Distinct Metabolites in Osteopenia and Osteoporosis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
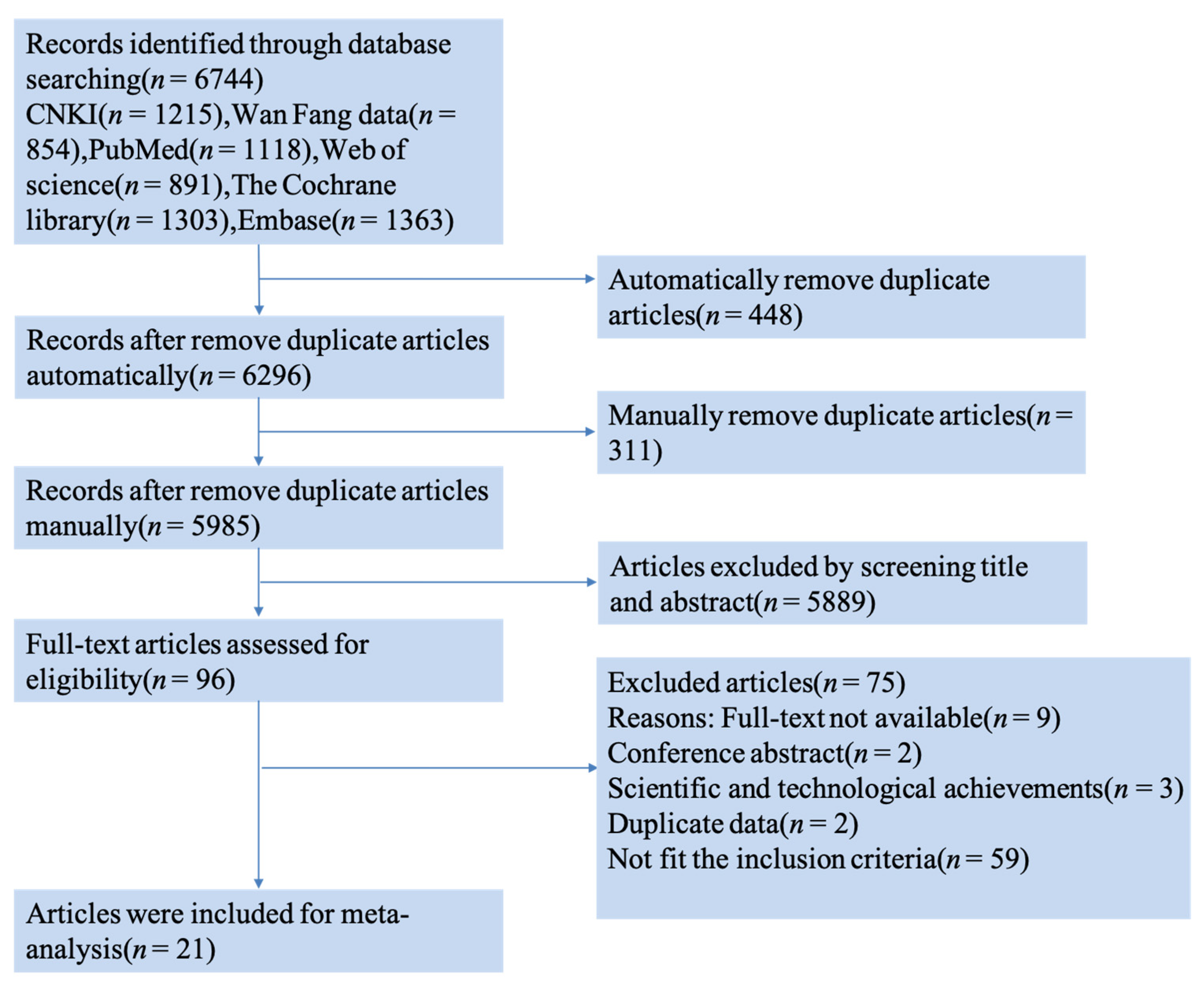
3.1. Literature Search Results
3.2. Characteristics of Included Studies
3.3. Risk of Bias of Included Studies
3.4. Qualitative Synthesis
3.5. Amino Acids
3.6. Lipid Metabolites
3.7. Carbohydrate Metabolites
3.8. Other Metabolites
3.9. Metabolites and Traditional Chinese Medicine Syndrome
3.10. Pathways Analysis
3.11. Meta-Analysis for Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, Itc17–Itc32. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, T.; Dockery, F. Osteoporosis and fracture risk in older people. Clin. Med. 2014, 14, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Yong, E.L.; Logan, S. Menopausal osteoporosis: Screening, prevention and treatment. Singap. Med. J. 2021, 62, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Karaguzel, G.; Holick, M.F. Diagnosis and treatment of osteopenia. Rev. Endocr. Metab. Disord. 2010, 11, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Zhenlin, Z. Guidelines for the Diagnosis and Treatment of Primary Osteoporosiss (2022). Chin. Gen. Med. 2023, 26, 1671–1691. [Google Scholar]
- Bandaru, S.; Hare, K.; Krueger, D.; Binkley, N. Do patients that fracture with normal DXA-measured BMD have normal bone? Arch. Osteoporos. 2020, 15, 70. [Google Scholar] [CrossRef]
- Ma, J.; Lin, X.; Chen, C.; Li, S.; Zhang, S.; Chen, Z.; Li, D.; Zhao, F.; Yang, C.; Yin, C.; et al. Circulating miR-181c-5p and miR-497-5p Are Potential Biomarkers for Prognosis and Diagnosis of Osteoporosis. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef]
- Delaney, M.F. Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am. J. Obstet. Gynecol. 2006, 194, S12–S23. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Reid, I.R. Should we prescribe calcium or vitamin D supplements to treat or prevent osteoporosis? Climacteric 2015, 18 (Suppl. S2), 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Bolland, M.J. Calcium supplementation in osteoporosis: Useful or harmful? Eur. J. Endocrinol. 2018, 178, D13–D25. [Google Scholar] [CrossRef] [PubMed]
- Thudium, C.S.; Löfvall, H.; Karsdal, M.A.; Bay-Jensen, A.C.; Bihlet, A.R. Protein biomarkers associated with pain mechanisms in osteoarthritis. J. Proteom. 2019, 190, 55–66. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Kelsey, J.L. Risk factors for osteoporosis and associated fractures. Public Health Rep. 1989, 104, 14–20. [Google Scholar]
- Muñoz, M.; Robinson, K.; Shibli-Rahhal, A. Bone Health and Osteoporosis Prevention and Treatment. Clin. Obstet. Gynecol. 2020, 63, 770–787. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Sun, Y.; Wu, M.; Ma, Y.; Yang, L.; Meng, C.; Zhong, L.; Hossain, M.A.; Peng, B. Prediction model for the risk of osteoporosis incorporating factors of disease history and living habits in physical examination of population in Chongqing, Southwest China: Based on artificial neural network. BMC Public Health 2021, 21, 991. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Brown, J.P.; Don-Wauchope, A.; Douville, P.; Albert, C.; Vasikaran, S.D. Current use of bone turnover markers in the management of osteoporosis. Clin. Biochem. 2022, 109–110, 1–10. [Google Scholar] [CrossRef]
- Jain, S.; Camacho, P. Use of bone turnover markers in the management of osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 366–372. [Google Scholar] [CrossRef]
- Yu, X.H.; Cao, R.R.; Yang, Y.Q.; Zhang, L.; Lei, S.F.; Deng, F.Y. Systematic evaluation for the causal effects of blood metabolites on osteoporosis: Genetic risk score and Mendelian randomization. Front. Public Health 2022, 10, 905178. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Yavropoulou, M.P.; Makras, P. Postmenopausal osteoporosis coexisting with other metabolic diseases: Treatment considerations. Maturitas 2021, 147, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, D. Metabolomics study of oral cancers. Metabolomics 2019, 15, 22. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, H.; Li, G.H.; Long, M.T.; Cheung, C.L.; Vasan, R.S.; Hsu, Y.H.; Kiel, D.P.; Liu, C.T. Metabolomics Insights into Osteoporosis Through Association with Bone Mineral Density. J. Bone Miner. Res. 2021, 36, 729–738. [Google Scholar] [CrossRef]
- Lv, H.; Jiang, F.; Guan, D.; Lu, C.; Guo, B.; Chan, C.; Peng, S.; Liu, B.; Guo, W.; Zhu, H.; et al. Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. Int. J. Mol. Sci. 2016, 17, 2018. [Google Scholar] [CrossRef]
- Mei, Z.; Dong, X.; Qian, Y.; Hong, D.; Xie, Z.; Yao, G.; Qin, A.; Gao, S.; Hu, J.; Liang, L.; et al. Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine 2020, 62, 103111. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Vrabel, M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Rev. Espaola Nutr. Humana Dietética 2009, 18, e123. [Google Scholar]
- Wells, G.A.; Shea, B.J.; O’Connell, D.; Peterson, J.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis; University of Liverpool: Liverpool, UK, 2000. [Google Scholar]
- Favaro Zeola, L.; Soares, P.V.; Cunha-Cruz, J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J. Dent. 2019, 81, 1–6. [Google Scholar] [CrossRef]
- Lim, S.K. Altered Hydroxylation of Estrogen in Patients with Postmenopausal Osteopenia. J. Clin. Endocrinol. Metab. 1997, 82, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, D.; Zhao, A.; Hou, X.; Zheng, X.; Chen, P.; Bao, Y.; Jia, W.; Hu, C.; Zhang, Z.L. Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporos. Int. 2019, 30, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Aleidi, S.M.; Alnehmi, E.A.; Alshaker, M.; Masood, A.; Benabdelkamel, H.; Al-Ansari, M.M.; Abdel Rahman, A.M. A Distinctive Human Metabolomics Alteration Associated with Osteopenic and Osteoporotic Patients. Metabolites 2021, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Pontes, T.A.; Barbosa, A.D.; Silva, R.D.; Melo-Junior, M.R.; Silva, R.O. Osteopenia-osteoporosis discrimination in postmenopausal women by 1H NMR-based metabonomics. PLoS ONE 2019, 14, e0217348. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bao, J.; An, G.; Ouyang, G.; Zhang, P.; Wang, C.; Ying, H.; Ouyang, P.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. Biosyst. 2016, 12, 2265–2275. [Google Scholar] [CrossRef]
- Yu, L.; Qi, H.; An, G.; Bao, J.; Ma, B.; Zhu, J.; Ouyang, G.; Zhang, P.; Fan, H.; Zhang, Q. Association between metabolic profiles in urine and bone mineral density of pre- and postmenopausal Chinese women. Menopause 2019, 26, 94–102. [Google Scholar] [CrossRef]
- Yin, H.; Zhu, T.C.; Zhang, Y.F.; Wang, J.W. Metabolomics-based screening for potential serum biomarkers of kidney yang defi ciency syndrome in osteoporosis patients: A pilot study. Asian J. Surg. 2022, 45, 2494–2495. [Google Scholar] [CrossRef]
- Poór, V.; Bufa, A.; Bíró, I.; Wilhelm, F.; Juricskay, S. Examination of sex steroids in the urines of postmenopausal women with osteoporosis. Chromatographia 2004, 60, S165–S168. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Miyamoto, H.; Mori, T.; Yoshida, S.; Fujie, A.; et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone 2017, 95, 1–4. [Google Scholar] [CrossRef]
- Deng, D.; Pan, C.; Wu, Z.; Sun, Y.; Liu, C.; Xiang, H.; Yin, P.; Shang, D. An Integrated Metabolomic Study of Osteoporosis: Discovery and Quantification of Hyocholic Acids as Candidate Markers. Front. Pharmacol. 2021, 12, 725341. [Google Scholar] [CrossRef]
- Cao, X.; Deng, L.; Zhou, G.; Wang, L.; Han, Y.; Li, G. Disorder of serum lipid metabolism in patients with postmenopausal osteoporosis based on untargeted lipidomics. Int. J. Clin. Exp. Med. 2021, 14, 789–799. [Google Scholar]
- Kou, J.; He, C.; Cui, L.; Zhang, Z.; Wang, W.; Tan, L.; Liu, D.; Zheng, W.; Gu, W.; Xia, N. Discovery of Potential Biomarkers for Postmenopausal Osteoporosis Based on Untargeted GC/LC-MS. Front. Endocrinol. 2022, 13, 849076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, K.; Du, J.; Ou, M.; Hou, J.; Wang, D.; Wang, J.; Zhang, W.; Sun, G. Serum concentrations of neonicotinoids and their characteristic metabolites in elderly population from South China: Association with osteoporosis. Environ. Res. 2022, 203, 111772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Shen, H.; Su, K.J.; Zhang, J.G.; Tian, Q.; Zhao, L.J.; Qiu, C.; Zhang, Q.; Garrett, T.J.; Liu, J.; et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr. Metab. 2018, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- You, Y.S.; Lin, C.Y.; Liang, H.J.; Lee, S.H.; Tsai, K.S.; Chiou, J.M.; Chen, Y.C.; Tsao, C.K.; Chen, J.H. Association between the metabolome and low bone mineral density in Taiwanese women determined by (1)H NMR spectroscopy. J. Bone Miner. Res. 2014, 29, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Fujie, A.; Morita, M.; Watanabe, R.; Tando, T.; et al. Metabolomics-based profiles predictive of low bone mass in menopausal women. Bone Rep. 2018, 9, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, J.W.; Zhang, Y.F.; Ma, Y.; Zhu, T.C.; Chen, H.; Wang, G.X.; Dou, W.W.; Su, Q.J. Screening study of the serum metabolite biomarkers in osteoporosis patients with liver-kidney Yin deficiency syndrome by UPLC-MS. Chin. J. Osteoporos. 2021, 27, 1316–1322. [Google Scholar]
- Zhu, T.C. Clinical Metabolomics of Primary Osteoporosis Based on Yin/Yang Deficiency Syndrome. Master’s Thesis, Nanjing University of Traditional Chinese Medicine, Nanjing, China, 2020. [Google Scholar]
- Li, X.F. Research of Common Cyndrome Factors for Postmenopausal Osteoporosis Based on Metabolomics. Master’s Thesis, Shandong University of Traditional Chinese Medicine, Nanjing, China, 2019. [Google Scholar]
- Guo, S.N.; Qi, X.N.; Yao, X.S.; Ren, L.; Chen, W.N. Metabolomics analysis based on UPLC-MS /MS in primary osteoporosis with Kidney-Yang deficiency syndrome. Chin. J. Osteoporos. 2022, 28, 1410–1415. [Google Scholar]
- Suzuki, A.; Iwata, J. Amino acid metabolism and autophagy in skeletal development and homeostasis. Bone 2021, 146, 115881. [Google Scholar] [CrossRef]
- Cui, Z.; Feng, H.; He, B.; He, J.; Tian, Y. Relationship Between Serum Amino Acid Levels and Bone Mineral Density: A Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 763538. [Google Scholar] [CrossRef]
- Panahi, N.; Fahimfar, N.; Roshani, S.; Arjmand, B.; Gharibzadeh, S.; Shafiee, G.; Migliavacca, E.; Breuille, D.; Feige, J.N.; Grzywinski, Y.; et al. Association of amino acid metabolites with osteoporosis, a metabolomic approach: Bushehr elderly health program. Metabolomics 2022, 18, 63. [Google Scholar] [CrossRef]
- Shen, L.; Hu, G.; Karner, C.M. Bioenergetic Metabolism In Osteoblast Differentiation. Curr. Osteoporos. Rep. 2022, 20, 53–64. [Google Scholar] [CrossRef]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 675385. [Google Scholar] [CrossRef]
- Bihuniak, J.D.; Insogna, K.L. The effects of dietary protein and amino acids on skeletal metabolism. Mol. Cell Endocrinol. 2015, 410, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, H.; Fox, T.; Eagles, J.; Satoh, H.; Nozawa, H.; Okiyama, A.; Morinaga, Y.; Fairweather-Tait, S.J. Acute effect of poly-gamma-glutamic acid on calcium absorption in post-menopausal women. J. Am. Coll. Nutr. 2007, 26, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Yoou, M.S.; Kim, H.M.; Jeong, H.J. Efficacy of proline in the treatment of menopause. Exp. Biol. Med. 2016, 241, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Khare, P.; Nagar, H.K.; Raghuwanshi, N.; Srivastava, R. Hydroxyproline: A Potential Biochemical Marker and Its Role in the Pathogenesis of Different Diseases. Curr. Protein Pept. Sci. 2016, 17, 596–602. [Google Scholar] [CrossRef]
- da Silva, R.A.; de Camargo Andrade, A.F.; da Silva Feltran, G.; Fernandes, C.; de Assis, R.I.F.; Ferreira, M.R.; Andia, D.C.; Zambuzzi, W.F. The role of triiodothyronine hormone and mechanically-stressed endothelial cell paracrine signalling synergism in gene reprogramming during hBMSC-stimulated osteogenic phenotype in vitro. Mol. Cell Endocrinol. 2018, 478, 151–167. [Google Scholar] [CrossRef]
- Yamada, M.; Tsukimura, N.; Ikeda, T.; Sugita, Y.; Att, W.; Kojima, N.; Kubo, K.; Ueno, T.; Sakurai, K.; Ogawa, T. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials 2013, 34, 6147–6156. [Google Scholar] [CrossRef]
- Brakspear, K.S.; Mason, D.J. Glutamate signaling in bone. Front. Endocrinol. 2012, 3, 97. [Google Scholar] [CrossRef]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pan, Y.; Li, X.; Wang, L.; Liu, M.; Tu, P.; Wu, C.; Xiao, J.; Han, Q.; Da, W.; et al. Quercetin Attenuates Osteoporosis in Orchiectomy Mice by Regulating Glucose and Lipid Metabolism via the GPRC6A/AMPK/mTOR Signaling Pathway. Front. Endocrinol. 2022, 13, 849544. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yu, X. Lipid metabolism disorders and bone dysfunction-interrelated and mutually regulated (review). Mol. Med. Rep. 2015, 12, 783–794. [Google Scholar] [CrossRef]
- During, A. Osteoporosis: A role for lipids. Biochimie 2020, 178, 49–55. [Google Scholar] [CrossRef]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feskanich, D.; Willett, W.C.; Eliassen, A.H.; Wu, T. Association between global biomarkers of oxidative stress and hip fracture in postmenopausal women: A prospective study. J. Bone Miner. Res. 2014, 29, 2577–2583. [Google Scholar] [CrossRef]
- Kwak, H.B.; Lee, S.W.; Li, Y.J.; Kim, Y.A.; Han, S.Y.; Jhon, G.J.; Kim, H.H.; Lee, Z.H. Inhibition of osteoclast differentiation and bone resorption by a novel lysophosphatidylcholine derivative, SCOH. Biochem. Pharmacol. 2004, 67, 1239–1248. [Google Scholar] [CrossRef]
- Jung, H.J.; Im, S.S.; Song, D.K.; Bae, J.H. Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells. BMB Rep. 2017, 50, 323–328. [Google Scholar] [CrossRef]
- Shi, Y. The investigation of energy metabolism in osteoblasts and osteoclasts. Hua Xi Kou Qiang Yi Xue Za Zhi 2021, 39, 501–509. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Li, Z.; Zhang, C.; Liu, P.; Yao, W.; Jia, T. Study on Neuroendocrine-Immune Function of Cistanche deserticola and Its Rice Wine Steaming Products in Glucocorti-coid-Induced Rat Model. Evid. Based Complement. Altern. Med. 2020, 2020, 5321976. [Google Scholar] [CrossRef]
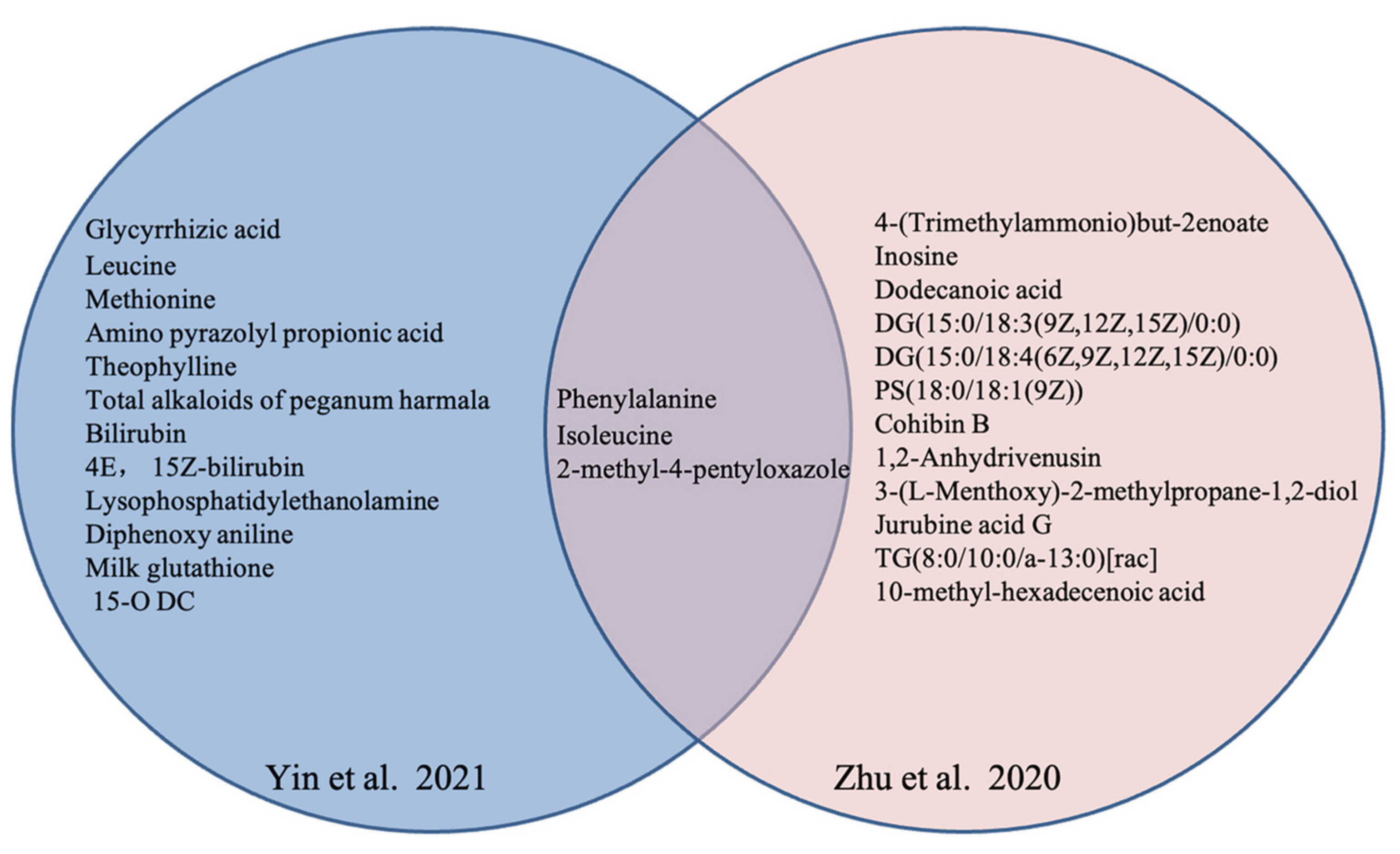

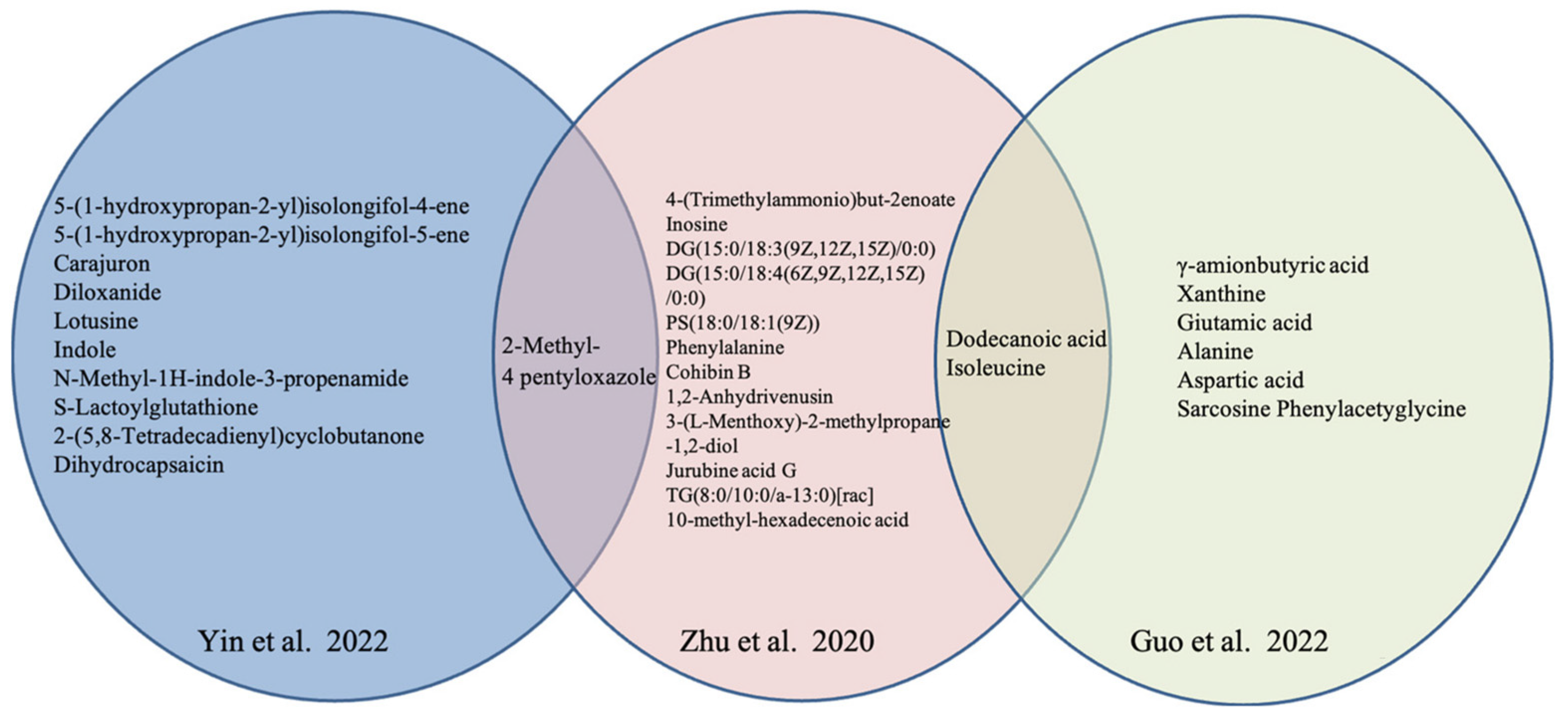
| References | Country | Study Design | No. of OP or ON/Control | Age of OP or ON/Control (years) | BMI of OP or ON/Control (m/kg2) | Technique | Biological Sample | Key Findings | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|
|
Yin et al., 2021 [48] | China | case-control | 30 OP vs. 30 control | 66.1 ± 7.5/64.3 ± 6.3 | 24.34 ± 2.99/24.11 ± 2.87 | UPLC/MS | blood | 15 different metabolites in OP with Yin deficiency syndrome: Glycocholic Acid, Bilirubin, Diloxanide, etc. | 7 | |
| Zhu 2020 [49] | China | case-control | 30 OP(A) vs. 30 OP(I) 30 control | 65.47 ± 7.54/66.1 ± 7.47 /55.97 ± 9.47 | 22.93 ± 3.42/24.34 ± 2.99 /24.11 ± 2.87 | UPLC/MS | serum | 15 different metabolites | 10 (↑) *: Inosine, Lucidenic acid G, etc. | 7 |
| 5 (↓) *: Dodecanoic acid, Cohibin B, etc. | ||||||||||
|
Li 2020 [50] | China | case-control | 120 OP vs. 18 control | 46–87 | 14.69–33.33 | HNMR | serum | 20 different metabolites: Glutamine, Leucine, etc. | 6 | |
|
Guo et al., 2022 [51] | China | case-control | 20 OP vs. 12 control | 62.7 ± 2.2/47.5 ± 5.4 | NA/NA * | UPLC/MS/MS | serum | 157 different metabolites | 93 (↑): L-isoleucine, γ-Aminobutyric acid, etc. 64 (↓): Alanine, Glutamate, etc. | 7 |
|
Yin et al., 2022 [38] | China | case-control | 30 OP vs. 30 control | 65.47 ± 7.54/55.97 ± 9.47 | 22.93 ± 3.42/24.11 ± 2.87 | UPLC/MS | serum | 11 potential metabolite biomarkers of KYADS: Indole, Lotusine, etc. | 6 | |
|
Poor et al., 2003 [39] | Hungary | case-control | 11 OP vs. 13 control | 53.8 ± 4.9/56.6 ± 5.7 | NA/NA | capillary gas chromatography | urine | 8 Urinary steroid different metabolites: Tetrahydro-corticosterone, 11-O-androsterone, etc. | 6 | |
|
Wang et al., 2019 [33] | China | case-control | Male: 40 OP vs. 46 ON vs. 46 control Female: 60 OP vs. 61 ON vs. 61 control | Male: 66.9 ± 2.9/67.2 ± 1.3/67.4 ± 1.4 Female: 60.7 ± 3.9/60.8 ± 4.0/60.1 ± 4.2 | Male: 23.3 ± 2.5/23.4 ± 2.5/23.4 ± 2.4 Female: 26.8 ± 3.5/26.7 ± 3.5/26.7 ± 3.5 | LC-MS/MS | blood | Male: 8 metabolites in males showed significant differences between the three groups Female: 12 metabolites showed significant differences between the three groups | 8 | |
|
Miyamoto et al., 2017 [40] | Japan | case-control | 5 OP vs. 42 control | 55.83 ± 3.6/56.34 ± 3.5 | 23.09 ± 1.8/22.25 ± 2.53 | LC/MS | serum | protein metabolism | (↓) Gly-Gly, cystine (↑) hydroxyproline | 6 |
|
Aleidi et al., 2021 [34] | Jordan | case-control | 25 OP vs. 22 ON vs. 22 control | 66.16 ± 1.78/64.64 ± 1.72 /54.82 ± 1.03 | 30.70 ± 1.4/30.38 ± 1.84 /32.21 ± 1.1 | UPLC/MS | serum | 94 dysregulated metabolites: | 52 (↑) 42 (↓) | 8 |
|
Deng et al., 2021 [41] | China | case-control | 32 OP vs. 32 control | 60.47 ± 12.39/60.59 ± 14.14 | NA/NA | UHPLC-HRMS | serum | The differential metabolites | (↑) PE, TG(18:0/18:0/18:0), cyclic Melatonin, etc. (↓): LPC, 4-Hydroxyproline, etc. | 9 |
|
Cao et al., 2021 [42] | China | case-control | 36 OP vs. 55 control | 57.51 ± 4.59 | NA/NA | LC-MS | blood | 10 different lipid metabolites: | 6 (↑): PC (18:0/20:4), TG (16:0/10:0/20:4), CL (19:0/18:2/20:0/22:6), CL (75:4), PC (36:5), Tand G (54:4) 4 (↓): PC (36:2), CL (22:3/18:0/18:0/20:4), LPC (18:1), SM (d16:0/18:1) | 7 |
|
Kou et al., 2022 [43] | China | case-control | 50 OP vs. 50 control | 69.3 ± 9.3/66.3 ± 10 | 23.8 ± 3.2/23.5 ± 4.4 | GC/LC-MS | serum | 18 different metabolites | 8 | |
|
Pontes et al., 2019 [35] | Brazil | case-control | 24 OP vs. 26 ON vs. 28 control | 60. 8 ± 6.0/61.88 ± 7.9/ 60.38 ± 6.2 | 25.58 ± 4.8/27.20 ± 5.2/ 25.35 ± 3.4 | H NMR | serum | 9 different metabolites OP | 6 (↑): Cholesterol, Leucine, isoleucine, Lactate, Unsaturated lipids, Allantoin 3 (↓): Tyrosine, Choline, Taurine | 7 |
|
Zhang et al., 2022 [44] | China | case-control | 120 OP vs. 80 control | 71/70 | NA/NA | LC-MS/MS | serum | (↑) NEOs and their metabolites | 7 | |
|
LIM et al., 1997 [32] | Korea | case-control | 34 ON vs. 25 control | 56.8 ± 0.4/57.2 ± 0.4 | 23.15 ± 0.36/24.38 ± 0.36 | GC-MS | urinary | 18 estrogen metabolites: | 7 | |
|
Qi et al., 2016 [36] | China | case-control | 67 OP vs. 114 ON vs. 79 control | 58.37 ± 4.78/57.03 ± 4.53/ 54.43 ± 4.9 | 23.52 ± 3.39/23.56 ± 3.05/ 24.75 ± 3.21 | GC-MS | serum | 12 different metabolites between low BMD and control 5 free fatty acids (LA, Oleic acid, AA and 11, 14-Eicosadienoic acid) correlations with BMD | 8 | |
|
Zhao et al., 2018 [45] | USA | case-control | 65 OP vs. 71 control | 31.2 ± 4.9/31.8 ± 55.3 | 21.9 ± 2.5/29.7 ± 8.6 | LC-MS | serum | 14 metabolites, 7 amino acids and amino acid derivatives, 5 lipids (including three bile acids), and 2 organic acids were significantly associated with the risk for low BMD | 7 | |
|
Yu et al., 2018 [37] | China | case-control | 77 OP vs. 92 ON vs. 71 control | 57.97 ± 4.07/56.72 ± 4.79/ 54.71 ± 4.81 | 23.12 ± 3.08/23.01 ± 2.98/ 24.73 ± 3.14 | GC–MS | Urine | 17 different metabolites | 8 | |
|
You et al., 2014 [46] | China | cross-sectional study | Premenopausal: 134 OP vs. 349 control Postmenopausal: 77 OP vs. 41 control | Premenopausal: 44.7 ± 0.29/44.9 ± 0.19 Postmenopausal: 52.5 ± 0.29/50.7 ± 0.47 | Premenopausal: 21.2 ± 0.27/22.5 ± 0.17 Postmenopausal: 21.8 ± 0.56/24.3 ± 0.60 | GC–MS | blood | 7 different metabolites | 2 (↑): Acetate, Glutamine 5 (↓): Lactate, Acetone, Lipids, VLDLs, Glucose | 9 |
|
Mei et al., 2020 [27] | China | case-control | Discovery set: 83 OP vs. 205 ON vs. 413 control Replication set: 107 OP vs. 68 ON vs. 103 control | Discovery set: 63.0 ± 9.1/59.0 ± 10.8/ 52.9 ± 12 Replication set: 70.3 ± 9.5/66.5 ± 13.9/ 62.6 ± 12.7 | Discovery set: 22.8 ± 2.9/24.2 ± 3.3/ 24.7 ± 3.2 Replication set: 22.4 ± 3.7/23.2 ± 3.2/ 24.3 ± 3.7 | LC-MS | blood | 47 different metabolites (13 amino acids, 2 carboxylic acids, 14 glycerophospholipids, 3 purines and purine derivatives, 7 sphingolipids, and 8 others) | 9 | |
|
Miyamoto et al., 2018 [47] | Japan | case-control | 33 OP vs. 46 control | 39–61 | NA/NA | LC/MS | serum | 24 different metabolites | 8 | |
| Category | Metabolites | Variation Trend | Reference |
|---|---|---|---|
| Amino Acids | glutamine | ↓ * | Wang et al. [33] |
| ↑ * | Zhao et al. [45] | ||
| ↑ | You et al. [46] | ||
| ↑ | Miyamoto et al. [40,47] | ||
| hydroxyproline | ↑ | Wang et al. [33] | |
| ↑ | Miyamoto et al. (2017) [40] | ||
| ↓ | Deng et al. [41] | ||
| ↑ | Miyamoto et al. (2018) [47] | ||
| gly-gly | ↓ | Miyamoto et al. (2017) [40] | |
| ↓ | Kou et al. [43] | ||
| ↓ | Miyamoto et al. (2018) [47] | ||
| cystine | ↓ | Miyamoto et al. (2017) [40] | |
| ↓ | Zhao et al. [45] | ||
| ↓ | Miyamoto et al. (2018) [47] | ||
| taurine | ↓ | Pontes et al. [35] | |
| ↑ | Zhao et al. [45] | ||
| ↓ | Yu et al. [37] | ||
| Lipid Metabolites | PC | ↑ | Aleidi et al. [34] |
| ↑ | Cao et al. [42] | ||
| ↑ | Kou et al. [43] | ||
| LPC | ↑ | Wang et al. [33] | |
| ↓ | Deng et al. [41] | ||
| ↓ | Cao et al. [42] | ||
| ↑ | Kou et al. [43] | ||
| ↑ | Miyamoto et al. (2018) [47] | ||
| SM | ↓ | Cao et al. [42] | |
| ↓ | Kou et al. [43] | ||
| Carbohydrate Metabolites | glucose | ↓ | Kou et al. [43] |
| ↓ | You et al. [46] | ||
| Other Metabolites | lactate | ↑ | Kou et al. [43] |
| ↑ | Pontes et al. [35] | ||
| ↓ | You et al. [46] | ||
| succinic | ↑ | Deng et al. [41] | |
| ↑ | Zhao et al. [45] | ||
| ↑ | Yu et al. [37] |
| Study | Pathways | Analysis Methods |
|---|---|---|
| Yin et al., 2021 [48] | Bile secretion | Enrichment analysis of KEGG signaling pathway |
| Secondary bile acid biosynthesis | ||
| Cholesterol metabolism | ||
| Caffeine metabolism | ||
| Pyruvate metabolism | ||
| Primary bile acid biosynthesis | ||
| Li et al., 2020 [50] | Valine, leucine, and isoleucine biosynthesis and degradation | Enrichment analysis and topology analysis |
| Aminoacyl-tRNA biosynthesis | ||
| Glycolysis or Gluconeogenesis | ||
| Glycerophospholipid metabolism | ||
| Glyoxylate and dicarboxylate metabolism | ||
| TCA cycle | ||
| Taurine and hypotaurine metabolism | ||
| Guo et al., 2022 [51] | Tryptophan metabolism | Enrichment analysis of KEGG signaling pathway |
| Glutathione metabolism | ||
| Phospholipase D signaling pathway | ||
| Arginine, proline with alanine metabolism | ||
| Aleidi et al. 2021 [34] | Histidine metabolism | The pathway analysis module |
| Aminoacyl-tRNA biosynthesis | ||
| Glyoxylate and dicarboxylate metabolism | ||
| Biosynthesis of unsaturated fatty acids | ||
| Deng et al., 2021 [41] | Lipids pathways | NA |
| Cao et al., 2021 [42] | Choline metabolism | The bubble diagram of pathway enrichment analysis |
| Glycerophospholipid metabolism | ||
| Retrograde endocannabinoid signaling | ||
| Linoleic acid metabolism | ||
| Alpha-linolenic acid metabolism | ||
| Arachidonic acid metabolism | ||
| Kou et al., 2022 [43] | Glucose metabolism | Database searching (KEGG) and consulting relevant literature |
| Amino acids metabolism | ||
| Choline metabolism | ||
| Inflammatory response | ||
| Zhao et al., 2018 [45] | Alanine, aspartate, and glutamate metabolism | MetaboAnalyst 3.0 software |
| Butanoate metabolism | ||
| Taurine and hypotaurine metabolism | ||
| Aminoacyl-tRNA biosynthesis | ||
| Glutathione metabolism | ||
| Primary bile acid biosynthesis | ||
| Glycine, serine, and threonine metabolism | ||
| Yu et al., 2018 [37] | Taurine metabolism | MetaboAnalyst 3.0 software |
| β-alanine metabolism | ||
| Galactose metabolism | ||
| TCA cycle | ||
| Proparoate metabolism | ||
| Nitrogen metabolism | ||
| Butanoate metabolism | ||
| Miyamoto et al., 2018 [47] | TCA cycle | NA |
| Urea cycle | ||
| Pentose phosphate pathway |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Han, X.; Shi, J.; Liao, Z.; Zhang, Y.; Li, Y.; Jiang, M.; Liu, M. Distinct Metabolites in Osteopenia and Osteoporosis: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4895. https://doi.org/10.3390/nu15234895
Wang Y, Han X, Shi J, Liao Z, Zhang Y, Li Y, Jiang M, Liu M. Distinct Metabolites in Osteopenia and Osteoporosis: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(23):4895. https://doi.org/10.3390/nu15234895
Chicago/Turabian StyleWang, Yuhe, Xu Han, Jingru Shi, Zeqi Liao, Yuanyue Zhang, Yuanyuan Li, Miao Jiang, and Meijie Liu. 2023. "Distinct Metabolites in Osteopenia and Osteoporosis: A Systematic Review and Meta-Analysis" Nutrients 15, no. 23: 4895. https://doi.org/10.3390/nu15234895
APA StyleWang, Y., Han, X., Shi, J., Liao, Z., Zhang, Y., Li, Y., Jiang, M., & Liu, M. (2023). Distinct Metabolites in Osteopenia and Osteoporosis: A Systematic Review and Meta-Analysis. Nutrients, 15(23), 4895. https://doi.org/10.3390/nu15234895