Comparative Analysis of Gut Microbiota from Rats Induced by Se Deficiency and T-2 Toxin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Construction and Sample Collection
2.2. T-2 Toxin Solution Preparation
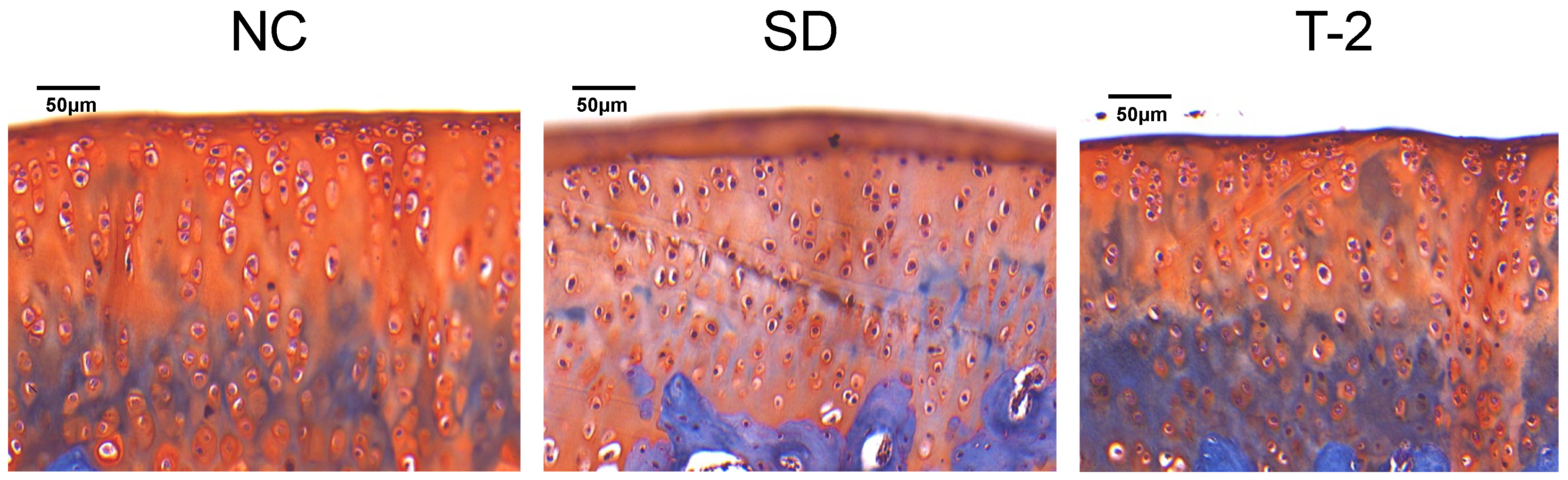
2.3. Safranin O/Fast Green Staining
2.4. DNA Extraction of Gut Microbiota
2.5. 16S rDNA Gene Sequencing
2.6. Statistical Analysis
3. Results
3.1. Pathological Changes in the Knee Joint of the Rats
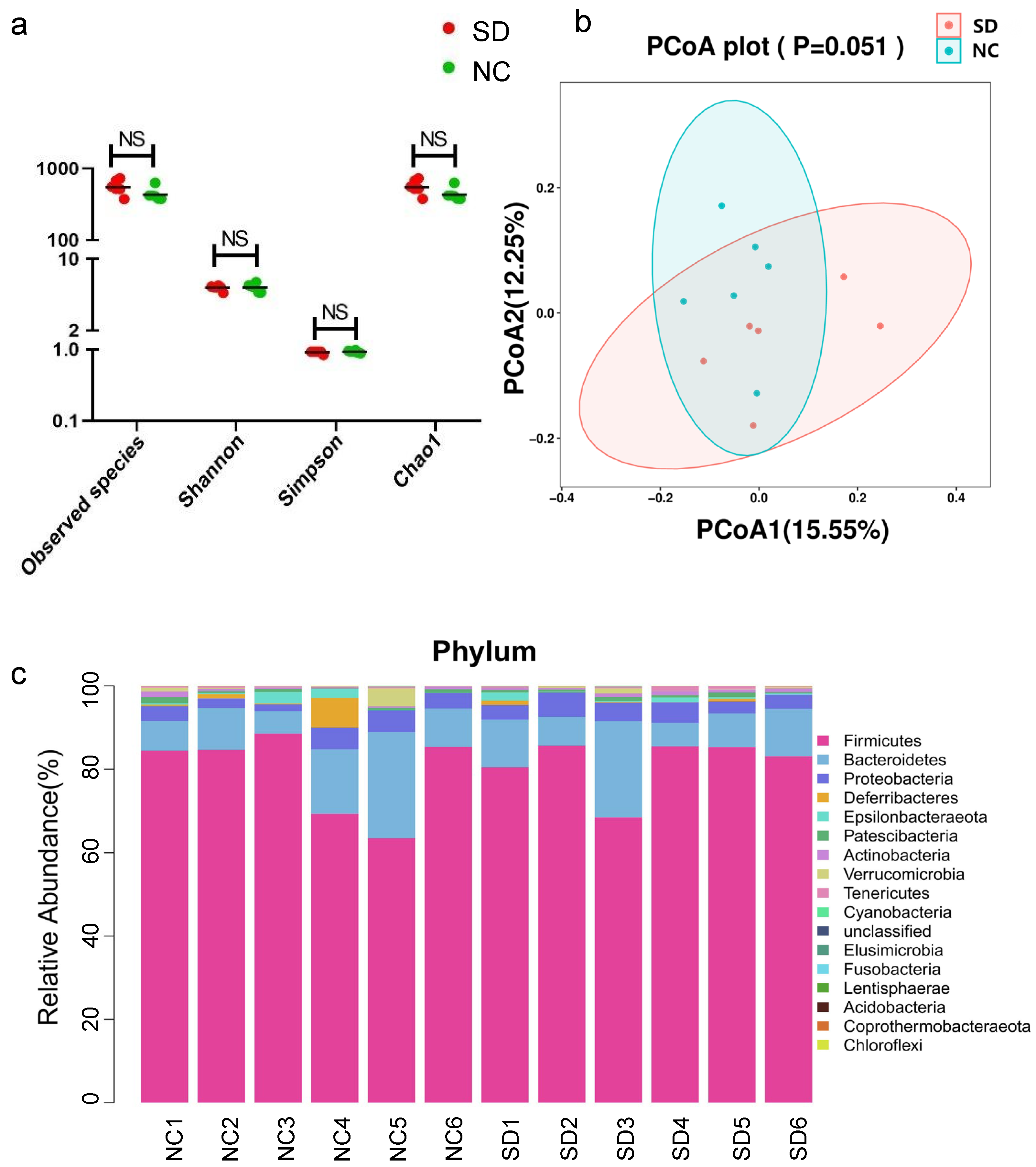
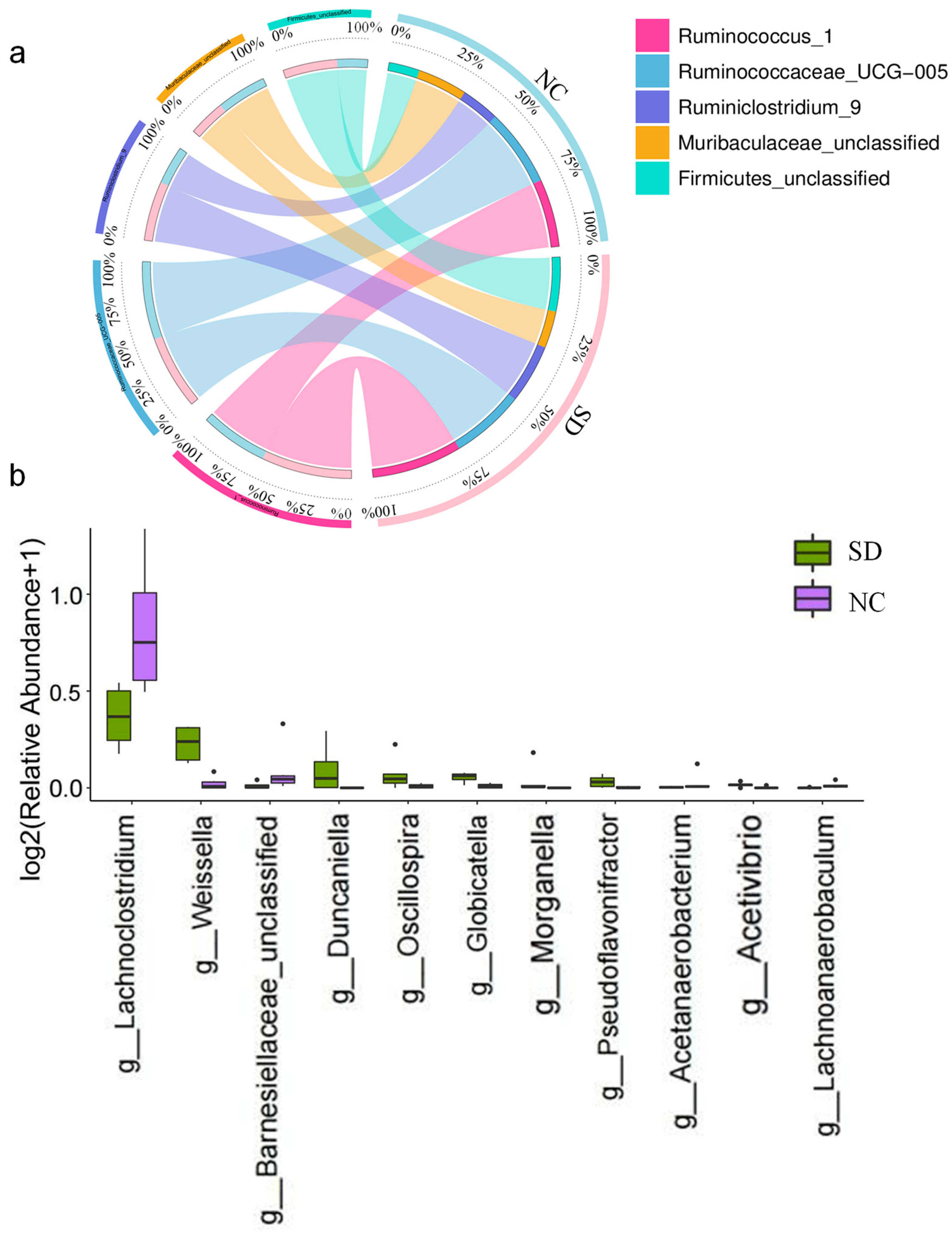
3.2. Analysis of Gut Microbiota in Rats Exposed to Se Deficiency vs. Negative Control Rats
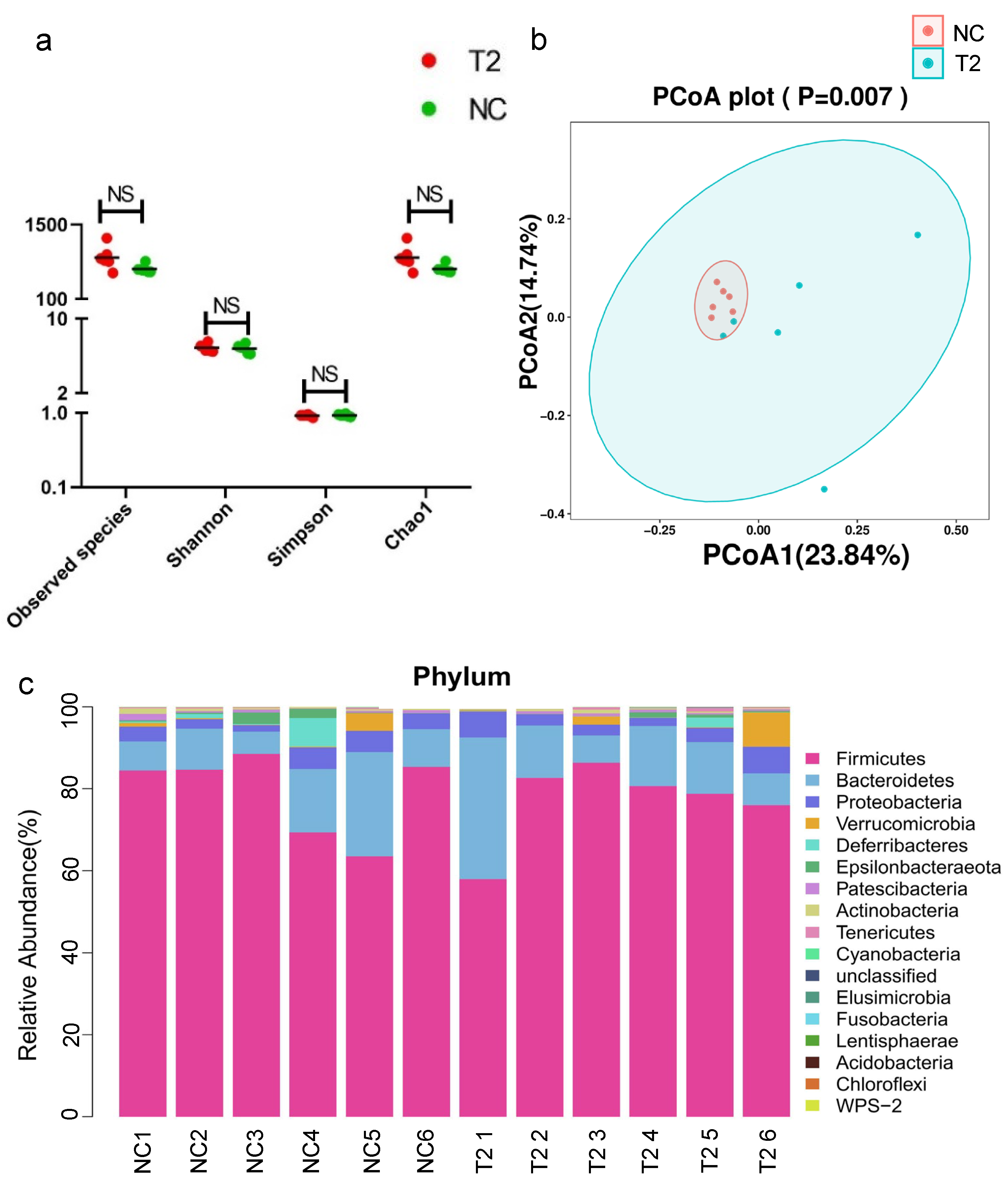
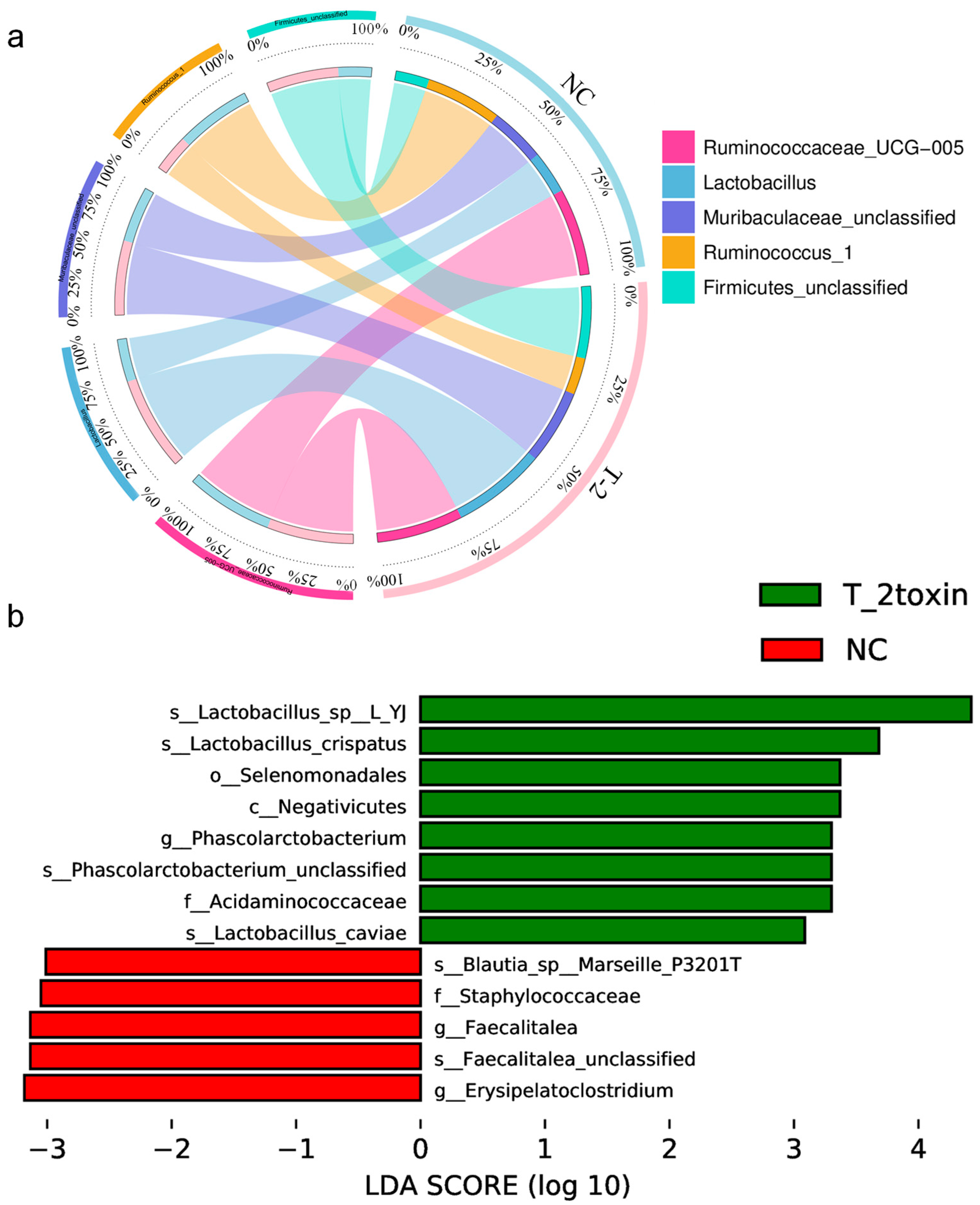
3.3. Analysis of Gut Microbiota in Rats Exposed to T-2 Toxin vs. Negative Control Rats
3.4. Analysis of Gut Microbiota in Rats Exposed to Se Deficiency vs. T-2 Toxin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Yu, J.; Liu, H.; Liu, Y.; Liu, N.; Cao, Y.; Zhang, X.; Sun, D. Endemic Kashin-Beck disease: A food-sourced osteoarthropathy. Semin. Arthritis Rheum. 2020, 50, 366–372. [Google Scholar] [CrossRef]
- Shi, T.; Fu, X.; Wang, F.; Zhang, X.; Cai, Y.; Wu, X.; Sun, L. The WNT/β-catenin signalling pathway induces chondrocyte apoptosis in the cartilage injury caused by T-2 toxin in rats. Toxicon 2021, 204, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Chen, X.; Qi, M.; Du, X.; Xia, Z.; Liu, Q.; Sun, N.; Shi, C.; Zhang, R. Status and potential diagnostic roles of essential trace elements in Kashin- Beck disease patients. J. Trace Elem. Med. Biol. 2022, 69, 126880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, B.; Luo, K. Differences of selenium and other trace elements abundances between the Kaschin-Beck disease area and nearby non-Kaschin-Beck disease area, Shaanxi Province, China. Food Chem. 2022, 373, 131481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Li, H.; Yang, L.; Li, Y.; Kong, C. Spatial distribution and determinants of health loss from Kashin-Beck disease in Bin County, Shaanxi Province, China. BMC Public Health 2021, 21, 387. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin-The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules 2021, 26, 6868. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Guo, X.; Pang, Q.; Wu, S.; Wu, C.; Xu, P.; Bai, Y. The role of mitochondria in T-2 toxin-induced human chondrocytes apoptosis. PLoS ONE 2014, 9, e108394. [Google Scholar] [CrossRef]
- Shi, Y.; Shao, X.; Sun, M.; Ma, J.; Li, B.; Zou, N.; Li, F. MiR-140 is involved in T-2 toxin-induced matrix degradation of articular cartilage. Toxicon 2023, 222, 106987. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.; Yang, L.; Li, Y.; Wei, B.; Wang, W.; Gong, H.; Guo, M.; Nima, C.; Zhao, S.; et al. Trace Element Levels in Scalp Hair of School Children in Shigatse, Tibet, an Endemic Area for Kaschin-Beck Disease (KBD). Biol. Trace Elem. Res. 2017, 180, 15–22. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Prieto, F.J.; Xu, H.; Ortiz-Alvarez, L.; Di, X.; Kohler, I.; Jurado-Fasoli, L.; Rubio-Lopez, J.; Plaza-Díaz, J.; Vilchez-Vargas, R.; Link, A.; et al. The relative abundance of fecal bacterial species belonging to the Firmicutes and Bacteroidetes phyla is related to plasma levels of bile acids in young adults. Metabolomics 2023, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome 2019, 7, 19. [Google Scholar] [CrossRef]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Lai, H.C.; Lin, T.L.; Chen, T.W.; Kuo, Y.L.; Chang, C.J.; Wu, T.R.; Shu, C.C.; Tsai, Y.H.; Swift, S.; Lu, C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 2022, 71, 309–321. [Google Scholar] [CrossRef]
- Ramires, L.C.; Santos, G.S.; Ramires, R.P.; da Fonseca, L.F.; Jeyaraman, M.; Muthu, S.; Lana, A.V.; Azzini, G.; Smith, C.S.; Lana, J.F. The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? Int. J. Mol. Sci. 2022, 23, 1494. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, M.A.; Donica, M.; Baker, L.W.; Stevenson, M.E.; Annan, A.C.; Beth Humphrey, M.; James, J.A.; Sawalha, A.H. Genome-Wide DNA Methylation Study Identifies Significant Epigenomic Changes in Osteoarthritic Subchondral Bone and Similarity to Overlying Cartilage. Arthritis Rheumatol. 2016, 68, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, J.M.; Sellam, J.; Maugars, Y.; Berenbaum, F. Cartilage-gut-microbiome axis: A new paradigm for novel therapeutic opportunities in osteoarthritis. RMD Open 2019, 5, e001037. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Liu, G.; Wu, T.; Du, L. Selenium for preventing Kashin-Beck osteoarthropathy in children: A meta-analysis. Osteoarthr. Cartil. 2009, 17, 144–151. [Google Scholar] [CrossRef]
- Peng, A.; Wang, W.H.; Wang, C.X.; Wang, Z.J.; Rui, H.F.; Wang, W.Z.; Yang, Z.W. The role of humic substances in drinking water in Kashin-Beck disease in China. Environ. Health Perspect. 1999, 107, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol. Nutr. Food Res. 2017, 61, 1600680. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J.; Legge, R.; Benson, A.K.; Hatfield, D.L.; Gladyshev, V.N. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhang, Y.; Wang, Z.L.; Xue, S.H.; Li, S.Y.; Zhou, X.R.; Zhang, M.; Fang, Q.; Wang, W.J.; Chen, C.; et al. Increased Chondrocyte Apoptosis in Kashin-Beck Disease and Rats Induced by T-2 Toxin and Selenium Deficiency. Biomed. Environ. Sci. 2017, 30, 351–362. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Y.; Liu, Y.; Chen, F.; Chen, S.; Zhang, F.; Wang, C.; Li, S.; Hu, M.; Huang, R.; et al. Comparative Analysis of Differentially Expressed Genes in Chondrocytes from Rats Exposed to Low Selenium and T-2 Toxin. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Wang, H.L.; Dai, L.L.; Zong, W.H.; Wang, Y.Z.; Bi, J.; Han, W.; Dong, G.J. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Chiang, H.C.; Gordon, J.I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 2008, 4, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Zhang, H.; Lee, Y.K.; Zhai, Q.; Chen, W. Roles of intestinal bacteroides in human health and diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 3518–3536. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, Q.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. A potential species of next-generation probiotics? The dark and light sides of Bacteroides fragilis in health. Food Res. Int. 2019, 126, 108590. [Google Scholar] [CrossRef]
- Fehlner-Peach, H.; Magnabosco, C.; Raghavan, V.; Scher, J.U.; Tett, A.; Cox, L.M.; Gottsegen, C.; Watters, A.; Wiltshire-Gordon, J.D.; Segata, N.; et al. Distinct Polysaccharide Utilization Profiles of Human Intestinal Prevotella copri Isolates. Cell Host Microbe 2019, 26, 680–690.e685. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Su, Y.; Li, T. An update on T2-toxins: Metabolism, immunotoxicity mechanism and human assessment exposure of intestinal microbiota. Heliyon 2022, 8, e10012. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Asha; Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.Y.; Jhun, J.; Jung, K.; Park, S.H.; Yang, C.W.; Cho, Y.; Kim, S.J.; Cho, M.L. Lactobacillus acidophilus ameliorates pain and cartilage degradation in experimental osteoarthritis. Immunol. Lett. 2018, 203, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Guo, C.; Wang, D.; Zhang, C.; Hua, L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: A randomised double-blind, placebo-controlled clinical trial. Benef. Microbes 2017, 8, 697–703. [Google Scholar] [CrossRef]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus gnavus: Friend or foe for human health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, H.; Guan, F.; Xue, S.; Song, D.; Chen, J.; Wang, Z. T-2 Toxin Alters the Levels of Collagen II and Its Regulatory Enzymes MMPs/TIMP-1 in a Low-Selenium Rat Model of Kashin-Beck Disease. Biol. Trace Elem. Res. 2016, 169, 237–246. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship With Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, X.; Pi, S.; Chang, J.; Dou, X.; Yan, S.; Song, X.; Chen, Y.; Zeng, X.; Zhu, L.; et al. Dietary supplementation with biogenic selenium nanoparticles alleviate oxidative stress-induced intestinal barrier dysfunction. NPJ Sci. Food 2022, 6, 30. [Google Scholar] [CrossRef] [PubMed]
| SD | T-2 | |
|---|---|---|
| Phylum | ||
| Firmicutes | 81.39% | 77.06% |
| Bacteroidetes | 11.11% | 14.85% |
| Proteobacteria | 4.17% | 3.95% |
| Actinobacteria | 0.70% | 0.50% |
| Verrucomicrobia | 0.36% | 1.81% |
| Genus | ||
| Ruminococcus_1 | 12.62% | 4.58% |
| Ruminococcaceae_UCG-005 | 10.31% | 10.75% |
| Ruminiclostridium_9 | 8.26% | 4.74% |
| Lactobacillus | 2.82% | 11.71% |
| Muribaculaceae_unclassified | 5.16% | 9.26% |
| Firmicutes_unclassified | 7.55% | 8.95% |
| SD | T-2 | |
|---|---|---|
| (A) * | ||
| Weissella | Up (3.45) | up (3.06) |
| Acetivibrio | up (2.89) | up (5.30) |
| Pseudoflavonifractor | up (3.49) | up (4.61) |
| Acetanaerobacterium | down (−2.93) | down (−Inf) |
| (B) ** | ||
| Morganella (Inf) | Veillonella (2.03) | |
| Globicatella (2.46) | Ruminococcaceae_UCG-011 (Inf) | |
| Duncaniella (Inf) | Eubacterium_xylanophilum_group (1.98) | |
| Oscillospira (3.05) | Phascolarctobacterium (0.49) | |
| (C) *** | ||
| Lachnoanaerobaculum (−4.49) | Hydrogenoanaerobacterium (-Inf) | |
| Barnesiellaceae_unclassified (−3.11) | Rikenella (−3.98) | |
| Lachnoclostridium (−1.44) | Phocea (−5.03) | |
| Aerococcus (−4.86) | ||
| DTU014_unclassified (−3.49) | ||
| Erysipelatoclostridium (−1.69) | ||
| Faecalitalea (−3.30) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Gong, Y.; Zhang, Y.; Li, S.; Wang, C.; Yuan, Y.; Lv, X.; Liu, Y.; Chen, F.; Chen, S.; et al. Comparative Analysis of Gut Microbiota from Rats Induced by Se Deficiency and T-2 Toxin. Nutrients 2023, 15, 5027. https://doi.org/10.3390/nu15245027
Wu Y, Gong Y, Zhang Y, Li S, Wang C, Yuan Y, Lv X, Liu Y, Chen F, Chen S, et al. Comparative Analysis of Gut Microbiota from Rats Induced by Se Deficiency and T-2 Toxin. Nutrients. 2023; 15(24):5027. https://doi.org/10.3390/nu15245027
Chicago/Turabian StyleWu, Yifan, Yi Gong, Yu Zhang, Shujin Li, Chaowei Wang, Yuequan Yuan, Xi Lv, Yanli Liu, Feihong Chen, Sijie Chen, and et al. 2023. "Comparative Analysis of Gut Microbiota from Rats Induced by Se Deficiency and T-2 Toxin" Nutrients 15, no. 24: 5027. https://doi.org/10.3390/nu15245027
APA StyleWu, Y., Gong, Y., Zhang, Y., Li, S., Wang, C., Yuan, Y., Lv, X., Liu, Y., Chen, F., Chen, S., Zhang, F., Guo, X., Wang, X., Ning, Y., & Zhao, H. (2023). Comparative Analysis of Gut Microbiota from Rats Induced by Se Deficiency and T-2 Toxin. Nutrients, 15(24), 5027. https://doi.org/10.3390/nu15245027







