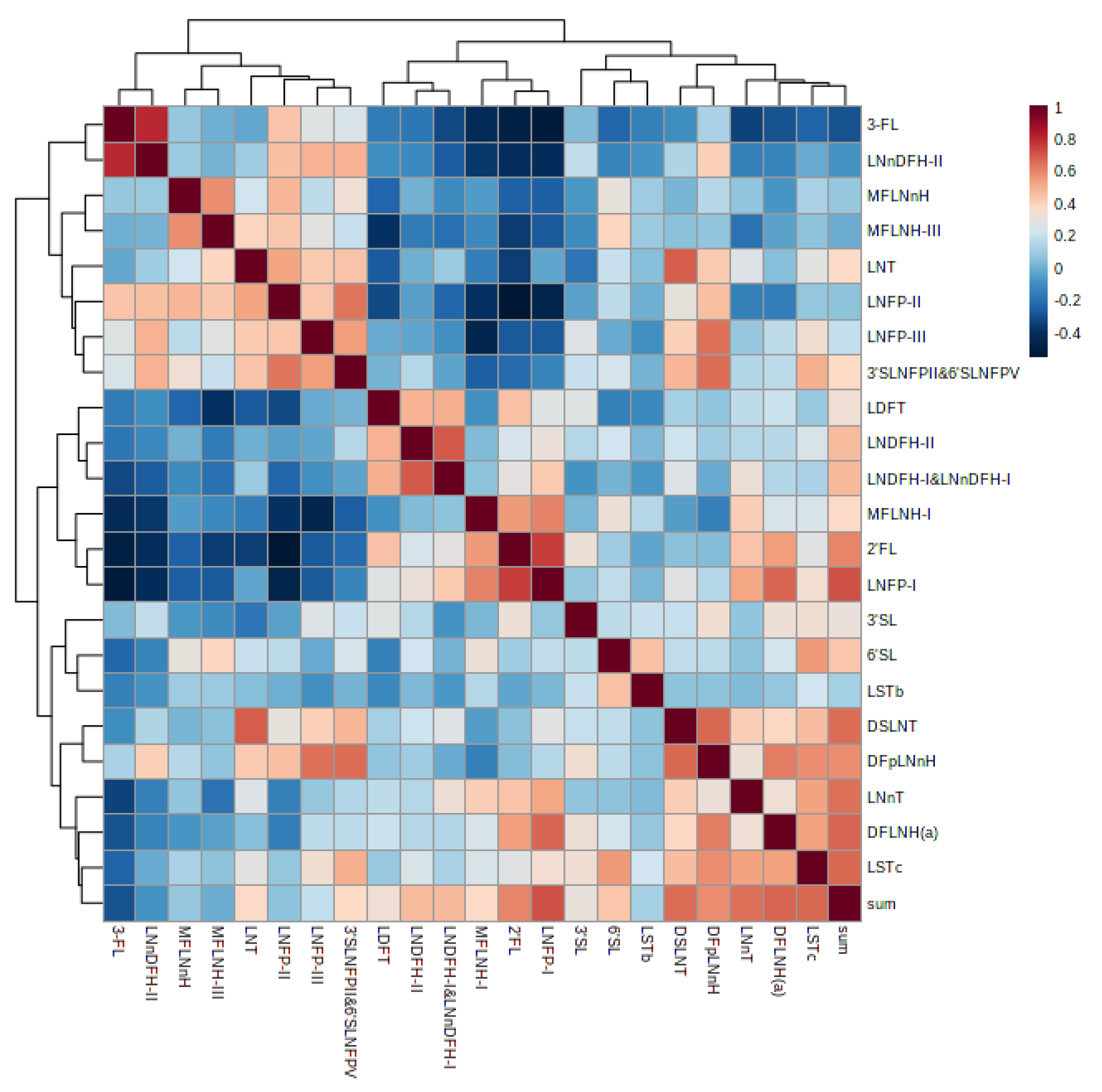
3.6. Correlation of Human Milk Oligosaccharides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Triantis, V.; Bode, L.; van Neerven, R.J.J. Immunological Effects of Human Milk Oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.R.; Chow, J.M.; Buck, R.H. Multifunctional Benefits of Prevalent HMOs: Implications for Infant Health. Nutrients 2021, 13, 3364. [Google Scholar] [CrossRef]
- Okburan, G.; Kızıler, S. Human milk oligosaccharides as prebiotics. Pediatr. Neonatol. 2023. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2016, 69 (Suppl. S2), 42–51. [Google Scholar] [CrossRef]
- Liu, F.; Simpson, A.B.; D’Costa, E.; Bunn, F.S.; van Leeuwen, S.S. Sialic acid, the secret gift for the brain. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Abbas, S.; Keir, A.K.; Makrides, M.; Klein, L.D.; Grzeskowiak, L.E.; McPhee, A.J.; Rumbold, A.R. Tailoring Human Milk Oligosaccharides to Prevent Necrotising Enterocolitis Among Preterm Infants. Front. Nutr. 2021, 8, 702888. [Google Scholar] [CrossRef]
- Kobata, A. Structures and application of oligosaccharides in human milk. Proc. Jpn. Academy Ser. B Phys. Biol. Sci. 2010, 86, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; et al. Human Milk Oligosaccharides: 2’-Fucosyllactose (2’-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Lozano, S.; de Vos, P. Relationship Between Oligosaccharides and Glycoconjugates Content in Human Milk and the Development of the Gut Barrier. Compr. Rev. Food Sci. Food Saf. 2019, 18, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Tonon, K.M.; de Morais, M.B.; Abrão, A.C.F.V.; Miranda, A.; Morais, T.B. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Chen, X.; Pang, X.; Zhang, S.; Obaroakpo, J.U.; Shilong, J.; Lu, J.; Lv, J. Absolute quantification of twelve oligosaccharides in human milk using a targeted mass spectrometry-based approach. Carbohydr. Polym. 2019, 219, 328–333. [Google Scholar] [CrossRef]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; García-Mantrana, I.; Bertua-Ríos, B.; Martínez-Costa, C.; Borsch, C.; Rudloff, S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef]
- Meredith-Dennis, L.; Xu, G.; Goonatilleke, E.; Lebrilla, C.B.; Underwood, M.A.; Smilowitz, J.T. Composition and Variation of Macronutrients, Immune Proteins, and Human Milk Oligosaccharides in Human Milk from Nonprofit and Commercial Milk Banks. J. Hum. Lact. 2018, 34, 120–129. [Google Scholar] [CrossRef]
- Nijman, R.M.; Liu, Y.; Bunyatratchata, A.; Smilowitz, J.T.; Stahl, B.; Barile, D. Characterization and Quantification of Oligosaccharides in Human Milk and Infant Formula. J. Agric. Food Chem. 2018, 66, 6851–6859. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef]
- Thurl, S.; Henker, J.; Siegel, M.; Tovar, K.; Sawatzki, G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J. 1997, 14, 795–799. [Google Scholar] [CrossRef]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.A.; Yang, Z.Y. An on-line database for human milk composition in China. Asia Pac. J. Clin. Nutr. 2016, 25, 818–825. [Google Scholar] [CrossRef]
- Li, J.; Bi, Y.; Zheng, Y.; Cao, C.; Yu, L.; Yang, Z.; Chai, W.; Yan, J.; Lai, J.; Liang, X. Development of high-throughput UPLC-MS/MS using multiple reaction monitoring for quantitation of complex human milk oligosaccharides and application to large population survey of secretor status and Lewis blood group. Food Chem. 2022, 397, 133750. [Google Scholar] [CrossRef]
- Yan, J.; Ding, J.; Jin, G.; Duan, Z.; Yang, F.; Li, D.; Zhou, H.; Li, M.; Guo, Z.; Chai, W.; et al. Profiling of Human Milk Oligosaccharides for Lewis Epitopes and Secretor Status by Electrostatic Repulsion Hydrophilic Interaction Chromatography Coupled with Negative-Ion Electrospray Tandem Mass Spectrometry. Anal. Chem. 2019, 91, 8199–8206. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Soyyılmaz, B.; Mikš, M.H.; Röhrig, C.H.; Matwiejuk, M.; Meszaros-Matwiejuk, A.; Vigsnæs, L.K. The Mean of Milk: A Review of Human Milk Oligosaccharide Concentrations throughout Lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Luo, G.; Lu, R.; Shi, W.; Cheng, H.; Lu, Y.; Jin, K.; Yang, C.; Wang, Z.; Long, J.; et al. Distribution of Lewis and Secretor polymorphisms and corresponding CA19-9 antigen expression in a Chinese population. FEBS Open Bio 2017, 7, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Reverri, E.J.; Devitt, A.A.; Kajzer, J.A.; Baggs, G.E.; Borschel, M.W. Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2’-Fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Leusink-Muis, T.; Kettelarij, N.; van Ark, I.; Blijenberg, B.; Hesen, N.A.; Stahl, B.; Overbeek, S.A.; Garssen, J.; Folkerts, G.; et al. Human Milk Oligosaccharide 2’-Fucosyllactose Improves Innate and Adaptive Immunity in an Influenza-Specific Murine Vaccination Model. Front. Immunol. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Hoeflinger, J.L.; Davis, S.R.; Chow, J.; Miller, M.J. In vitro impact of human milk oligosaccharides on Enterobacteriaceae growth. J. Agric. Food Chem. 2015, 63, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Nanthakumar, N.N.; Newburg, D.S. The Human Milk Oligosaccharide 2’-Fucosyllactose Quenches Campylobacter jejuni-Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Zehra, S.; Khambati, I.; Vierhout, M.; Mian, M.F.; Buck, R.; Forsythe, P. Human Milk Oligosaccharides Attenuate Antigen-Antibody Complex Induced Chemokine Release from Human Intestinal Epithelial Cell Lines. J. Food Sci. 2018, 83, 499–508. [Google Scholar] [CrossRef]
- Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Martínez-Lara, E.; Blanco, S.; Martín, M.J.; Castanys, E.; Buck, R.; et al. Effects of a human milk oligosaccharide, 2’-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J. Nutr. Biochem. 2015, 26, 455–465. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of 3-FL (3-Fucosyllactose) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. Eur. Food Saf. Auth. 2021, 19, e06662. [Google Scholar] [CrossRef]
- Shi, R.; Ma, J.; Yan, Q.; Yang, S.; Fan, Z.; Jiang, Z. Biochemical characterization of a novel α-L-fucosidase from Pedobacter sp. and its application in synthesis of 3’-fucosyllactose and 2’-fucosyllactose. Appl. Microbiol. Biotechnol. 2020, 104, 5813–5826. [Google Scholar] [CrossRef]
- Musumeci, M.; Simpore, J.; D’Agata, A.; Sotgiu, S.; Musumeci, S. Oligosaccharides in colostrum of Italian and Burkinabe women. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 372–378. [Google Scholar] [CrossRef]
- Erney, R.M.; Malone, W.T.; Skelding, M.B.; Marcon, A.A.; Kleman-Leyer, K.M.; O’Ryan, M.L.; Ruiz-Palacios, G.; Hilty, M.D.; Pickering, L.K.; Prieto, P.A. Variability of human milk neutral oligosaccharides in a diverse population. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean ± SD or Proportion (%) |
|---|---|
| Maternal age (years) | 26.8 ± 4.2 (n = 481) |
| Maternal BMI (kg/m2) | 23.3 ± 3.5 (n = 481) |
| Infant’s gender (boy) | 54.3% (n = 261) |
| Preterm | 4.4% (n = 21) |
| Birth weight of term infants (g) | 3491.3 ± 1060.9 (n = 460) |
| Birth weight of preterm infants (g) | 2845.2 ± 544.0 (n = 21) |
| C-section rate | 45.7% (n = 220) |
| Lactation stage | |
| Colostrum | 33.7% (n = 162) |
| Transitional milk | 35.3% (n = 170) |
| Mature milk | 31.0% (n = 149) |
| Residential area | |
| Rural area | 36.8% (n = 177) |
| Urban area | 63.2% (n = 304) |
| Geographical region | |
| Coastal area | 31.8% (n = 153) |
| Inland area | 68.2% (n = 328) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Yan, J.; Bi, Y.; Shuttleworth, P.W.; Wang, Y.; Jiang, S.; Wang, J.; Duan, Y.; Lai, J.; Yang, Z. Human Milk Oligosaccharides Are Associated with Lactation Stage and Lewis Phenotype in a Chinese Population. Nutrients 2023, 15, 1408. https://doi.org/10.3390/nu15061408
Ren X, Yan J, Bi Y, Shuttleworth PW, Wang Y, Jiang S, Wang J, Duan Y, Lai J, Yang Z. Human Milk Oligosaccharides Are Associated with Lactation Stage and Lewis Phenotype in a Chinese Population. Nutrients. 2023; 15(6):1408. https://doi.org/10.3390/nu15061408
Chicago/Turabian StyleRen, Xiangnan, Jingyu Yan, Ye Bi, Paul William Shuttleworth, Ye Wang, Shan Jiang, Jie Wang, Yifan Duan, Jianqiang Lai, and Zhenyu Yang. 2023. "Human Milk Oligosaccharides Are Associated with Lactation Stage and Lewis Phenotype in a Chinese Population" Nutrients 15, no. 6: 1408. https://doi.org/10.3390/nu15061408
APA StyleRen, X., Yan, J., Bi, Y., Shuttleworth, P. W., Wang, Y., Jiang, S., Wang, J., Duan, Y., Lai, J., & Yang, Z. (2023). Human Milk Oligosaccharides Are Associated with Lactation Stage and Lewis Phenotype in a Chinese Population. Nutrients, 15(6), 1408. https://doi.org/10.3390/nu15061408





