Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients—A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
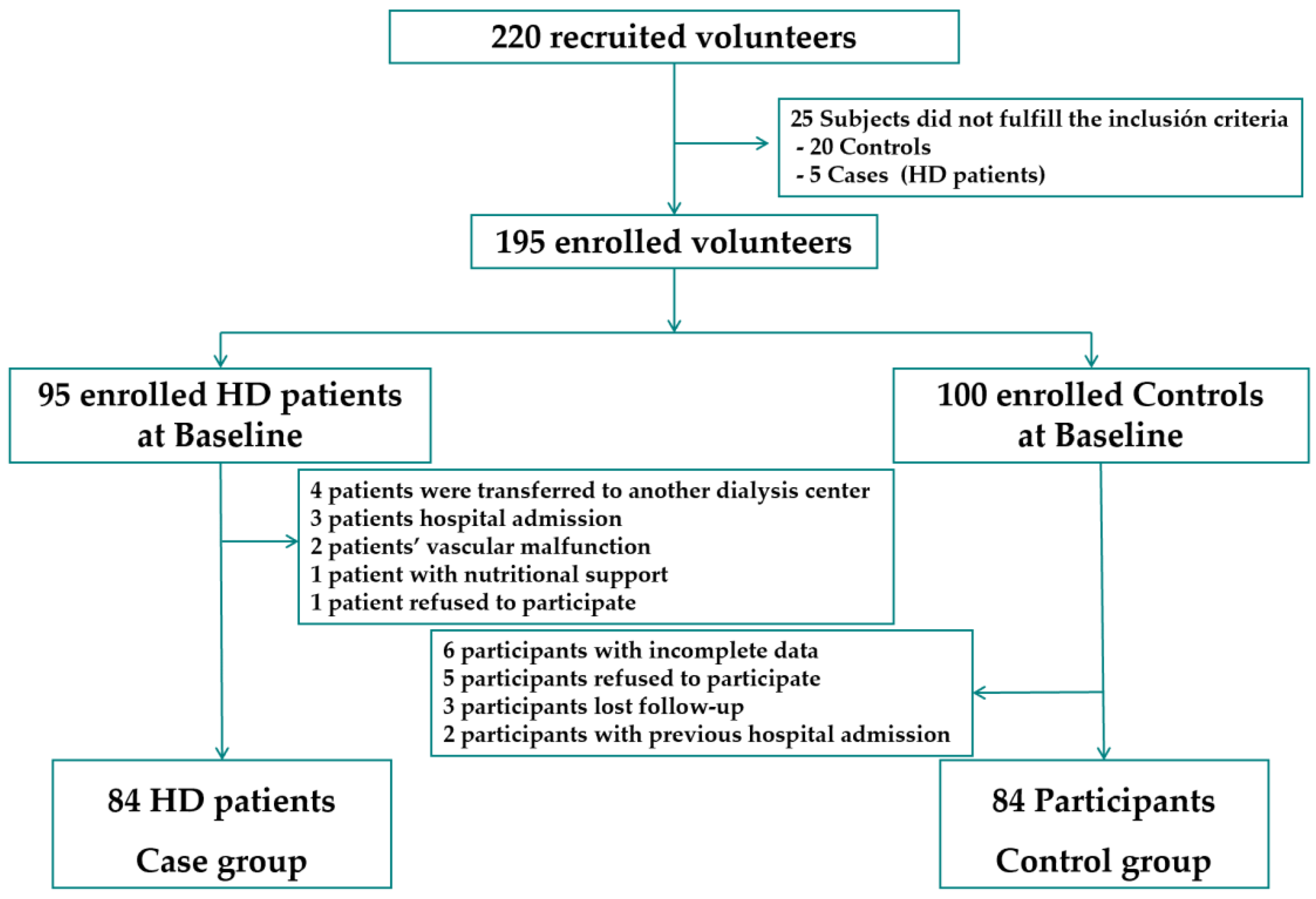
2.1. Study Design and Participants
2.2. Sample Size
2.3. Study Protocol and Data Collection
2.4. Anthropometric Measurements
2.5. Analysis of Body Composition
2.6. Laboratory Parameters
2.7. Geriatric Nutritional Risk Index
2.8. Statistical Analysis
3. Results
3.1. Global Data and Comparison between Cases and Controls
3.2. Univariate Conditional Regression Analyses
3.3. Multivariate Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs. 2022. Available online: https://population.un.org/wpp/ (accessed on 28 September 2023).
- GBD. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and all-cause mortality in older adults: A meta-analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, T.; Mehrotra, R.; Park, J.; Streja, E.; Dukkipati, R.; Nissenson, A.R.; Ma, J.Z.; Kovesdy, C.P.; Kalantar-Zadeh, K. Effect of Age and Dialysis Vintage on Obesity Paradox in Long-term Hemodialysis Patients. Am. J. Kidney Dis. 2013, 63, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Giovannucci, E.L. Body composition and mortality in the general population: A review of epidemiologic studies. Exp. Biol. Med. 2018, 243, 1275–1285. [Google Scholar] [CrossRef]
- de Mutsert, R.; Snijder, M.B.; van der Sman-de Beer, F.; Seidell, J.C.; Boeschoten, E.W.; Krediet, R.T.; Dekker, J.M.; Vandenbroucke, J.P.; Dekker, F.W. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J. Am. Soc. Nephrol. 2007, 18, 967–974. [Google Scholar] [CrossRef]
- Ladhani, M.; Craig, J.C.; Irving, M.; Clayton, P.A.; Wong, G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2017, 32, 439–449. [Google Scholar] [CrossRef]
- Kim, C.S.; Han, K.D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Body Mass Index and Waist Circumference with All-Cause Mortality in Hemodialysis Patients. J. Clin. Med. 2020, 9, 1289. [Google Scholar] [CrossRef]
- Sabatino, A.; Avesani, C.M.; Regolisti, G.; Adinolfi, M.; Benigno, G.; Delsante, M.; Fiaccadori, E.; Gandolfini, I. Sarcopenic obesity and its relation with muscle quality and mortality in patients on chronic hemodialysis. Clin. Nutr. 2023, 42, 1359–1368. [Google Scholar] [CrossRef]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef]
- Leij-Halfwerk, S.; Verwijs, M.H.; van Houdt, S.; Borkent, J.W.; Guaitoli, P.R.; Pelgrim, T.; Heymans, M.W.; Power, L.; Visser, M.; Corish, C.A.; et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults >/=65 years: A systematic review and meta-analysis. Maturitas 2019, 126, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Liao, Y.; Peng, Z.; Liu, F.; Wang, Q.; Yang, W. Association of muscle wasting with mortality risk among adults: A systematic review and meta-analysis of prospective studies. J. Cachexia Sarcopenia Muscle 2023, 14, 1596–1612. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Khan, T.A.; Aune, D.; Emadi, A.; Shab-Bidar, S. Body fat and risk of all-cause mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Int. J. Obes. 2022, 46, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Garagarza, C.; Valente, A.; Caetano, C. Low body cell mass index in hemodialysis patients: Association with clinical parameters and survival. Hemodial. Int. 2020, 24, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z. Effect of bioimpedance-defined overhydration parameters on mortality and cardiovascular events in patients undergoing dialysis: A systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211031063. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Chiang, J.M.; Kittiskulnam, P.; Sheshadri, A.; Grimes, B.; Segal, M.; Kaysen, G.A.; Johansen, K.L. Longitudinal Assessment of Body Composition and Its Association With Survival Among Participants of the ACTIVE/ADIPOSE Study. J. Ren. Nutr. 2022, 32, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Ruperto, M.; Barril, G. The Extracellular Mass to Body Cell Mass Ratio as a Predictor of Mortality Risk in Hemodialysis Patients. Nutrients 2022, 14, 1659. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Muller-Bolla, K.; Ferrari, C.; Stanga, Z.; Nydegger, U.E.; Risch, L.; Risch, M. Mortality risk factors in community-dwelling, subjectively healthy, Swiss older adults: Update after 8-years follow-up. BMC Geriatr. 2023, 23, 303. [Google Scholar] [CrossRef]
- Kang, S.S.; Chang, J.W.; Park, Y. Nutritional Status Predicts 10-Year Mortality in Patients with End-Stage Renal Disease on Hemodialysis. Nutrients 2017, 9, 399. [Google Scholar] [CrossRef]
- Lukowsky, L.R.; Kheifets, L.; Arah, O.A.; Nissenson, A.R.; Kalantar-Zadeh, K. Patterns and predictors of early mortality in incident hemodialysis patients: New insights. Am. J. Nephrol. 2012, 35, 548–558. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Santin, F.; Brito, F.; Lindholm, B.; Stenvinkel, P.; Avesani, C.M. Nutritional status of older patients on hemodialysis: Which nutritional markers can best predict clinical outcomes? Nutrition 2019, 65, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Qureshi, A.R.; Heimburger, O.; Barany, P.; Wang, K.; Pecoits-Filho, R.; Stenvinkel, P.; Lindholm, B. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney Dis. 2006, 47, 139–148. [Google Scholar] [CrossRef]
- Huang, J.C.; Tsai, Y.C.; Wu, P.Y.; Lee, J.J.; Chen, S.C.; Chiu, Y.W.; Hsu, Y.L.; Chang, J.M.; Chen, H.C. Independent Association of Overhydration with All-Cause and Cardiovascular Mortality Adjusted for Global Left Ventricular Longitudinal Systolic Strain and E/E′ Ratio in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, J.; Zhou, Z.; Liu, J.; Li, C.; Liu, C. Impact of serum albumin level and variability on short-term cardiovascular-related and all-cause mortality in patients on maintenance hemodialysis. Medicine 2021, 100, e27666. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Limonta, D.; Pusani, C.; Vanotti, A. Feasible use of estimated height for predicting outcome by the geriatric nutritional risk index in long-term care resident elderly. Gerontology 2007, 53, 184–186. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Cereda, E.; Zagami, A.; Vanotti, A.; Piffer, S.; Pedrolli, C. Geriatric Nutritional Risk Index and overall-cause mortality prediction in institutionalised elderly: A 3-year survival analysis. Clin. Nutr. 2008, 27, 717–723. [Google Scholar] [CrossRef]
- Baumeister, S.E.; Fischer, B.; Doring, A.; Koenig, W.; Zierer, A.; John, J.; Heier, M.; Meisinger, C. The Geriatric Nutritional Risk Index predicts increased healthcare costs and hospitalization in a cohort of community-dwelling older adults: Results from the MONICA/KORA Augsburg cohort study, 1994–2005. Nutrition 2011, 27, 534–542. [Google Scholar] [CrossRef]
- Tsuneyoshi, S.; Matsukuma, Y.; Kawai, Y.; Hiyamuta, H.; Yamada, S.; Kitamura, H.; Tanaka, S.; Taniguchi, M.; Tsuruya, K.; Nakano, T.; et al. Association between geriatric nutritional risk index and stroke risk in hemodialysis patients: 10-Years outcome of the Q-Cohort study. Atherosclerosis 2021, 323, 30–36. [Google Scholar] [CrossRef]
- Matsukuma, Y.; Tanaka, S.; Taniguchi, M.; Nakano, T.; Masutani, K.; Hirakata, H.; Kitazono, T.; Tsuruya, K. Association of geriatric nutritional risk index with infection-related mortality in patients undergoing hemodialysis: The Q-Cohort Study. Clin. Nutr. 2019, 38, 279–287. [Google Scholar] [CrossRef]
- Takahashi, H.; Ito, Y.; Ishii, H.; Aoyama, T.; Kamoi, D.; Kasuga, H.; Yasuda, K.; Maruyama, S.; Matsuo, S.; Murohara, T.; et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J. Cardiol. 2014, 64, 32–36. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, S.; Fukuma, S.; Nakano, T.; Tsuruya, K.; Inaba, M. Geriatric Nutritional Risk Index (GNRI) and Creatinine Index Equally Predict the Risk of Mortality in Hemodialysis Patients: J-DOPPS. Sci. Rep. 2020, 10, 5756. [Google Scholar] [CrossRef]
- Panichi, V.; Cupisti, A.; Rosati, A.; Di, G.A.; Scatena, A.; Menconi, O.; Bozzoli, L.; Bottai, A. Geriatric nutritional risk index is a strong predictor of mortality in hemodialysis patients: Data from the Riscavid cohort. J. Nephrol. 2014, 27, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, M.; Zhang, Y.; Nie, L.; He, T.; Wang, Y.; Huang, Y.; Feng, B.; Zhang, J.; Zhao, J. Association of Geriatric Nutritional Risk Index with Mortality in Hemodialysis Patients: A Meta-Analysis of Cohort Studies. Kidney Blood Press. Res. 2018, 43, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef]
- Esquius, M.; Schwartz, S.; Lopez Hellin, J.; Andreu, A.L.; Garcia, E. Anthropometric reference parameters for the aged population. Med. Clin. 1993, 100, 692–698. [Google Scholar]
- World Obesity Federation. World Obesity Atlas. 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 28 August 2023).
- Wold health Organization (W.H.O). Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. 2011. Available online: https://apps.who.int/iris/handle/10665/44583 (accessed on 28 July 2023).
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Dumler, F.; Kilates, C. Use of bioelectrical impedance techniques for monitoring nutritional status in patients on maintenance dialysis. J. Ren. Nutr. 2000, 10, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Lazarus, J.M.; Lew, N.L.; Ma, L.; Lowrie, E.G. Bioimpedance norms for the hemodialysis population. Kidney Int. 1997, 52, 1617–1621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J. Statistic at Square One, 12th ed.; Wiley-Blackwell: Hoboken, NJ, USA; London, UK, 2021. [Google Scholar]
- Rahimlu, M.; Shab-Bidar, S.; Djafarian, K. Body Mass Index and All-cause Mortality in Chronic Kidney Disease: A Dose-response Meta-analysis of Observational Studies. J. Ren. Nutr. 2017, 27, 225–232. [Google Scholar] [CrossRef]
- Beddhu, S.; Pappas, L.M.; Ramkumar, N.; Samore, M. Effects of body size and body composition on survival in hemodialysis patients. J. Am. Soc. Nephrol. 2003, 14, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wong, P.Y.; Chung, Y.L.; Chow, S.K.; Cheung, W.H.; Law, S.W.; Chan, J.C.N.; Wong, R.M.Y. Deciphering the “obesity paradox” in the elderly: A systematic review and meta-analysis of sarcopenic obesity. Obes. Rev. 2023, 24, e13534. [Google Scholar] [CrossRef]
- Wizemann, V.; Wabel, P.; Chamney, P.; Zaluska, W.; Moissl, U.; Rode, C.; Malecka-Masalska, T.; Marcelli, D. The mortality risk of overhydration in haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 1574–1579. [Google Scholar] [CrossRef]
- Obi, Y.; Rhee, C.M.; Mathew, A.T.; Shah, G.; Streja, E.; Brunelli, S.M.; Kovesdy, C.P.; Mehrotra, R.; Kalantar-Zadeh, K. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J. Am. Soc. Nephrol. 2016, 27, 3758–3768. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Li, H.; Yu, P.; Yuan, L.; Hao, C.; Chen, J.; Kalantar-Zadeh, K. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am. J. Nephrol. 2014, 40, 140–150. [Google Scholar] [CrossRef]
- Moist, L.M.; Port, F.K.; Orzol, S.M.; Young, E.W.; Ostbye, T.; Wolfe, R.A.; Hulbert-Shearon, T.; Jones, C.A.; Bloembergen, W.E. Predictors of loss of residual renal function among new dialysis patients. J. Am. Soc. Nephrol. 2000, 11, 556–564. [Google Scholar] [CrossRef]
- Daugirdas, J.T.; Greene, T.; Rocco, M.V.; Kaysen, G.A.; Depner, T.A.; Levin, N.W.; Chertow, G.M.; Ornt, D.B.; Raimann, J.G.; Larive, B.; et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013, 83, 949–958. [Google Scholar] [CrossRef]
- Gunal, A.I.; Kirciman, E.; Guler, M.; Yavuzkir, M.; Celiker, H. Should the preservation of residual renal function cost volume overload and its consequence left ventricular hypertrophy in new hemodialysis patients? Ren. Fail. 2004, 26, 405–409. [Google Scholar] [CrossRef]
- Kong, J.H.; Davies, M.R.P.; Mount, P.F. Relationship between residual kidney function and symptom burden in haemodialysis patients. Intern. Med. J. 2021, 51, 52–61. [Google Scholar] [CrossRef]
- Wang, Z.; Deurenberg, P.; Wang, W.; Pietrobelli, A.; Baumgartner, R.N.; Heymsfield, S.B. Hydration of fat-free body mass: New physiological modeling approach. Am. J. Physiol. 1999, 276, E995–E1003. [Google Scholar] [CrossRef]
- Canaud, B.; Morena-Carrere, M.; Leray-Moragues, H.; Cristol, J.P. Fluid Overload and Tissue Sodium Accumulation as Main Drivers of Protein Energy Malnutrition in Dialysis Patients. Nutrients 2022, 14, 4489. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, C.R.; Park, K.H.; Hwang, J.H.; Kim, S.H. Predicting clinical outcomes using phase angle as assessed by bioelectrical impedance analysis in maintenance hemodialysis patients. Nutrition 2017, 41, 7–13. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Song, J.; Ma, L.; Wang, Y.; Mei, Q.; Jiang, W. Total body water/fat-free mass ratio as a valuable predictive parameter for mortality in maintenance hemodialysis patients. Medicine 2022, 101, e29904. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Kovesdy, C.P.; Bross, R.; Kopple, J.D.; Kalantar-Zadeh, K. Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am. J. Clin. Nutr. 2008, 88, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Ruperto, M.; Barril, G. Nutritional Status, Body Composition, and Inflammation Profile in Older Patients with Advanced Chronic Kidney Disease Stage 4-5: A Case-Control Study. Nutrients 2022, 14, 3650. [Google Scholar] [CrossRef] [PubMed]
- Barril, G.; Nogueira, A.; Alvarez-Garcia, G.; Nunez, A.; Sanchez-Gonzalez, C.; Ruperto, M. Nutritional Predictors of Mortality after 10 Years of Follow-Up in Patients with Chronic Kidney Disease at a Multidisciplinary Unit of Advanced Chronic Kidney Disease. Nutrients 2022, 14, 3848. [Google Scholar] [CrossRef]
- Ruperto, M.; Sanchez-Muniz, F.J.; Barril, G. Predictors of protein-energy wasting in haemodialysis patients: A cross-sectional study. J. Hum. Nutr. Diet. 2014, 29, 38–47. [Google Scholar] [CrossRef]
- Tsai, M.T.; Liu, H.C.; Huang, T.P. The impact of malnutritional status on survival in elderly hemodialysis patients. J. Chin. Med. Assoc. 2016, 79, 309–313. [Google Scholar] [CrossRef]
- Ishii, H.; Takahashi, H.; Ito, Y.; Aoyama, T.; Kamoi, D.; Sakakibara, T.; Umemoto, N.; Kumada, Y.; Suzuki, S.; Murohara, T. The Association of Ankle Brachial Index, Protein-Energy Wasting, and Inflammation Status with Cardiovascular Mortality in Patients on Chronic Hemodialysis. Nutrients 2017, 9, 416. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chiu, Y.L.; Hsu, S.P.; Pai, M.F.; Yang, J.Y.; Peng, Y.S. Fetuin A/nutritional status predicts cardiovascular outcomes and survival in hemodialysis patients. Am. J. Nephrol. 2014, 40, 233–241. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Kubrusly, M.; Lima, A.T.; Torres, D.M.; Cavalcante, N.M.; Jeronimo, A.L.; Oliveira, T.C. Correlation between nutritional markers and appetite self-assessments in hemodialysis patients. J. Ren. Nutr. 2015, 25, 301–307. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yu, Q.; Yuan, W. Value of the Geriatric Nutritional Risk Index in predicting mortality of elderly patients undergoing hemodialysis. Zhonghua Yi Xue Za Zhi 2015, 95, 3741–3745. [Google Scholar]
- Szeto, C.C.; Kwan, B.C.; Chow, K.M.; Law, M.C.; Li, P.K. Geriatric nutritional risk index as a screening tool for malnutrition in patients on chronic peritoneal dialysis. J. Ren. Nutr. 2010, 20, 29–37. [Google Scholar] [CrossRef]
- Kuo, I.C.; Huang, J.C.; Wu, P.Y.; Chen, S.C.; Chang, J.M.; Chen, H.C. A Low Geriatric Nutrition Risk Index Is Associated with Progression to Dialysis in Patients with Chronic Kidney Disease. Nutrients 2017, 9, 1228. [Google Scholar] [CrossRef]




| Variable | HD Patients (n = 84) | Controls (n = 84) | p-Value |
|---|---|---|---|
| Male, n (%) | 40.0 (47.60) | 40.0 (47.60) | - |
| Age (years) | 76.40 ± 4.04 | 77.26 ± 3.75 | 0.150 |
| DM n; (%) | 23.0 (13.70) | 14 (7.60) | 0.095 |
| BW (kg) | 66.72 ± 13.34 | 66.90 ± 11.27 | 0.920 |
| SBW (%) | 100.42 ± 21.30 | 119.86 ± 21.84 | <0.001 |
| BMI (kg/m2) | 25.44 ± 4.87 | 28.54 ± 5.12 | <0.001 |
| WC (cm) | 96.04 ± 12.97 | 102.20 ± 11.39 | 0.001 |
| SKF (%) | 121.60 ± 55.64 | 144.79 ± 45.92 | <0.001 |
| MAMC (%) | 95.21 ± 10.51 | 110.33 ± 16.01 | <0.001 |
| Resistance (Ω) | 506.36 ± 68.18 | 605.01 ± 72.31 | <0.001 |
| Reactance (χc) | 40.98 ± 10.02 | 51.12 ± 6.00 | <0.001 |
| FFM (kg) | 46.27 ± 9.69 | 40.76 ± 5.98 | 0.078 |
| MM (kg) | 23.89 ± 5.69 | 27.11 ± 4.49 | <0.001 |
| FM (kg) | 20.34 ± 9.95 | 26.52 ± 8.37 | 0.008 |
| Exchangeable Na/K | 1.32 ± 0.35 | 0.93 ± 0.18 | <0.001 |
| TBW (L) | 35.94 ± 6.91 | 32.37 ± 4.28 | 0.100 |
| ECW (L) | 18.98 ± 3.80 | 14.68 ± 2.54 | <0.001 |
| ICW (L) | 16.96 ± 4.46 | 17.68 ± 3.01 | 0.010 |
| BCM (kg) | 18.52 ± 4.86 | 21.94 ± 3.89 | <0.001 |
| PA (°) | 4.26 ± 0.70 | 5.41 ± 0.90 | <0.001 |
| Variable | HD Patients (n = 84) | Controls (n = 84) | p-Value |
|---|---|---|---|
| s-Cholesterol (mg/dL) | 157.33 ± 42.41 | 169.97 ± 37.14 | 0.153 |
| s-Triglycerides (mg/dL) | 152.76 ± 85.97 | 112.64 ± 45.23 | <0.001 |
| s-Creatinine (mg/dL) | 3.82 ± 1.33 | 0.95 ± 0.28 | <0.001 |
| s-Phosphorous (mg/dL) | 4.69 ± 0.77 | 4.12 ± 0.39 | <0.001 |
| s-Albumin (g/dL) | 3.73 ± 0.42 | 3.98 ± 0.30 | 0.030 |
| s-Prealbumin (mg/dL) | 26.40 ± 8.52 | 18.51 ± 3.56 | 0.030 |
| s-Transferrin (mg/dL) | 171.36 ± 30.97 | 210.52 ± 25.72 | <0.001 |
| s-Ferritin (ηg/mL) | 511.86 ± 452.91 | 106.16 ± 94.46 | <0.001 |
| s-CRP (mg/dL) | 1.21 ± 0.91 | 0.64 ± 0.50 | <0.001 |
| Hemoglobin (g/dL) | 12.10 ± 1.39 | 12.52 ± 1.36 | 0.466 |
| Total lymphocyte count (×103/mm3) | 1361.79 ± 499.41 | 1947.10 ± 731.92 | 0.073 |
| GNRI (points) | 97.55 ± 11.32 | 108.47 ± 10.65 | <0.001 |
| Variable | OR | St Error | 95%CI | p-Value |
|---|---|---|---|---|
| BMI (kg/m2) | 0.841 | 0.037 | 0.771 to 0.918 | <0.001 |
| WC (cm) | 0.956 | 0.014 | 0.928 to 0.985 | 0.003 |
| FFM (%) | 1.164 | 0.041 | 1.086 to 1.247 | <0.001 |
| MM (%) | 0.902 | 0.024 | 0.855 to 0.952 | <0.001 |
| FM (%) | 0.889 | 0.023 | 0.843 to 0.938 | <0.001 |
| TBW (%) | 1.165 | 0.041 | 1.086 to 1.251 | <0.001 |
| ECW (%) | 1.278 | 0.063 | 1.160 to 1.408 | <0.001 |
| ICW (%) | 0.785 | 0.038 | 0.713 to 0.865 | <0.001 |
| BCM (%) | 0.857 | 0.026 | 0.807 to 0.910 | <0.001 |
| PA (°) | 0.157 | 0.061 | 0.073 to 0.337 | <0.001 |
| Total cholesterol (mg/dL) | 0.987 | 0.005 | 0.977 to 0.997 | 0.011 |
| s-Triglycerides (mg/dL) | 1.007 | 0.003 | 1.002 to 1.013 | 0.011 |
| s-Phosphorous (mg/dL) | 2.396 | 0.742 | 1.305 to 4.398 | 0.005 |
| s-Albumin (g/dL) | 0.341 | 0.155 | 0.139 to 0.833 | 0.018 |
| s-Prealbumin (mg/dL) | 0.756 | 0.077 | 0.682 to 0.861 | <0.001 |
| s-Transferrin (mg/dL) | 0.956 | 0.008 | 0.938 to 0.973 | <0.001 |
| s-Ferritin (ηg/mL) | 1.010 | 0.002 | 1.006 to 1.014 | <0.001 |
| s-CRP (mg/dL) | 1.704 | 0.281 | 1.233 to 2.355 | <0.001 |
| Total lymphocyte count (×103/mm3) | 0.998 | 0.003 | 0.998 to 0.999 | <0.001 |
| GNRI (points) | 0.881 | 0.023 | 0.844 to 0.934 | <0.001 |
| Variable | OR | St Error | 95%CI | p-Value |
|---|---|---|---|---|
| Age (<75 years) | 0.119 | 0.604 | 0.036 to 0.388 | <0.001 |
| BMI (≥23 kg/m2) | 0.169 | 0.612 | 0.051 to 0.562 | 0.004 |
| ECW (%) | 1.162 | 0.047 | 1.061 to 1.273 | 0.001 |
| PA (°) | 0.099 | 0.516 | 0.036 to 0.271 | <0.001 |
| s-Albumin (≥3.8 g/dL) | 0.251 | 0.634 | 0.073 to 0.870 | 0.029 |
| s-CRP (<1 mg/dL) | 0.056 | 0.736 | 0.013 to 0.235 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruperto, M.; Barril, G. Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients—A Case–Control Study. Nutrients 2023, 15, 5036. https://doi.org/10.3390/nu15245036
Ruperto M, Barril G. Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients—A Case–Control Study. Nutrients. 2023; 15(24):5036. https://doi.org/10.3390/nu15245036
Chicago/Turabian StyleRuperto, Mar, and Guillermina Barril. 2023. "Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients—A Case–Control Study" Nutrients 15, no. 24: 5036. https://doi.org/10.3390/nu15245036
APA StyleRuperto, M., & Barril, G. (2023). Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients—A Case–Control Study. Nutrients, 15(24), 5036. https://doi.org/10.3390/nu15245036







