Naturally Bicarbonated Water Supplementation Does Not Improve Anaerobic Cycling Performance or Blood Gas Parameters in Active Men and Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Research Design
2.2. Participants
2.3. Anthropometrics and Body Composition
2.4. Dietary Monitoring
2.5. O2peak Assessment
2.6. Repeated Cycling Sprints
2.7. Rating of Perceived Exertion and Visual Analog Scale
2.8. Supplementation Protocol
2.9. Blood Lactate and Blood Gas Measurements
2.10. Statistical Analysis
3. Results
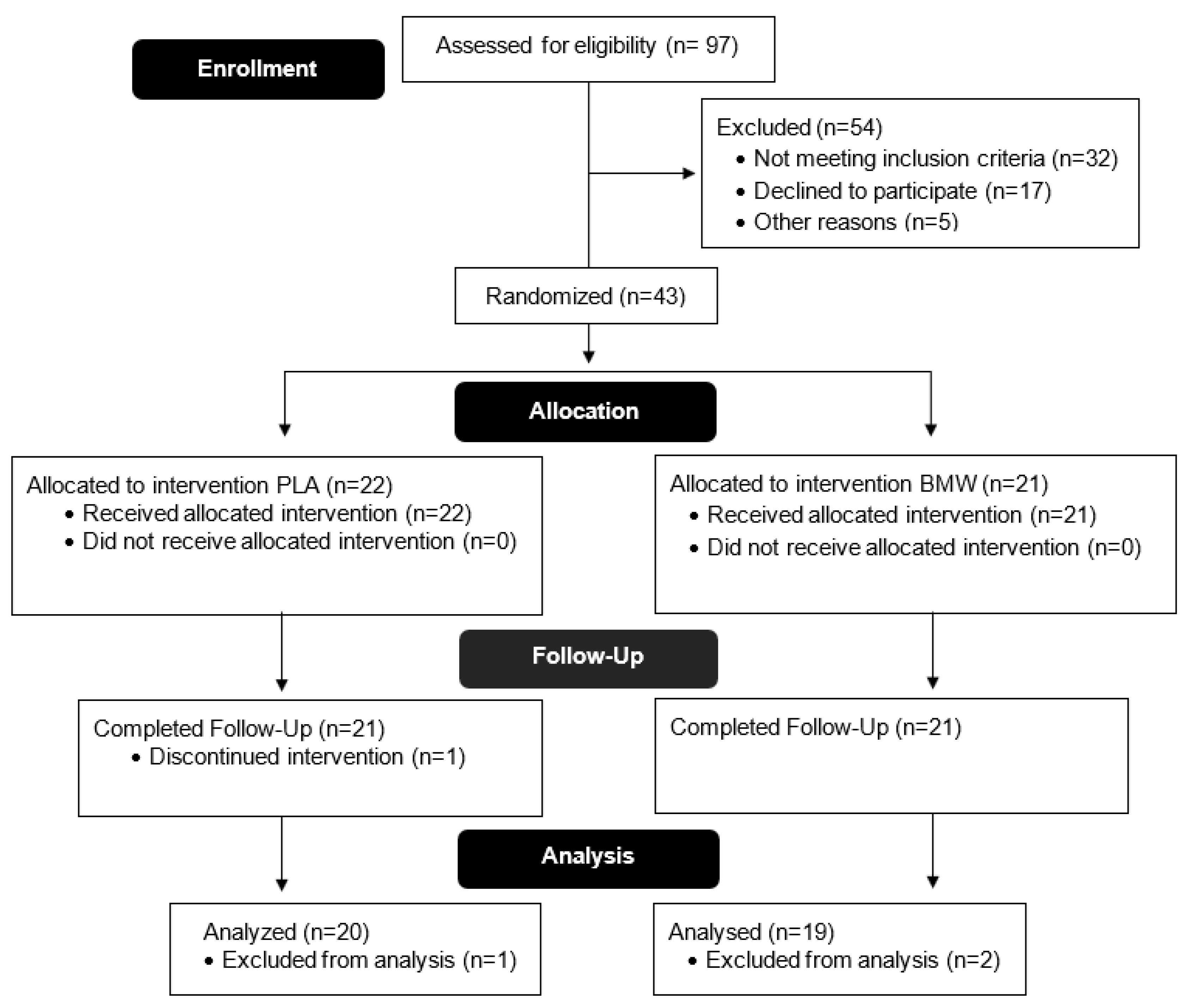
3.1. Participant Demographics
3.2. Dietary Intake
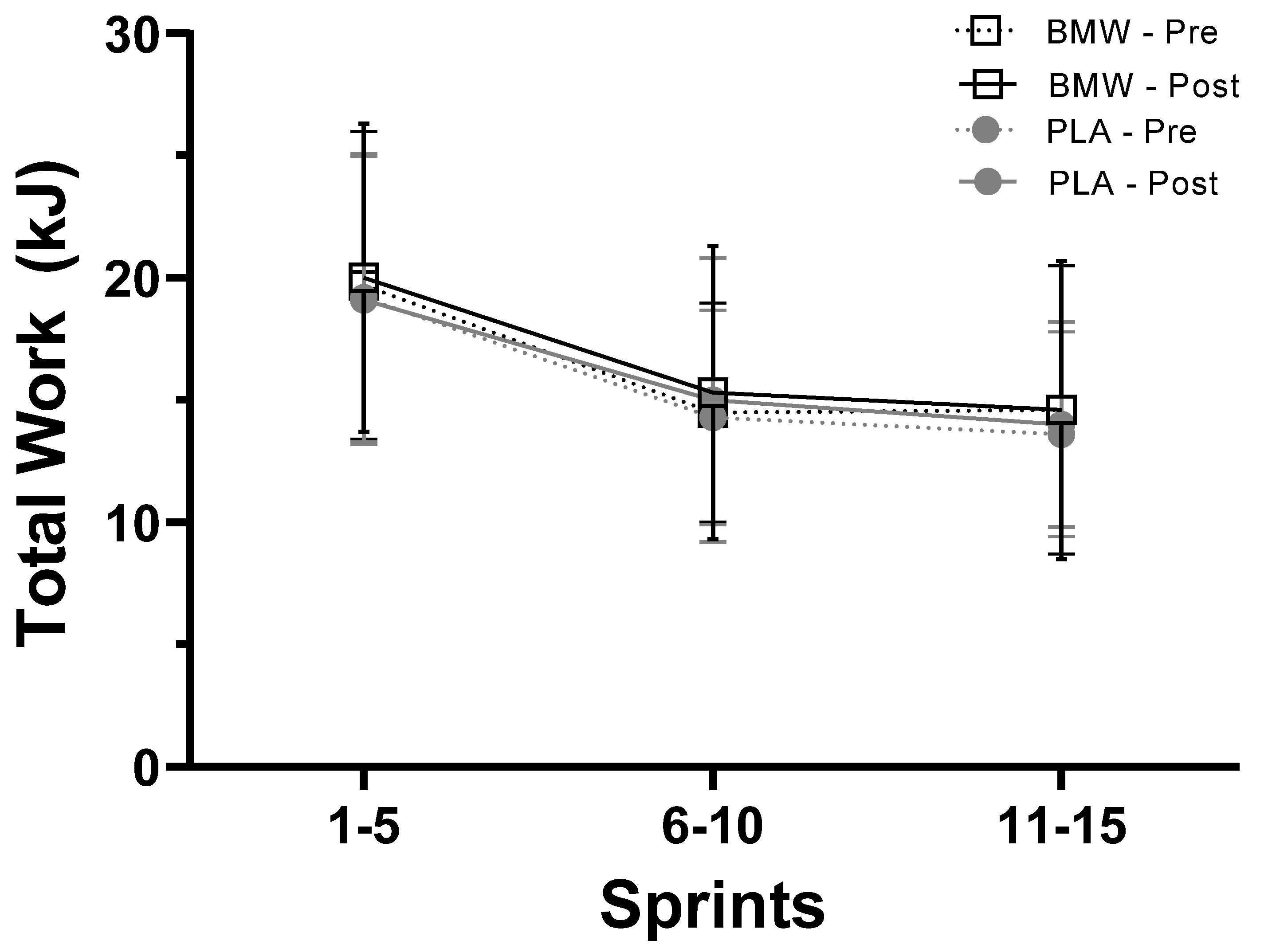
3.3. Total Work
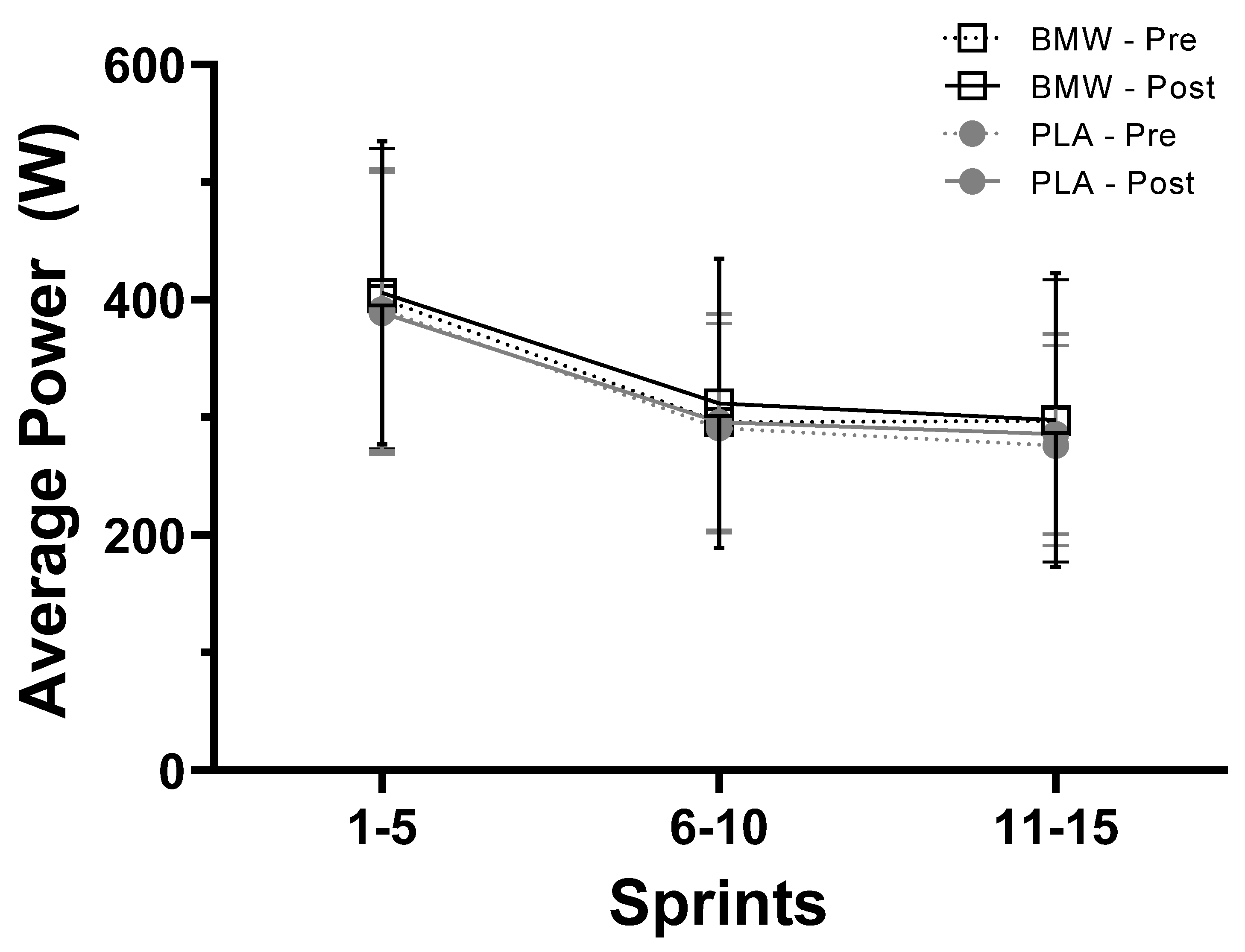
3.4. Average Power
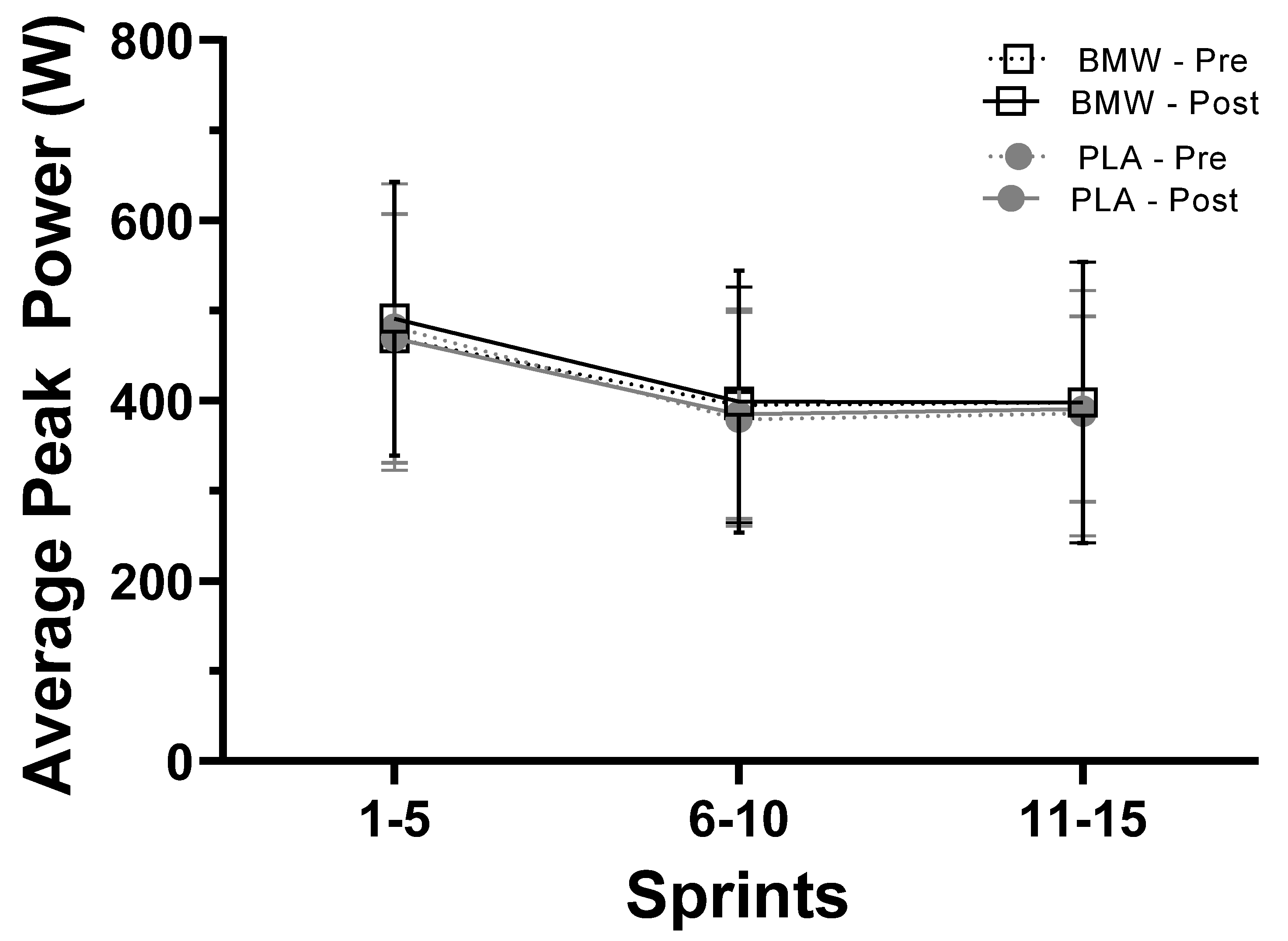
3.5. Average Peak Power
3.6. Rating of Perceived Exertion and Visual Analog Scales of Fatigue
3.7. Lactate, pH, and Other Blood Gas Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishop, D.J. Fatigue during intermittent-sprint exercise. Clin. Exp. Pharmacol. Physiol. 2012, 39, 836–841. [Google Scholar] [CrossRef]
- Noakes, T.D. From catastrophe to complexity: A novel model of integrative central neural regulation of effort and fatigue during exercise in humans: Summary and conclusions. Br. J. Sports Med. 2005, 39, 120–124. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Hermansen, L.; Osnes, J.-B. Blood and muscle pH after maximal exercise in man. J. Appl. Physiol. 1972, 32, 304–308. [Google Scholar] [CrossRef]
- Burke, L.M.; Pyne, D.B. Bicarbonate loading to enhance training and competitive performance. Int. J. Sports Physiol. Perform. 2007, 2, 93–97. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. Bicarbonate buffering of lactic acid generated during exercise. J. Appl. Physiol. 1986, 60, 472–478. [Google Scholar] [CrossRef]
- Dennig, H.; Talbott, J.; Edwards, H.; Dill, D. Effect of acidosis and alkalosis upon capacity for work. J. Clin. Investig. 1931, 9, 601–613. [Google Scholar] [CrossRef]
- McCartney, N.; Heigenhauser, G.; Jones, N.L. Power output and fatigue of human muscle in maximal cycling exercise. J. Appl. Physiol. 1983, 55, 218–224. [Google Scholar] [CrossRef]
- Peart, D.J.; Siegler, J.C.; Vince, R.V. Practical recommendations for coaches and athletes: A meta-analysis of sodium bicarbonate use for athletic performance. J. Strength Cond. Res. 2012, 26, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Requena, B.; Zabala, M.; Padial, P.; Feriche, B. Sodium bicarbonate and sodium citrate: Ergogenic aids? J. Strength Cond. Res. 2005, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.C.; Marshall, P.W.; Bishop, D.; Shaw, G.; Green, S. Mechanistic insights into the efficacy of sodium bicarbonate supplementation to improve athletic performance. Sports Med.-Open 2016, 2, 41. [Google Scholar] [CrossRef]
- Applegate, E. Effective nutritional ergogenic aids. Int. J. Sport Nutr. 1999, 9, 229–239. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jager, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Linderman, J.K.; Gosselink, K.L. The effects of sodium bicarbonate ingestion on exercise performance. Sports Med. 1994, 18, 75–80. [Google Scholar] [CrossRef]
- Matson, L.G.; Tran, Z.V. Effects of sodium bicarbonate ingestion on anaerobic performance: A meta-analytic review. Int. J. Sport Nutr. 1993, 3, 2–28. [Google Scholar] [CrossRef]
- Lindh, A.M.; Peyrebrune, M.C.; Ingham, S.A.; Bailey, D.M.; Folland, J.P. Sodium bicarbonate improves swimming performance. Int. J. Sports Med. 2008, 29, 519–523. [Google Scholar] [CrossRef]
- Marriott, M.; Krustrup, P.; Mohr, M. Ergogenic effects of caffeine and sodium bicarbonate supplementation on intermittent exercise performance preceded by intense arm cranking exercise. J. Int. Soc. Sports Nutr. 2015, 12, 13. [Google Scholar] [CrossRef]
- Jones, N.L.; Sutton, J.R.; Taylor, R.; Toews, C.J. Effect of pH on cardiorespiratory and metabolic responses to exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 43, 959–964. [Google Scholar] [CrossRef]
- McNaughton, L.R. Bicarbonate ingestion: Effects of dosage on 60 s cycle ergometry. J. Sports Sci. 1992, 10, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L.; McLay-Cooke, R.T.; Brown, R.C.; Gray, A.R.; Fairbairn, K.A. Increased blood pH but not performance with sodium bicarbonate supplementation in elite rugby union players. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.; Robinson, A.L.; Sparks, S.A.; Bridge, C.A.; Bentley, D.J.; McNaughton, L.R. The Effects of Novel Ingestion of Sodium Bicarbonate on Repeated Sprint Ability. J. Strength Cond. Res. 2016, 30, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.C.; Marshall, P.W.; Bray, J.; Towlson, C. Sodium bicarbonate supplementation and ingestion timing: Does it matter? J. Strength Cond. Res. 2012, 26, 1953–1958. [Google Scholar] [CrossRef]
- Grgic, J.; Pedisic, Z.; Saunders, B.; Artioli, G.G.; Schoenfeld, B.J.; McKenna, M.J.; Bishop, D.J.; Kreider, R.B.; Stout, J.R.; Kalman, D.S.; et al. International Society of Sports Nutrition position stand: Sodium bicarbonate and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 61. [Google Scholar] [CrossRef]
- Gurton, W.H.; Gough, L.A.; Sparks, S.A.; Faghy, M.A.; Reed, K.E. Sodium Bicarbonate Ingestion Improves Time-to-Exhaustion Cycling Performance and Alters Estimated Energy System Contribution: A Dose-Response Investigation. Front. Nutr. 2020, 7, 154. [Google Scholar] [CrossRef]
- Chycki, J.; Kostrzewa, M.; Maszczyk, A.; Zajac, A. Chronic Ingestion of Bicarbonate-Rich Water Improves Anaerobic Performance in Hypohydrated Elite Judo Athletes: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 4948. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Stotler, B.A.; Kratz, A. Analytical and clinical performance of the epoc blood analysis system: Experience at a large tertiary academic medical center. Am. J. Clin. Pathol. 2013, 140, 715–720. [Google Scholar] [CrossRef]
- Chycki, J.; Kurylas, A.; Maszczyk, A.; Golas, A.; Zajac, A. Alkaline water improves exercise-induced metabolic acidosis and enhances anaerobic exercise performance in combat sport athletes. PLoS ONE 2018, 13, e0205708. [Google Scholar] [CrossRef]
- Douroudos, I.I.; Fatouros, I.G.; Gourgoulis, V.; Jamurtas, A.Z.; Tsitsios, T.; Hatzinikolaou, A.; Margonis, K.; Mavromatidis, K.; Taxildaris, K. Dose-related effects of prolonged NaHCO3 ingestion during high-intensity exercise. Med. Sci. Sports Exerc. 2006, 38, 1746–1753. [Google Scholar] [CrossRef]
- Edge, J.; Bishop, D.; Goodman, C. Effects of chronic NaHCO3 ingestion during interval training on changes to muscle buffer capacity, metabolism, and short-term endurance performance. J. Appl. Physiol. 2006, 101, 918–925. [Google Scholar] [CrossRef]
- Zhou, N.; Fan, Y.; Kong, X.; Wang, X.; Wang, J.; Wu, H. Effects of serial and acute enteric-coated sodium bicarbonate supplementation on anaerobic performance, physiological profile, and metabolomics in healthy young men. Front. Nutr. 2022, 9, 931671. [Google Scholar] [CrossRef]
- Costill, D.L.; Verstappen, F.; Kuipers, H.; Janssen, E.; Fink, W. Acid-base balance during repeated bouts of exercise: Influence of HCO3. Int. J. Sports Med. 1984, 5, 228–231. [Google Scholar] [CrossRef]
- Artioli, G.G.; Gualano, B.; Coelho, D.F.; Benatti, F.B.; Gailey, A.W.; Lancha, A.H. Does sodium-bicarbonate ingestion improve simulated judo performance? Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 206–217. [Google Scholar] [CrossRef]
- Price, M.; Moss, P.; Rance, S. Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med. Sci. Sports Exerc. 2003, 35, 1303–1308. [Google Scholar] [CrossRef]
- Joyce, S.; Minahan, C.; Anderson, M.; Osborne, M. Acute and chronic loading of sodium bicarbonate in highly trained swimmers. Eur. J. Appl. Physiol. 2012, 112, 461–469. [Google Scholar] [CrossRef]
- Vanhatalo, A.; McNaughton, L.R.; Siegler, J.; Jones, A.M. Effect of induced alkalosis on the power-duration relationship of “all-out” exercise. Med. Sci. Sports Exerc. 2010, 42, 563–570. [Google Scholar] [CrossRef]
- Zabala, M.; Peinado, A.B.; Calderón, F.J.; Sampedro, J.; Castillo, M.J.; Benito, P.J. Bicarbonate ingestion has no ergogenic effect on consecutive all out sprint tests in BMX elite cyclists. Eur. J. Appl. Physiol. 2011, 111, 3127–3134. [Google Scholar] [CrossRef]
- Boegman, S.; Stellingwerff, T.; Shaw, G.; Clarke, N.; Graham, K.; Cross, R.; Siegler, J.C. The Impact of Individualizing Sodium Bicarbonate Supplementation Strategies on World-Class Rowing Performance. Front. Nutr. 2020, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. Sodium bicarbonate improves 4 km time trial cycling performance when individualised to time to peak blood bicarbonate in trained male cyclists. J. Sports Sci. 2018, 36, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Stecker, R.A.; Harty, P.S.; Jagim, A.R.; Candow, D.G.; Kerksick, C.M. Timing of ergogenic aids and micronutrients on muscle and exercise performance. J. Int. Soc. Sports Nutr. 2019, 16, 37. [Google Scholar] [CrossRef]
- Ferreira, L.H.B.; Smolarek, A.C.; Chilibeck, P.D.; Barros, M.P.; McAnulty, S.R.; Schoenfeld, B.J.; Zandona, B.A.; Souza-Junior, T.P. High doses of sodium bicarbonate increase lactate levels and delay exhaustion in a cycling performance test. Nutrition 2019, 60, 94–99. [Google Scholar] [CrossRef]
- Horswill, C.A.; Costill, D.L.; Fink, W.J.; Flynn, M.G.; Kirwan, J.P.; Mitchell, J.B.; Houmard, J.A. Influence of sodium bicarbonate on sprint performance: Relationship to dosage. Med. Sci. Sports Exerc. 1988, 20, 566–569. [Google Scholar] [CrossRef]
- George, K.P.; MacLaren, D.P. The effect of induced alkalosis and acidosis on endurance running at an intensity corresponding to 4 mM blood lactate. Ergonomics 1988, 31, 1639–1645. [Google Scholar] [CrossRef]
- Gough, L.A.; Deb, S.K.; Sparks, A.; McNaughton, L.R. The Reproducibility of 4-km Time Trial (TT) Performance Following Individualised Sodium Bicarbonate Supplementation: A Randomised Controlled Trial in Trained Cyclists. Sports Med. Open 2017, 3, 34. [Google Scholar] [CrossRef][Green Version]
- Brisola, G.M.; Miyagi, W.E.; da Silva, H.S.; Zagatto, A.M. Sodium bicarbonate supplementation improved MAOD but is not correlated with 200- and 400-m running performances: A double-blind, crossover, and placebo-controlled study. Appl. Physiol. Nutr. Metab. 2015, 40, 931–937. [Google Scholar] [CrossRef]
- Bishop, D.; Edge, J.; Davis, C.; Goodman, C. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med. Sci. Sports Exerc. 2004, 36, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. Sodium bicarbonate supplementation improves severe-intensity intermittent exercise under moderate acute hypoxic conditions. Eur. J. Appl. Physiol. 2018, 118, 607–615. [Google Scholar] [CrossRef]
- McNaughton, L.R.; Ford, S.; Newbold, C. Effect of Sodium Bicarbonate Ingestion on High Intensity Exercise in Moderately Trained Women. J. Strength Cond. Res. 1997, 11, 98–102. [Google Scholar]
- Driller, M.W.; Gregory, J.R.; Williams, A.D.; Fell, J.W. The effects of serial and acute NaHCO3 loading in well-trained cyclists. J. Strength Cond. Res. 2012, 26, 2791–2797. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, L.; Backx, K.; Palmer, G.; Strange, N. Effects of chronic bicarbonate ingestion on the performance of high-intensity work. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Limmer, M.; de Marées, M.; Platen, P. Effects of daily ingestion of sodium bicarbonate on acid-base status and anaerobic performance during an altitude sojourn at high altitude: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Rimmer, S.; Osler, C.J.; Higgins, M.F. Ingestion of Sodium Bicarbonate (NaHCO). Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Hurst, P.; Schipof-Godart, L.; Szabo, A.; Raglin, J.; Hettinga, F.; Roelands, B.; Lane, A.; Foad, A.; Coleman, D.; Beedie, C. The Placebo and Nocebo effect on sports performance: A systematic review. Eur. J. Sport Sci. 2020, 20, 279–292. [Google Scholar] [CrossRef]
- Hurst, P.; Foad, A.; Coleman, D.; Beedie, C. Athletes Intending to Use Sports Supplements Are More Likely to Respond to a Placebo. Med. Sci. Sports Exerc. 2017, 49, 1877–1883. [Google Scholar] [CrossRef]
| Condition | Mean ± SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age (years) | SW | 28.7 ± 9.1 | 20 | 45 |
| BMW | 27.5 ± 7.0 | 20 | 44 | |
| Height (cm) | SW | 170.8 ± 7.9 | 157 | 185 |
| BMW | 168.9 ± 14.4 | 122.5 | 189 | |
| Weight (kg) | SW | 69.8 ± 12.5 | 46.5 | 94.5 |
| BMW | 68.2 ± 9.2 | 49.8 | 89 | |
| Fat Percent (%) | SW | 20.5 ± 8.5 | 7 | 35.7 |
| BMW | 19.7 ± 7.3 | 10 | 37 | |
| O2max (mL O2/kg/min) | SW | 42.1 ± 8.1 | 29.4 | 56.1 |
| BMW | 43.3 ± 7.3 | 28.7 | 57.2 | |
| METs | SW | 12.0 ± 2.3 | 8.4 | 16 |
| BMW | 12.4 ± 2.1 | 8.2 | 16.4 |
| Variable | Pre Supplementation | Post Supplementation | Between Group (p) | ||
|---|---|---|---|---|---|
| Energy (kcal/day) | SW | 2287 ± 729 | 2189 ± 714 | Time | 0.547 |
| BMW | 2135 ± 887 | 2096 ± 1084 | G × T | 0.796 | |
| Protein (g/day) | SW | 125.1 ± 52.3 | 102.6 ± 35.9 | Time | 0.114 |
| BMW | 104.2 ± 44.9 | 106.1 ± 58.6 | G × T | 0.065 | |
| Carbohydrates (g/day) | SW | 232.7 ± 94.8 | 251.8 ± 104.4 | Time | 0.626 |
| BMW | 230.1 ± 135.2 | 226.6 ± 155.6 | G × T | 0.455 | |
| Fat (g/day) | SW | 97.0 ± 35.2 | 86.7 ± 38.5 | Time | 0.216 |
| BMW | 91.0 ± 41.2 | 86.2 ± 40.1 | G × T | 0.645 | |
| Potassium (mg/day) | SW | 2909 ± 1456 | 2977 ± 1340 | Time | 0.272 |
| BMW | 2808 ± 1328 | 3025 ± 1692 | G × T | 0.563 | |
| Sodium (mg/day) | SW | 4614 ± 1523 | 4140 ± 1366 | Time | 0.415 |
| BMW | 3787 ± 1405 | 3837 ± 2096 | G × T | 0.316 | |
| Calcium (mg/day) | SW | 1246 ± 896 | 1097 ± 742 | Time | 0.989 |
| BMW | 1023 ± 628 | 1175 ± 776 | G × T | 0.141 | |
| Start | Sprint 3 | Sprint 6 | Sprint 9 | Sprint 12 | Sprint 15 | Between Group (p) | ||
|---|---|---|---|---|---|---|---|---|
| RPE | ||||||||
| SW | 6.2 ± 0.7 | 11.2 ± 2.2 | 14.1 ± 2.2 | 16.0 ± 2.4 | 17.3 ± 2.3 | 18.6 ± 2.0 | Time | <0.001 |
| BMW | 6.6 ± 1.2 | 11.4 ± 2.1 | 14.4 ± 2.0 | 16.5 ± 1.8 | 17.9 ± 1.7 | 18.9 ± 1.6 | G × T | 0.99 |
| Fatigue VAS | ||||||||
| SW | 4.3 ± 6.1 | 20.6 ± 13.2 | 49.5 ± 16.2 | 68.4 ± 17.8 | 80.2 ± 13.3 | 91.1 ± 18.7 | Time | <0.001 |
| BMW | 4.5 ± 4.9 | 28.7 ± 15.4 | 53.1 ± 15.9 | 69.5 ± 15.2 | 81.8 ± 13.5 | 88.4 ± 12.2 | G × T | 0.75 |
| Pre | Immediate Post | 5 Min Post | 10 Min Post | Between Group (p) | ||
|---|---|---|---|---|---|---|
| Lactate (mM) | ||||||
| SW | 2.1 ± 0.9 | 12.5 ± 3.1 | 13.8 ± 3.7 | 13.0 ± 4.2 | Time | <0.001 |
| BMW | 1.9 ± 0.9 | 12.5 ± 5.5 | 15.7 ± 4.4 | 15.0 ± 4.5 | G × T | 0.17 |
| pH | ||||||
| SW | 7.3 ± 0. | 7.17 ± 0.1 | 7.19 ± 0.1 | 7.21 ± 0.1 | Time | <0.001 |
| BMW | 7.3 ± 0. | 7.20 ± 0.1 | 7.20 ± 0.1 | 7.24 ± 0.1 | G × T | 0.85 |
| PCO2 (mmHg) | ||||||
| SW | 56.8 ± 6.6 | 60.3 ± 13.2 | 44.9 ± 9.0 | 41.6 ± 7.0 | Time | <0.001 |
| BMW | 55.0 ± 5.7 | 58.8 ± 12.1 | 39.8 ± 5.2 | 38.3 ± 5.0 | G × T | 0.55 |
| PO2 (mmHg) | ||||||
| SW | 22.5 ± 5.6 | 27.7 ± 13.3 | 49.3 ± 10.3 | 50.6 ± 15.6 | Time | <0.001 |
| BMW | 25.1 ± 6.1 | 24.6 ± 13.6 | 58.6 ± 16.2 | 57.5 ± 15.6 | G × T | 0.06 |
| HCO3 (mM) | ||||||
| SW | 28.7 ± 2.2 | 21.9 ± 3.2 | 16.9 ± 3.2 | 16.8 ± 3.1 | Time | <0.001 |
| BMW | 29.8 ± 2.0 | 23.1 ± 4.6 | 15.9 ± 3.8 | 16.6 ± 4.1 | G × T | 0.18 |
| CO2 (mM) | ||||||
| SW | 30.5 ± 2.4 | 23.7 ± 3.4 | 18.3 ± 3.3 | 18.1 ± 3.1 | Time | <0.001 |
| BMW | 31.5 ± 2.1 | 24.9 ± 4.6 | 17.2 ± 3.9 | 17.8 ± 4.2 | G × T | 0.18 |
| Base Excess (ECF) | ||||||
| SW | 2.5 ± 2.1 | −6.6 ± 3.6 | −11.3 ± 4.0 | −11.0 ± 4.3 | Time | <0.001 |
| BMW | 4.1 ± 1.9 | −4.9 ± 6.0 | −12.1 ± 5.0 | −10.8 ± 5.4 | G × T | 0.26 |
| Base Excess (B) | ||||||
| SW | 1.0 ± 1.5 | −7.7 ± 3.3 | −11.2 ± 3. 8 | −10.7 ± 4.3 | Time | <0.001 |
| BMW | 4.1 ± 1.9 | −4.9 ± 6.0 | −12.1 ± 5.0 | −10.8 ± 5.4 | G × T | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagele, A.M.; Boring, J.L.; Moon, J.M.; Sunderland, K.L.; Mumford, P.W.; Kerksick, C.M. Naturally Bicarbonated Water Supplementation Does Not Improve Anaerobic Cycling Performance or Blood Gas Parameters in Active Men and Women. Nutrients 2023, 15, 5052. https://doi.org/10.3390/nu15245052
Hagele AM, Boring JL, Moon JM, Sunderland KL, Mumford PW, Kerksick CM. Naturally Bicarbonated Water Supplementation Does Not Improve Anaerobic Cycling Performance or Blood Gas Parameters in Active Men and Women. Nutrients. 2023; 15(24):5052. https://doi.org/10.3390/nu15245052
Chicago/Turabian StyleHagele, Anthony M., Johnathan L. Boring, Jessica M. Moon, Kyle L. Sunderland, Petey W. Mumford, and Chad M. Kerksick. 2023. "Naturally Bicarbonated Water Supplementation Does Not Improve Anaerobic Cycling Performance or Blood Gas Parameters in Active Men and Women" Nutrients 15, no. 24: 5052. https://doi.org/10.3390/nu15245052
APA StyleHagele, A. M., Boring, J. L., Moon, J. M., Sunderland, K. L., Mumford, P. W., & Kerksick, C. M. (2023). Naturally Bicarbonated Water Supplementation Does Not Improve Anaerobic Cycling Performance or Blood Gas Parameters in Active Men and Women. Nutrients, 15(24), 5052. https://doi.org/10.3390/nu15245052









