Nutritional Modulation of Hepcidin in the Treatment of Various Anemic States
Abstract
:1. Introduction
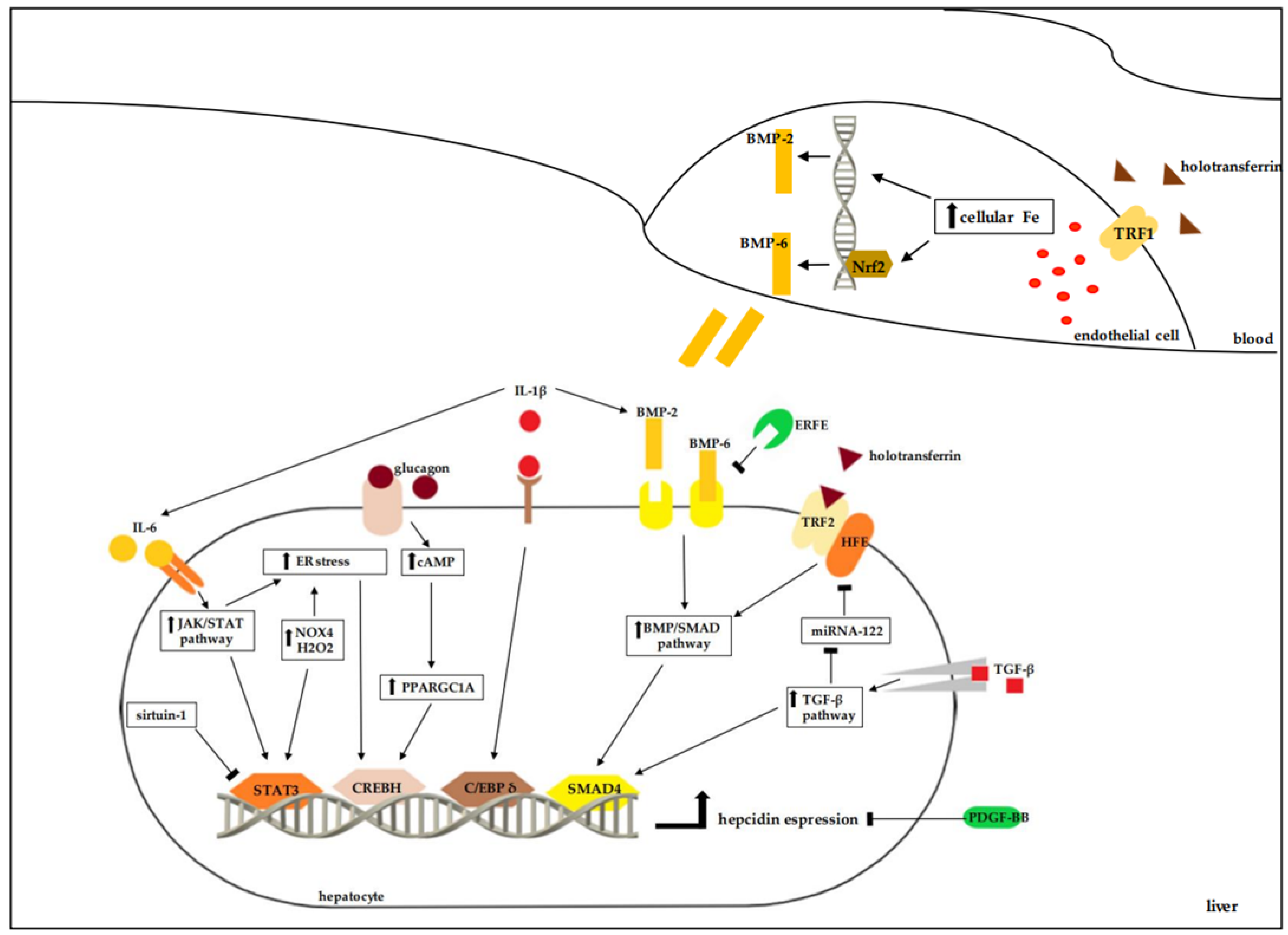
2. Regulation of Hepcidin Release
3. Anemic States Characterized by the Increase in Hepcidin Release
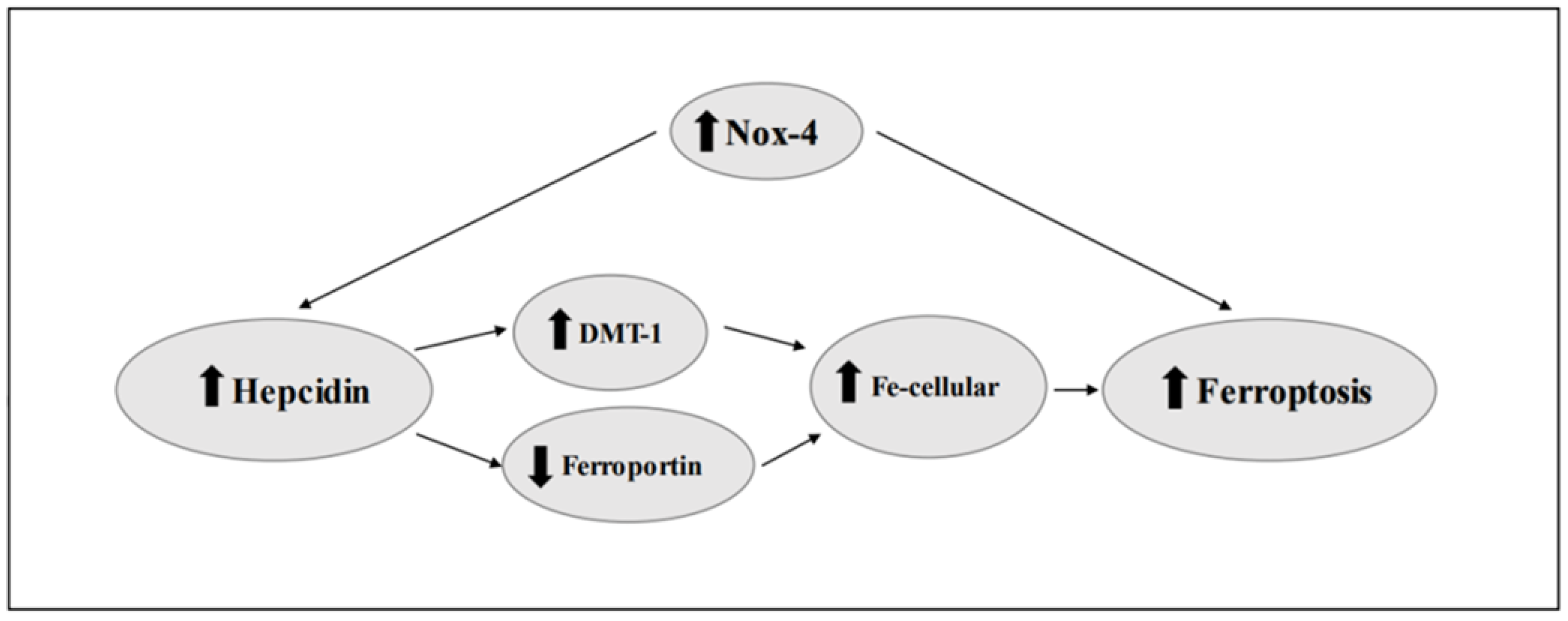
4. Ferroptosis and Hepcidin
5. Nutritional Modulation of Hepcidin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Olbina, G.; Girelli, D.; Nemeth, E.; Westerman, M. Immunoassay for human serum hepcidin. Blood 2008, 112, 4292–4297. [Google Scholar] [CrossRef]
- Troutt, J.S.; Rudling, M.; Persson, L.; Ståhle, L.; Angelin, B.; Butterfield, A.M.; Schade, A.E.; Cao, G.; Konrad, R.J. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin. Chem. 2012, 58, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar] [CrossRef]
- Billesbølle, C.B.; Azumaya, C.M.; Kretsch, R.C.; Powers, A.S.; Gonen, S.; Schneider, S.; Arvedson, T.; Dror, R.O.; Cheng, Y.; Manglik, A. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature 2020, 586, 807–811. [Google Scholar] [CrossRef]
- Ginzburg, Y.Z. Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019, 110, 17–45. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Ruwe, T.A.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Wang, K.; Wang, H.; Wu, Q.; Yang, C.; Yu, Y.; Ni, P.; Zhong, Y.; Song, Z.; et al. RNF217 regulates iron homeostasis through its E3 ubiquitin ligase activity by modulating ferroportin degradation. Blood 2021, 138, 689–705. [Google Scholar] [CrossRef]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef]
- Canali, S.; Wang, C.Y.; Zumbrennen-Bullough, K.B.; Bayer, A.; Babitt, J.L. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am. J. Hematol. 2017, 92, 1204–1213. [Google Scholar] [CrossRef]
- Andriopoulos, B., Jr.; Corradini, E.; Xia, Y.; Faasse, S.A.; Chen, S.; Grgurevic, L.; Knutson, M.D.; Pietrangelo, A.; Vukicevic, S.; Lin, H.Y.; et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 2009, 41, 482–487. [Google Scholar] [CrossRef]
- Canali, S.; Zumbrennen-Bullough, K.B.; Core, A.B.; Wang, C.Y.; Nairz, M.; Bouley, R.; Swirski, F.K.; Babitt, J.L. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 2017, 129, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.J.; Duarte, T.L.; Arezes, J.; Garcia-Santos, D.; Hamdi, A.; Pasricha, S.R.; Armitage, A.E.; Mehta, H.; Wideman, S.; Santos, A.G.; et al. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat. Metab. 2019, 1, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Alfaro-Magallanes, V.M.; Babitt, J.L. Bone morphogenic proteins in iron homeostasis. Bone 2020, 138, 115495. [Google Scholar] [CrossRef]
- Gao, J.; Chen, J.; Kramer, M.; Tsukamoto, H.; Zhang, A.S.; Enns, C.A. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009, 9, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.S.; Xiong, S.; Tsukamoto, H.; Enns, C.A. Localization of iron metabolism-related mRNAs in rat liver indicate that HFE is expressed predominantly in hepatocytes. Blood 2004, 103, 1509–1514. [Google Scholar] [CrossRef]
- Kawabata, H.; Yang, R.; Hirama, T.; Vuong, P.T.; Kawano, S.; Gombart, A.F.; Koeffler, H.P. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J. Biol. Chem. 1999, 274, 20826–20832. [Google Scholar] [CrossRef]
- Waheed, A.; Parkkila, S.; Saarnio, J.; Fleming, R.E.; Zhou, X.Y.; Tomatsu, S.; Britton, R.S.; Bacon, B.R.; Sly, W.S. Association of HFE protein with transferrin receptor in crypt enterocytes of human duodenum. Proc. Natl. Acad. Sci. USA 1999, 96, 1579–1584. [Google Scholar] [CrossRef]
- Chen, J.; Chloupková, M.; Gao, J.; Chapman-Arvedson, T.L.; Enns, C.A. HFE modulates transferrin receptor 2 levels in hepatoma cells via interactions that differ from transferrin receptor 1-HFE interactions. J. Biol. Chem. 2007, 282, 36862–36870. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Rozier, M.; Meynard, D.; Odhiambo, A.; Lin, H.Y.; Feng, Q.; Migas, M.C.; Britton, R.S.; Babitt, J.L.; Fleming, R.E. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology 2011, 141, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Li, C.; Xu, X.; Zheng, Y.; Xiao, C.; Zerfas, P.; Cooperman, S.; Eckhaus, M.; Rouault, T.; Mishra, L.; et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005, 2, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Huang, H.C.; Tang, K.S.; Su, L.T.; Huang, Y.H.; Huang, H.C.; Chen, I.L. Elevated Urinary Hepcidin Level and Hypoferremia in Infants with Febrile Urinary Tract Infection: A Prospective Cohort Study. Children 2023, 10, 870. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Wisniewski, O.W.; Kolodziejski, P.; Krauss, H. Role of hepcidin in physiology and pathophysiology. Emerging experimental and clinical evidence. J. Physiol. Pharmacol. 2021, 72, 23–33. [Google Scholar] [CrossRef]
- Kanamori, Y.; Murakami, M.; Sugiyama, M.; Hashimoto, O.; Matsui, T.; Funaba, M. Interleukin-1β (IL-1β) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein δ (C/EBPδ) expression in hepatocytes. J. Biol. Chem. 2017, 292, 10275–10287. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Vecchi, C.; Montosi, G.; Zhang, K.; Lamberti, I.; Duncan, S.A.; Kaufman, R.J.; Pietrangelo, A. ER stress controls iron metabolism through induction of hepcidin. Science 2009, 325, 877–880. [Google Scholar] [CrossRef]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069. [Google Scholar] [CrossRef]
- Wang, M.; Xue, Q.; Li, X.; Krohn, K.; Ziesche, S.; Ceglarek, U.; Blüher, M.; Keller, M.; Yaskolka Meir, A.; Heianza, Y.; et al. Circulating Levels of microRNA-122 and Hepatic Fat Change in Response to Weight-Loss Interventions: CENTRAL Trial. J. Clin. Endocrinol. Metab. 2022, 107, e1899–e1906. [Google Scholar] [CrossRef]
- Castoldi, M.; Vujic Spasic, M.; Altamura, S.; Elmén, J.; Lindow, M.; Kiss, J.; Stolte, J.; Sparla, R.; D’Alessandro, L.A.; Klingmüller, U.; et al. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J. Clin. Investig. 2011, 121, 1386–1396. [Google Scholar] [CrossRef]
- Paluschinski, M.; Kordes, C.; Vucur, M.; Buettner, V.; Roderburg, C.; Xu, H.C.; Shinte, P.V.; Lang, P.A.; Luedde, T.; Castoldi, M. Differential Modulation of miR-122 Transcription by TGFβ1/BMP6: Implications for Nonresolving Inflammation and Hepatocarcinogenesis. Cells 2023, 12, 1955. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.W.; Lin, J.Y.; Miao, R.; Zhong, J.C. Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases. Cardiovasc. Toxicol. 2020, 20, 463–473. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598, Erratum in Inflammation 2021, 44, 2142. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wang, M.; Tang, W.; Shen, Z.; Miao, L.; Wu, W.; Li, C.; Wang, X.; Xin, X.; Zhu, Y.Z. Hydrogen Sulfide Attenuates Inflammatory Hepcidin by Reducing IL-6 Secretion and Promoting SIRT1-Mediated STAT3 Deacetylation. Antioxid. Redox Signal. 2016, 24, 70–83. [Google Scholar] [CrossRef]
- Zhang, A.S.; Enns, C.A. Molecular mechanisms of normal iron homeostasis. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Arezes, J.; Foy, N.; McHugh, K.; Sawant, A.; Quinkert, D.; Terraube, V.; Brinth, A.; Tam, M.; LaVallie, E.R.; Taylor, S.; et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 2018, 132, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Nachbaur, D.; Schroll, A.; Nairz, M.; Seifert, M.; Demetz, E.; Haschka, D.; Mitterstiller, A.M.; Kleinsasser, A.; Burtscher, M.; et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut 2014, 63, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Rausch, V.; Peccerella, T.; Millonig, G.; Seitz, H.K.; Mueller, S. Hypoxia enhances H2O2-mediated upregulation of hepcidin: Evidence for NOX4-mediated iron regulation. Redox Biol. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Abreu, R.; Essler, L.; Giri, P.; Quinn, F. Interferon-gamma promotes iron export in human macrophages to limit intracellular bacterial replication. PLoS ONE 2020, 15, e0240949. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Anemia of inflammation. Hematol. Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin. Interv. Aging 2014, 9, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Matsuo, T.; Kodama, K.; Nakao, K.; Asada, R. Reduced erythropoietin secretion in senile anemia. Am. J. Hematol. 1992, 41, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Yang, C.-C.; Pan, S.-Y.; Chou, Y.-H.; Chang, F.-C.; Lai, C.-F.; Tsai, M.-H.; Hsu, H.-L.; Lin, C.-H.; Chiang, W.-C.; et al. DNA methyltransferase inhibition restores erythropoietin production in fibrotic murine kidneys. J. Clin. Investig. 2016, 126, 721–731. [Google Scholar] [CrossRef]
- Kroot, J.J.; Laarakkers, C.M.; Geurts-Moespot, A.J.; Grebenchtchikov, N.; Pickkers, P.; van Ede, A.E.; Peters, H.P.; van Dongen-Lases, E.; Wetzels, J.F.; Sweep, F.C.; et al. Immunochemical and mass-spectrometry-based serum hepcidin assays for iron metabolism disorders. Clin. Chem. 2010, 56, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Mehdad, S.; Benaich, S.; Hamdouchi, A.E.; Bouhaddou, N.; Azlaf, M.; Menchawy, I.E.; Belghiti, H.; Benkirane, H.; Lahmam, H.; Barkat, A.; et al. Association between overweight and anemia in Moroccan adolescents: A cross-sectional study. Pan Afr. Med. J. 2022, 41, 156. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Cho, Y.; Cho, I.Y.; Ahn, J. Association between Obesity and Anemia in a Nationally Representative Sample of South Korean Adolescents: A Cross-Sectional Study. Healthcare 2022, 10, 1055. [Google Scholar] [CrossRef]
- Alkazemi, D.; Rahman, A.; Habra, B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci. Rep. 2021, 11, 3034. [Google Scholar] [CrossRef]
- Qin, Y.; Melse-Boonstra, A.; Pan, X.; Yuan, B.; Dai, Y.; Zhao, J.; Zimmermann, M.B.; Kok, F.J.; Zhou, M.; Shi, Z. Anemia in relation to body mass index and waist circumference among Chinese women. Nutr. J. 2013, 12, 10. [Google Scholar] [CrossRef]
- Crivelli, M.; Wyss, K.; Grize, L.; Matthys, B.; Aebi, T.; Zemp, E. Are overweight and obesity in children risk factors for anemia in early childhood? Results from a national nutrition survey in Tajikistan. Int. J. Public Health 2018, 63, 491–499. [Google Scholar] [CrossRef]
- Purdy, J.C.; Shatzel, J.J. The hematologic consequences of obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.; Matak, P.; McKie, A.T.; Sharp, P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J. Nutr. 2007, 137, 2366–2370. [Google Scholar] [CrossRef]
- Panichsillaphakit, E.; Suteerojntrakool, O.; Pancharoen, C.; Nuchprayoon, I.; Chomtho, S. The Association between Hepcidin and Iron Status in Children and Adolescents with Obesity. J. Nutr. Metab. 2021, 2021, 9944035. [Google Scholar] [CrossRef]
- Sanad, M.; Osman, M.; Gharib, A. Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: A case control study. Ital. J. Pediatr. 2011, 37, 34. [Google Scholar] [CrossRef] [PubMed]
- Sal, E.; Yenicesu, I.; Celik, N.; Pasaoglu, H.; Celik, B.; Pasaoglu, O.T.; Kaya, Z.; Kocak, U.; Camurdan, O.; Bideci, A.; et al. Relationship between obesity and iron deficiency anemia: Is there a role of hepcidin? Hematology 2018, 23, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chang, C.C.; Chien, E.; Lin, S.S.; Cheng-Shiuan, T.; Bai, C.H.; Chao, K.C. Association between interleukin 1β and interleukin 10 concentrations: A cross-sectional study in young adolescents in Taiwan. BMC Pediatr. 2013, 13, 123. [Google Scholar] [CrossRef]
- González-Arce, L.M.; Lara-Riegos, J.C.; Pérez-Mendoza, G.J.; Rubí-Castellanos, R.; Vega-Marcín, M.; Valencia-Pacheco, G.; Torres-Romero, J.C.; González-Herrera, L. High expression levels of circulating microRNA-122 and microRNA-222 are associated with obesity in children with Mayan ethnicity. Am. J. Hum. Biol. 2021, 33, e23540. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.; Yun, C.; Li, X.; Huang, Z. MiR-122-5p regulates the pathogenesis of childhood obesity by targeting CPEB1. Obes. Res. Clin. Pract. 2022, 16, 206–213. [Google Scholar] [CrossRef]
- Murashima, M.; Tanaka, T.; Kasugai, T.; Tomonari, T.; Ide, A.; Ono, M.; Mizuno, M.; Suzuki, T.; Hamano, T. Sodium-glucose cotransporter 2 inhibitors and anemia among diabetes patients in real clinical practice. J. Diabetes Investig. 2022, 13, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Hare, G.M.T.; Zhang, Y.; Chin, K.; Thai, K.; Jacobs, E.; Cazorla-Bak, M.P.; Nghiem, L.; Wilson, D.F.; Vinogradov, S.A.; Connelly, K.A.; et al. Impact of sodium glucose linked cotransporter-2 inhibition on renal microvascular oxygen tension in a rodent model of diabetes mellitus. Physiol. Rep. 2021, 9, e14890. [Google Scholar] [CrossRef]
- Umino, H.; Hasegawa, K.; Minakuchi, H.; Muraoka, H.; Kawaguchi, T.; Kanda, T.; Tokuyama, H.; Wakino, S.; Itoh, H. High Basolateral Glucose Increases Sodium-Glucose Cotransporter 2 and Reduces Sirtuin-1 in Renal Tubules through Glucose Transporter-2 Detection. Sci. Rep. 2018, 8, 6791. [Google Scholar] [CrossRef]
- Thomas, M.C.; MacIsaac, R.J.; Tsalamandris, C.; Power, D.; Jerums, G. Unrecognized anemia in patients with diabetes: A cross-sectional survey. Diabetes Care 2003, 26, 1164–1169. [Google Scholar] [CrossRef]
- An, S.; Nedumaran, B.; Koh, H.; Joo, D.J.; Lee, H.; Park, C.S.; Harris, R.A.; Shin, K.S.; Djalilian, A.R.; Kim, Y.D. Enhancement of the SESN2-SHP cascade by melatonin ameliorates hepatic gluconeogenesis by inhibiting the CRBN-BTG2-CREBH signaling pathway. Exp. Mol. Med. 2023, 55, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Kück, M.; Radziwolek, L.; Kerling, A. Iron Deficiency in Adolescent and Young Adult German Athletes-A Retrospective Study. Nutrients 2022, 14, 4511. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, D.; Arieli, R.; Redlich, N.; Navot-Mintzer, D.; Constantini, N.W. Iron Deficiency and Anemia in Male and Female Adolescent Athletes Who Engage in Ball Games. J. Clin. Med. 2023, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Mackay, K.; Leabeater, A.; Clarke, A.; Schofield, K.; Driller, M. High Prevalence of Iron Deficiency Exhibited in Internationally Competitive, Non-Professional Female Endurance Athletes-A Case Study. Int. J. Environ. Res. Public Health 2022, 19, 16606. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.L.; Mawdsley, R.H. Sports anemia: A review of the literature. Am. J. Sports Med. 1986, 14, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Schena, F.; Salvagno, G.L.; Aloe, R.; Banfi, G.; Guidi, G.C. Foot-strike haemolysis after a 60-km ultramarathon. Blood Transfus. 2012, 10, 377–383. [Google Scholar] [CrossRef]
- Larsuphrom, P.; Latunde-Dada, G.O. Association of Serum Hepcidin Levels with Aerobic and Resistance Exercise: A Systematic Review. Nutrients 2021, 13, 393. [Google Scholar] [CrossRef]
- Banzet, S.; Sanchez, H.; Chapot, R.; Bigard, X.; Vaulont, S.; Koulmann, N. Interleukin-6 contributes to hepcidin mRNA increase in response to exercise. Cytokine 2012, 58, 158–161. [Google Scholar] [CrossRef]
- Soares, V.; Silveira de Avelar, I.; Espíndola Mota Venâncio, P.; Pires-Oliveira, D.A.A.; de Almeida Silva, P.H.; Rodrigues Borges, A.; Fonseca, G.P.E.F.; Noll, M. Acute Changes in Interleukin-6 Level During Four Days of Long-Distance Walking. J. Inflamm. Res. 2020, 13, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; McKay, A.K.A.; Pyne, D.B.; Guelfi, K.J.; McCormick, R.H.; Laarakkers, C.M.; Swinkels, D.W.; Garvican-Lewis, L.A.; Ross, M.L.R.; Sharma, A.P.; et al. Factors influencing the post-exercise hepcidin-25 response in elite athletes. Eur. J. Appl. Physiol. 2017, 117, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron status and the acute post-exercise hepcidin response in athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Agostinovna Nigretti, A.; Sellitri, A.; Scarfò, M.; Nota, A. Regular Sport Activity Is Able to Reduce the Level of Genomic Damage. Biology 2023, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Barney, D.E., Jr.; Gordon, B.S.; Hennigar, S.R. REDD1 deletion and treadmill running increase liver hepcidin and gluconeogenic enzymes in male mice. J. Nutr. Sci. 2023, 12, e49. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Geng, N.; Shi, B.J.; Li, S.L.; Zhong, Z.Y.; Li, Y.C.; Xua, W.L.; Zhou, H.; Cai, J.H. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3826–3836. [Google Scholar] [CrossRef]
- Bao, W.D.; Pang, P.; Zhou, X.T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, W.; Mao, M.; Zhao, J.; Chen, J.; Cheng, N. Deubiquitinase USP35 modulates ferroptosis in lung cancer via targeting ferroportin. Clin. Transl. Med. 2021, 11, e390. [Google Scholar] [CrossRef]
- Wei, D.; Ke, Y.Q.; Duan, P.; Zhou, L.; Wang, C.Y.; Cao, P. MicroRNA-302a-3p induces ferroptosis of non-small cell lung cancer cells via targeting ferroportin. Free Radic. Res. 2021, 55, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ostrowski, R.; Jiang, D.; Zhao, Q.; Liang, Y.; Che, X.; Zhao, J.; Xiang, X.; Qin, W.; He, Z. Hepcidin Promoted Ferroptosis through Iron Metabolism which Is Associated with DMT1 Signaling Activation in Early Brain Injury following Subarachnoid Hemorrhage. Oxidative Med. Cell. Longev. 2021, 2021, 9800794. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Jiang, J.; Kou, J. Screening of key genes related to ferroptosis and a molecular interaction network analysis in colorectal cancer using machine learning and bioinformatics. J. Gastrointest. Oncol. 2023, 14, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Guo, J.; Liu, S.; Ding, A.; Wang, G.; Li, W.; Zhang, Y.; Bian, X.; Zhao, S.; et al. Ferroptosis-related gene NOX4, CHAC1 and HIF1A are valid biomarkers for stomach adenocarcinoma. J. Cell. Mol. Med. 2022, 26, 1183–1193. [Google Scholar] [CrossRef]
- Altenhöfer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef]
- Nienaber, A.; Baumgartner, J.; Dolman, R.C.; Ozturk, M.; Zandberg, L.; Hayford, F.E.A.; Brombacher, F.; Blaauw, R.; Parihar, S.P.; Smuts, C.M.; et al. Omega-3 Fatty Acid and Iron Supplementation Alone, but Not in Combination, Lower Inflammation and Anemia of Infection in Mycobacterium Tuberculosis-Infected Mice. Nutrients 2020, 12, 2897. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.D.; Geng, M.Y.; Dou, S.R.; Wang, X.; Zhang, Z.H.; Chang, Y.Z. Caffeine Decreases Hepcidin Expression to Alleviate Aberrant Iron Metabolism under Inflammation by Regulating the IL-6/STAT3 Pathway. Life 2022, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Nazlić, J.; Jurić, D.; Mudnić, I.; Boban, Z.; Dželalija, A.M.; Tandara, L.; Šupe-Domić, D.; Gugo, K.; Boban, M. Effects of Moderate Consumption of Red Wine on Hepcidin Levels in Patients with Type 2 Diabetes Mellitus. Foods 2022, 11, 1881. [Google Scholar] [CrossRef]
- Arruda, S.F.; Ramos, L.V.; Barbosa, J.L.A.; Hankins, N.A.C.; Rodrigues, P.A.M.; Cunha, M.S.B.D. The Action of JAK/STAT3 and BMP/HJV/SMAD Signaling Pathways on Hepcidin Suppression by Tucum-do-Cerrado in a Normal and Iron-Enriched Diets. Nutrients 2020, 12, 1515. [Google Scholar] [CrossRef]
- Mayasari, N.R.; Bai, C.H.; Hu, T.Y.; Chao, J.C.; Chen, Y.C.; Huang, Y.L.; Wang, F.F.; Tinkov, A.A.; Skalny, A.V.; Chang, J.S. Associations of Food and Nutrient Intake with Serum Hepcidin and the Risk of Gestational Iron-Deficiency Anemia among Pregnant Women: A Population-Based Study. Nutrients 2021, 13, 3501. [Google Scholar] [CrossRef] [PubMed]
- Park, W.R.; Choi, B.; Kim, Y.J.; Kim, Y.H.; Park, M.J.; Kim, D.I.; Choi, H.S.; Kim, D.K. Melatonin Regulates Iron Homeostasis by Inducing Hepcidin Expression in Hepatocytes. Int. J. Mol. Sci. 2022, 23, 3593. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.W.; Nguyen, N.H.; Gibert, Y.; Motola, S.; Buckett, P.; Wessling-Resnick, M.; Fraenkel, E.; Fraenkel, P.G. The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology 2013, 58, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S. Quercetin inhibits intestinal non-haem iron absorption by regulating iron metabolism genes in the tissues. Eur. J. Nutr. 2019, 58, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, K.S.; Lim, D.; Yang, D.J.; Park, J.I.; Kim, K.W.; Jeong, J.H.; Choi, H.S.; Kim, D.K. Epigallocatechin-3-Gallate (EGCG)-Inducible SMILE Inhibits STAT3-Mediated Hepcidin Gene Expression. Antioxidants 2020, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; An, P.; Wu, Q.; Shen, X.; Shao, D.; Wang, H.; Zhang, Y.; Zhang, S.; Yao, H.; Min, J.; et al. The dietary flavonoid myricetin regulates iron homeostasis by suppressing hepcidin expression. J. Nutr. Biochem. 2016, 30, 53–61. [Google Scholar] [CrossRef]
- Lainé, F.; Laviolle, B.; Bardou-Jacquet, E.; Fatih, N.; Jezequel, C.; Collet, N.; Ropert, M.; Morcet, J.; Hamon, C.; Reymann, J.M.; et al. Curcuma decreases serum hepcidin levels in healthy volunteers: A placebo-controlled, randomized, double-blind, cross-over study. Fundam. Clin. Pharmacol. 2017, 31, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Nahdi, A.; Hammami, I.; Brasse-Lagnel, C.; Pilard, N.; Hamdaoui, M.H.; Beaumont, C.; El Maya, M. Influence of garlic or its main active component diallyl disulfide on iron bioavailability and toxicity. Nutr. Res. 2010, 30, 85–95. [Google Scholar] [CrossRef]
- López, M.; Quintero-Macías, L.; Huerta, M.; Rodríguez-Hernández, A.; Melnikov, V.; Cárdenas, Y.; Bricio-Barrios, J.A.; Sánchez-Pastor, E.; Gamboa-Domínguez, A.; Leal, C.; et al. Capsaicin Decreases Kidney Iron Deposits and Increases Hepcidin Levels in Diabetic Rats with Iron Overload: A Preliminary Study. Molecules 2022, 27, 7764. [Google Scholar] [CrossRef]
- Chiu, P.F.; Ko, S.Y.; Chang, C.C. Vitamin C affects the expression of hepcidin and erythropoietin receptor in HepG2 cells. J. Ren. Nutr. 2012, 22, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J. Clin. Transl. Endocrinol. 2014, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Huang, Y.; Wang, C. 1,25(OH)2D3 Inhibited Ferroptosis in Zebrafish Liver Cells (ZFL) by Regulating Keap1-Nrf2-GPx4 and NF-κB-hepcidin Axis. Int. J. Mol. Sci. 2021, 22, 11334. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, V.S.; Crozier, S.R.; D’Angelo, S.; Prentice, A.; Cooper, C.; Harvey, N.C.; Jones, K.S.; MAVIDOS Trial Group. The Effect of Vitamin D Supplementation on Hepcidin, Iron Status, and Inflammation in Pregnant Women in the United Kingdom. Nutrients 2019, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, K.; Ratkowski, W.; Wołyniec, W.; Kaczmarczyk, M.; Witek, K.; Żmijewski, P.; Renke, M.; Jastrzębski, Z.; Rosemann, T.; Nikolaidis, P.T.; et al. The Effect of Vitamin D3 Supplementation on Hepcidin, Iron, and IL-6 Responses after a 100 km Ultra-Marathon. Int. J. Environ. Res. Public Health 2020, 17, 2962. [Google Scholar] [CrossRef]
- Pistis, K.D.; Westerberg, P.A.; Qureshi, A.R.; Beshara, S.; Sterner, G.; Bárány, P.; Linde, T. The effect of high-dose vitamin D supplementation on hepcidin-25 and erythropoiesis in patients with chronic kidney disease. BMC Nephrol. 2023, 24, 20. [Google Scholar] [CrossRef]
- Arruda, S.F.; Siqueira, E.M.; de Valência, F.F. Vitamin A deficiency increases hepcidin expression and oxidative stress in rat. Nutrition 2009, 25, 472–478. [Google Scholar] [CrossRef]
- Han, L.; Liu, Y.; Lu, M.; Wang, H.; Tang, F. Retinoic acid modulates iron metabolism imbalance in anemia of inflammation induced by LPS via reversely regulating hepcidin and ferroportin expression. Biochem. Biophys. Res. Commun. 2018, 507, 280–285. [Google Scholar] [CrossRef]
- Restrepo-Gallego, M.; Díaz, L.E. Vitamin A does not influence mRNA expression of hormone hepcidin but other biomarkers of iron homeostasis in young male Wistar rats. Int. J. Vitam. Nutr. Res. 2022, 92, 223–230. [Google Scholar] [CrossRef]
- Baratz, E.; Protchenko, O.; Jadhav, S.; Zhang, D.; Violet, P.C.; Grounds, S.; Shakoury-Elizeh, M.; Levine, M.; Philpott, C.C. Vitamin E Induces Liver Iron Depletion and Alters Iron Regulation in Mice. J. Nutr. 2023, 153, 1866–1876. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Acute dietary carbohydrate manipulation and the subsequent inflammatory and hepcidin responses to exercise. Eur. J. Appl. Physiol. 2015, 115, 2521–2530. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Timing of post-exercise carbohydrate ingestion: Influence on IL-6 and hepcidin responses. Eur. J. Appl. Physiol. 2015, 115, 2215–2222. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Sim, M.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Seven days of high carbohydrate ingestion does not attenuate post-exercise IL-6 and hepcidin levels. Eur. J. Appl. Physiol. 2016, 116, 1715–1724. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.; Song, J.; Liu, S.; Wang, Y.; Su, X.; Wei, K.; Xu, Z.; Li, H.; Wang, Z. The prebiotic effects of soluble dietary fiber mixture on renal anemia and the gut microbiota in end-stage renal disease patients on maintenance hemodialysis: A prospective, randomized, placebo-controlled study. J. Transl. Med. 2022, 20, 599. [Google Scholar] [CrossRef] [PubMed]
- Cieślicka, M.; Ostapiuk-Karolczuk, J.; Buttar, H.S.; Dziewiecka, H.; Kasperska, A.; Skarpańska-Stejnborn, A. Effects of Long-Term Supplementation of Bovine Colostrum on Iron Homeostasis, Oxidative Stress, and Inflammation in Female Athletes: A Placebo-Controlled Clinical Trial. Nutrients 2022, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Liew, S.C. Folic acid and diseases—Supplement it or not? Rev. Assoc. Med. Bras. (1992) 2016, 62, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Rossander-Hulthén, L.; Sandström, B.; Sandberg, A.S. Improved zinc and iron absorption from breakfast meals containing malted oats with reduced phytate content. Br. J. Nutr. 1996, 76, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Mah, E.; Dioum, E.; Marwaha, A.; Shanmugam, S.; Malleshi, N.; Sudha, V.; Gayathri, R.; Unnikrishnan, R.; Anjana, R.M.; et al. The Role of Oat Nutrients in the Immune System: A Narrative Review. Nutrients 2021, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yun, W.; Wang, G.; Li, A.; Gao, J.; He, Q. Roles and mechanisms of garlic and its extracts on atherosclerosis: A review. Front. Pharmacol. 2022, 13, 954938. [Google Scholar] [CrossRef] [PubMed]
- Skolmowska, D.; Głąbska, D. Effectiveness of Dietary Intervention with Iron and Vitamin C Administered Separately in Improving Iron Status in Young Women. Int. J. Environ. Res. Public Health 2022, 19, 11877. [Google Scholar] [CrossRef] [PubMed]
- Righi, N.C.; Schuch, F.B.; De Nardi, A.T.; Pippi, C.M.; de Almeida Righi, G.; Puntel, G.O.; da Silva, A.M.V.; Signori, L.U. Effects of vitamin C on oxidative stress, inflammation, muscle soreness, and strength following acute exercise: Meta-analyses of randomized clinical trials. Eur. J. Nutr. 2020, 59, 2827–2839. [Google Scholar] [CrossRef]
- Syed, S.; Michalski, E.S.; Tangpricha, V.; Chesdachai, S.; Kumar, A.; Prince, J.; Ziegler, T.R.; Suchdev, P.S.; Kugathasan, S. Vitamin D Status Is Associated with Hepcidin and Hemoglobin Concentrations in Children with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1650–1658. [Google Scholar] [CrossRef]
- Moran-Lev, H.; Weisman, Y.; Cohen, S.; Deutsch, V.; Cipok, M.; Bondar, E.; Lubetzky, R.; Mandel, D. The interrelationship between hepcidin, vitamin D, and anemia in children with acute infectious disease. Pediatr. Res. 2018, 84, 62–65. [Google Scholar] [CrossRef]
- Apple, C.G.; Miller, E.S.; Kannan, K.B.; Stortz, J.A.; Cox, M.; Loftus, T.J.; Parvataneni, H.K.; Patrick, M.; Hagen, J.E.; Brakenridge, S.; et al. Vitamin D status is associated with hepcidin and hemoglobin concentrations in patients with severe traumatic injury. J. Trauma Acute Care Surg. 2020, 89, 1124–1130. [Google Scholar] [CrossRef]
- Koren, Y.; Lubetzky, R.; Mandel, D.; Ovental, A.; Deutsch, V.; Hadanny, A.; Moran-Lev, H. Anemia, Hepcidin, and Vitamin D in Healthy Preterm Infants: A Pilot Study. Am. J. Perinatol. 2023, 40, 508–512. [Google Scholar] [CrossRef]
- Mogire, R.M.; Muriuki, J.M.; Morovat, A.; Mentzer, A.J.; Webb, E.L.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Cutland, C.L.; Sirima, S.B.; et al. Vitamin D Deficiency and Its Association with Iron Deficiency in African Children. Nutrients 2022, 14, 1372. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Peng, Z.; Cheng, H.; Zhuang, Y.; Chi, P.; Alifu, X.; Zhou, H.; Mo, M.; Yu, Y. Association of Vitamin D in Different Trimester with Hemoglobin during Pregnancy. Nutrients 2022, 14, 2455. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.G.; Fabrini, S.P.; Alfenas, R.C.G. Low glycemic index diet reduces body fat and attenuates inflammatory and metabolic responses in patients with type 2 diabetes. Arch. Endocrinol. Metab. 2017, 61, 137–144. [Google Scholar] [CrossRef] [PubMed]
| Foods | Experimental Model | Dose and Duration | Effects on Hepcidin Release | Proposed Mechanisms | Reference |
|---|---|---|---|---|---|
| Omega-3 | |||||
| EPA/DHA | Mycobacterium tuberculosis-infected C3HeB/FeJ mice | AIN-93G control diet supplemented with EPA (44% of total fatty acids) and DHA (28% of total fatty acids) for 3 weeks | Decrease in hepcidin levels in plasma | Reduction in IL-1 and IL-6 | [89] |
| Polyphenols-enriched foods and isolated polyphenols | |||||
| Caffeine | 2-month-old C57BL/6N mice | Daily intragastric administration of caffeine (100 mg/kg body weight) for 7 days | Decrease in hepcidin levels in liver | Reduction in IL-6/STAT3 | [90] |
| Wine | Patients with type 2 diabetes | 300 mL of red wine daily for 3 weeks | Decrease in hepcidin levels in plasma | n.d. | [91] |
| Tucum-do-cerrado (Bactris setosa Mart.) | Wistar rats | AIN-93G diet supplemented with 150 g of the edible parts of the tucum-do-cerrado fruit/kg of diet for 12 weeks | Decrease in hepcidin levels in liver | Increase in Sirtuin-1 | [92] |
| Dark leafy vegetables | Pregnant women | n.a. | Increase in hepcidin levels in serum | n.d. | [93] |
| Melatonin | 8-week-old C57BL/6J mice | Intraperitoneal injection of melatonin (10 mg/kg) | Increase in hepcidin levels in serum Increase in hepcidin gene expression in liver | Increase in c-Jun pathway | [94] |
| Genistein | Zebrafish embryos Human hepatocellular carcinoma cells | 7 µM from 28 to 52 for Zebrafish embryos, 0–20 µM for HepG2 cells | Increase in hepcidin levels | Increase in STAT3 and SMAD4 | [95] |
| Quercetin | Male Sprague Dawley rats | Gavage or intraperitoneal injection (50 mg/kg) for 5 h or 10 days | Increase in hepcidin levels in liver | n.d. | [96] |
| Epigallocatenin3-gallate | Human and mouse hepatocytes | Treatment (100–200 ng)/intraperitoneal injection of epigallocatenin3-gallate (100 µM) | Decrease in hepcidin levels | Induction of small heterodimer partner-interacting leucine zipper protein | [97] |
| Myricetin | Human hepatocellular carcinoma cells, human embryonic kidney cells, Male C57BL/6 mice | 20 µg/mL for 12 h or intraperitoneal injection of quercetin (40 mL/kg) | Decrease in hepcidin levels | Modulation of BMP/SMAD signaling | [98] |
| Spices | |||||
| Curcuma | Healthy male volunteers | 6 g (corresponding to 120 mg of curcumin) for 0.5–48 h | Decrease in hepcidin levels in plasma | n.d. | [99] |
| Garlic | Male Wistar rats | Gavage (1 g/kg body weight) for 3 weeks | Decrease in hepcidin levels in liver | Increase in Sirtuin-1 | [100] |
| Capsaicin | Male Wistar rats treated with streptozotocin to induce diabetes | Daily subcutaneous injection (1 mg/kg) for 12 weeks | Increase in hepcidin levels in liver | n.d. | [101] |
| Vitamins | |||||
| Vitamin C | Human hepatocellular carcinoma cells | 50–100 µg/mL for 6 h | Decrease in hepcidin levels | n.d. | [102] |
| Vitamin D | Human hepatocellular carcinoma PBMC monocytes Male C57BL/6 mice Healthy volunteers | 5 nM for 6 h Single intraperitoneal injections (0.2 μg/g) Single dose of oral vitamin D2 (100,000 IU). | Decrease in hepcidin levels | Transcriptional suppression of hepcidin gene | [103] |
| THP-1 macrophage-like monocytic cells Patients affected by chronic kidney disease | 5–40 nM overnight 50,000 IU weekly for 12 weeks | Decrease in hepcidin levels | Reduction in IL-1 and IL-6 | [104] | |
| Zebrafish liver cells | 200 pM for 72 h | Decrease in hepcidin levels | Inhibition of ferroptosis and modulation of Keap1–Nrf2–GPX4 and NF-κB–pathways | [105] | |
| Pregnant women | 1000 IU daily for 14 weeks | Decrease in hepcidin levels in plasma | n.d. | [106] | |
| Ultra-marathon runner | 10,000 UI daily for 2 weeks | No significant variation in hepcicin levels | n.d. | [107] | |
| Patients affected by chronic kidney disease | 8000 IU of cholecalciferol daily for 12 weeks | No significant variation in hepcicin levels | n.d. | [108] | |
| Vitamin A | Wistar rats | AIN-93G diet with or without 4000 IU/kg of diet for 57 days | Increase in hepcidin levels in deficient animals | n.d. | [109] |
| BALB/c mice | 3 or 15 mg/kg of retinoic acid for 14 days | Decrease in hepcidin levels | Modulation of TLT-4/NF-κB–pathways | [110] | |
| Young male Wistar rats | 6 weeks | No significant variation in hepcicin levels | n.d. | [111] | |
| Vitamin E | C57Bl/6 male mice | 450 mg/kg for 18 days | Decrease in hepcidin levels in plasma | Reduction in Nrf2 pathway | [112] |
| Carbohydrates and fiber | |||||
| Complex carbohydrates | Endurance athletes | 3–10 g/kg | Increase in hepcidin levels in serum | Increase in IL-6 | [113] |
| Endurance athletes | 1.2 g/kg beverage (12 mL/kg, 10% carbohydrate beverage) | Increase in hepcidin levels in serum | Increase in IL-6 | [114] | |
| Endurance athletes | 3–8 g/kg | Variations time-dependent | Increase in IL-6 | [115] | |
| Dietary fiber | Patients affected by end-stage renal disease | 10 g daily of dietary fiber or potato starch for 8 weeks | No significant variation in hepcicin levels | n.d | [116] |
| Supplements | |||||
| Bovine colostrum | Highly trained athletes | 3.2 g (four capsules) daily for 6 months | Increase in hepcidin levels in serum | Increase in IL-6 | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Andrea, P.; Giampieri, F.; Battino, M. Nutritional Modulation of Hepcidin in the Treatment of Various Anemic States. Nutrients 2023, 15, 5081. https://doi.org/10.3390/nu15245081
D’Andrea P, Giampieri F, Battino M. Nutritional Modulation of Hepcidin in the Treatment of Various Anemic States. Nutrients. 2023; 15(24):5081. https://doi.org/10.3390/nu15245081
Chicago/Turabian StyleD’Andrea, Patrizia, Francesca Giampieri, and Maurizio Battino. 2023. "Nutritional Modulation of Hepcidin in the Treatment of Various Anemic States" Nutrients 15, no. 24: 5081. https://doi.org/10.3390/nu15245081
APA StyleD’Andrea, P., Giampieri, F., & Battino, M. (2023). Nutritional Modulation of Hepcidin in the Treatment of Various Anemic States. Nutrients, 15(24), 5081. https://doi.org/10.3390/nu15245081








