A Review of the Nutritional Approach and the Role of Dietary Components in Children with Autism Spectrum Disorders in Light of the Latest Scientific Research
Abstract
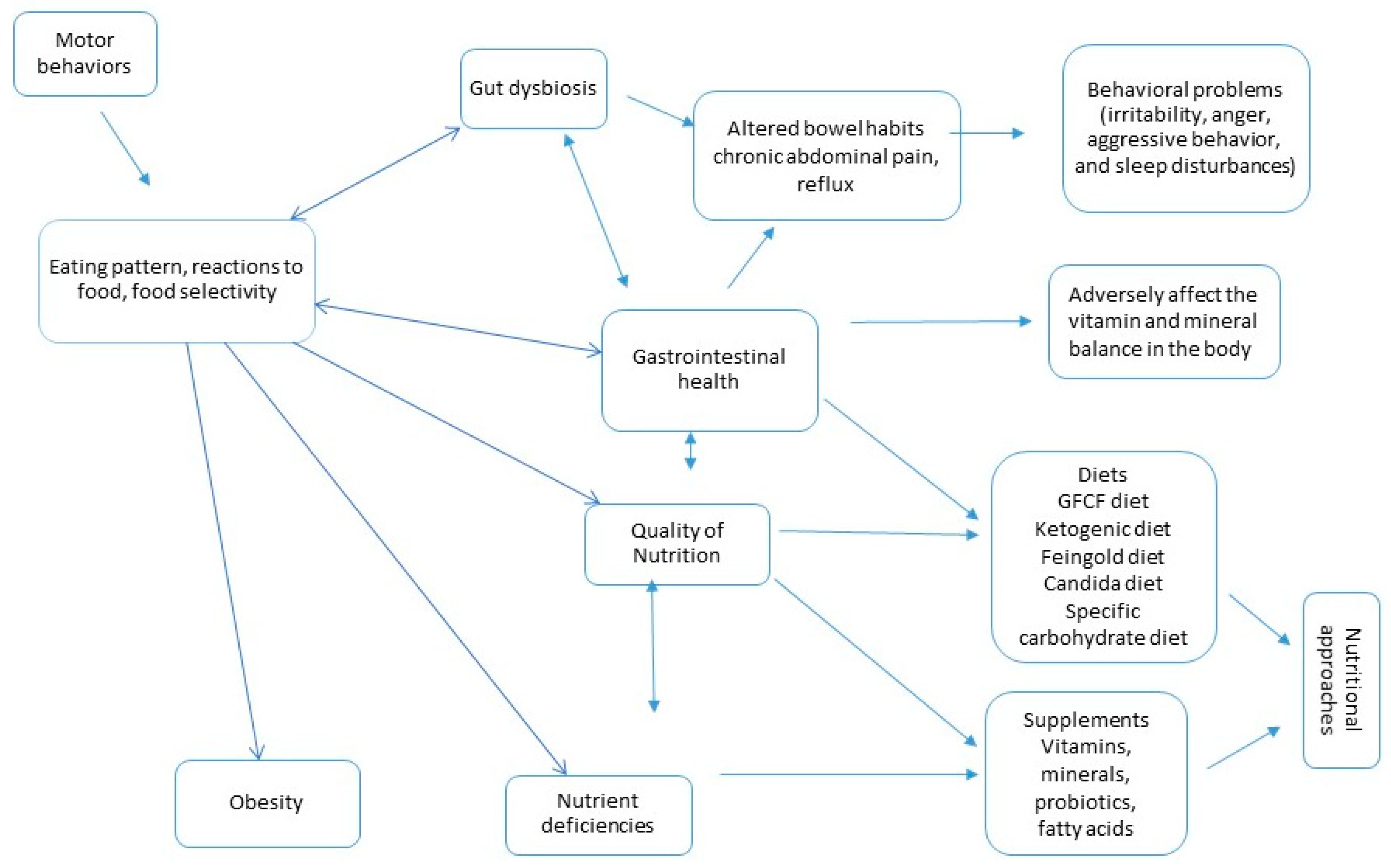
1. Introduction
2. Methods
3. Selective Eating as a Nutritional Problem
4. Nutritional Approaches
4.1. Diets
4.1.1. Gluten-Free and Casein-Free Diets
4.1.2. Ketogenic Diet
4.1.3. Feingold Diet
4.1.4. Candida Diet
4.1.5. Specific Carbohydrate Diet
4.2. Supplements
4.2.1. Vitamins and Minerals
4.2.2. Omega-3 Fatty Acids
4.2.3. Probiotics and Prebiotics
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almandil, N.B.; Alkuroud, D.N.; AbdulAzeez, S.; AlSulaiman, A.; Elaissari, A.; Borgio, J.F. Environmental and Genetic Factors in Autism Spectrum Disorders: Special Emphasis on Data from Arabian Studies. Int. J. Environ. Res. Public Health 2019, 16, 658. [Google Scholar] [CrossRef] [PubMed]
- Rylaarsdam, L.E.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef]
- CDC. What Is Autism Spectrum Disorder? CDC. 2023. Available online: https://www.cdc.gov/ncbddd/autism/facts.html (accessed on 25 August 2023).
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef] [PubMed]
- Mandecka, A.; Regulska-Ilow, B. The importance of nutritional management and education in the treatment of autism. Rocz. Państwowego Zakładu Hig. 2022, 73, 247–258. [Google Scholar] [CrossRef]
- Karhu, E.; Zukerman, R.; Eshraghi, R.S.; Mittal, J.; Deth, R.C.; Castejon, A.M.; Trivedi, M.; Mittal, R.; Eshraghi, A.A. Nutritional interventions for autism spectrum disorder. Nutr. Rev. 2019, 78, 515–531. [Google Scholar] [CrossRef]
- Cimmino, F.; Catapano, A.; Trinchese, G.; Cavaliere, G.; Culurciello, R.; Fogliano, C.; Penna, E.; Lucci, V.; Crispino, M.; Avallone, B.; et al. Dietary Micronutrient Management to Treat Mitochondrial Dysfunction in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2021, 22, 2862. [Google Scholar] [CrossRef] [PubMed]
- Leader, G.; Abberton, C.; Cunningham, S.; Gilmartin, K.; Grudzien, M.; Higgins, E.; Joshi, L.; Whelan, S.; Mannion, A. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Systematic Review. Nutrients 2022, 14, 1471. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, B.; Aishwarya, S.; Jancy, S.B.; Abhishek, G.S.; Babu, H.W.S.; Vijayakumar, P.; Narayanasamy, A.; Mariappan, S.; Sangeetha, R.; Gopalakrishnan, A.V.; et al. An anxious relationship between Autism Spectrum Disorder and Gut Microbiota: A tangled chemistry? J. Clin. Neurosci. 2022, 99, 169–189. [Google Scholar] [CrossRef]
- Krigsman, A.; Walker, S.J. Gastrointestinal disease in children with autism spectrum disorders: Etiology or consequence? World J. Psychiatry 2021, 11, 605–618. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Arora, G.; Sahni, G.; Kaur, M.; Singh, H.; Singh, B.; Kaur, S. Gut microbiota and Autism Spectrum Disorder: From pathogenesis to potential therapeutic perspectives. J. Tradit. Complement. Med. 2022, 13, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Hartman, R.E.; Patel, D. Dietary Approaches to the Management of Autism Spectrum Disorders. Adv. Neurobiol. 2020, 24, 547–571. [Google Scholar] [CrossRef]
- Isla Torres, F.C.; Guerrero Medina, A.C.D.; Gutiérrez Toribio, S.L.; Julián Guevara, K.K.; León Risco, K.B.; Huamán Saavedra, J.J. Therapeutic dietary approach to children with autistic spectrum disorder. J. Fac. Med. Humana 2022, 22, 865–877. [Google Scholar] [CrossRef]
- Van De Sande, M.M.H.; Van Buul, V.J.; Brouns, F.J.P.H. Autism and nutrition: The role of the gut–brain axis. Nutr. Res. Rev. 2014, 27, 199–214. [Google Scholar] [CrossRef]
- Bryant-Waugh, R.; Markham, L.; Kreipe, R.E.; Walsh, B.T. Feeding and eating disorders in childhood. Int. J. Eat. Disord. 2010, 43, 98–111. [Google Scholar] [CrossRef]
- Zucker, N.; Copeland, W.; Franz, L.; Carpenter, K.; Keeling, L.; Angold, A.; Egger, H. Psychological and Psychosocial Impairment in Preschoolers with Selective Eating. Pediatrics 2015, 136, e582–e590. [Google Scholar] [CrossRef]
- Ledford, J.R.; Gast, D.L. Feeding problems in children with autism spectrum disorders: A Review. Focus Autism Other Dev. Disabil. 2006, 21, 153–166. [Google Scholar] [CrossRef]
- Johnson, C.R.; Turner, K.; Stewart, P.A.; Schmidt, B.; Shui, A.; Macklin, E.; Reynolds, A.; James, J.; Johnson, S.L.; Courtney, P.M.; et al. Relationships Between Feeding Problems, Behavioral Characteristics and Nutritional Quality in Children with ASD. J. Autism Dev. Disord. 2014, 44, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Dowell, L.R.; Mahone, E.M.; Mostofsky, S.H. Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology 2009, 23, 563–570. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, A.-M.; Ragozzino, M.E.; Mosconi, M.W.; Shrestha, S.; Cook, E.H.; Sweeney, J.A. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology 2013, 27, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ung, D.; Selles, R.; Small, B.J.; Storch, E.A. A Systematic Review and Meta-Analysis of Cognitive-Behavioral Therapy for Anxiety in Youth with High-Functioning Autism Spectrum Disorders. Child Psychiatry Hum. Dev. 2014, 46, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Fujii, Y.; Hijikata, N. Support and development of autistic children with selective eating habits. Brain Dev. 2020, 42, 121–128. [Google Scholar] [CrossRef]
- Amin, N.; Tariq, M.; Arshad, M.; Cheema, M.A. Frequency of visual impairment in autistic children of autism school of Lahore, Pakistan. Br. J. Vis. Impair. 2023. [Google Scholar] [CrossRef]
- Leila Cherif, J.B.; Khemekhem, K.; Mkawer, S.; Ayadi, H.; Moalla, Y. Feeding Problems in Children with Autism Spectrum Disorders. J. Fam. Med. 2020, 1, 30–39. [Google Scholar] [CrossRef]
- Sharp, W.G.; Postorino, V.; McCracken, C.E.; Berry, R.C.; Criado, K.K.; Burrell, T.L.; Scahill, L. Dietary Intake, Nutrient Status, and Growth Parameters in Children with Autism Spectrum Disorder and Severe Food Selectivity: An Electronic Medical Record Review. J. Acad. Nutr. Diet. 2018, 118, 1943–1950. [Google Scholar] [CrossRef]
- Malhi, P.; Venkatesh, L.; Bharti, B.; Singhi, P. Feeding Problems and Nutrient Intake in Children with and without Autism: A Comparative Study. Indian J. Pediatr. 2017, 84, 283–288. [Google Scholar] [CrossRef]
- Postorino, V.; Sanges, V.; Giovagnoli, G.; Fatta, L.M.; De Peppo, L.; Armando, M.; Vicari, S.; Mazzone, L. Clinical differences in children with autism spectrum disorder with and without food selectivity. Appetite 2015, 92, 126–132. [Google Scholar] [CrossRef]
- Allen, S.L.; Smith, I.M.; Duku, E.; Vaillancourt, T.; Szatmari, P.; Bryson, S.; Fombonne, E.; Volden, J.; Waddell, C.; Zwaigenbaum, L.; et al. Behavioral Pediatrics Feeding Assessment Scale in Young Children with Autism Spectrum Disorder: Psychometrics and Associations with Child and Parent Variables. J. Pediatr. Psychol. 2015, 40, 581–590. [Google Scholar] [CrossRef]
- Zachor, D.A.; Ben-Itzchak, E. Specific Medical Conditions Are Associated with Unique Behavioral Profiles in Autism Spectrum Disorders. Front. Neurosci. 2016, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, M.; Santocchi, E.; Balboni, G.; Narzisi, A.; Bozza, M.; Fulceri, F.; Apicella, F.; Igliozzi, R.; Cosenza, A.; Tancredi, R.; et al. Behavioral Phenotype of ASD Preschoolers with Gastrointestinal Symptoms or Food Selectivity. J. Autism Dev. Disord. 2017, 47, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Saban-Bezalel, R.; Ben-Itzchak, E.; Stolar, O. Brief Report—Selective eating: Parental and day care professional perception of ASD symptom severity in toddlers and children over time. Res. Autism Spectr. Disord. 2021, 87, 101830. [Google Scholar] [CrossRef]
- Bourne, L.; Mandy, W.; Bryant-Waugh, R. Avoidant/restrictive food intake disorder and severe food selectivity in children and young people with autism: A scoping review. Dev. Med. Child Neurol. 2022, 64, 691–700. [Google Scholar] [CrossRef]
- Latif, A.; Heinz, P.; Cook, R. Iron Deficiency in Autism and Asperger Syndrome. Autism 2002, 6, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M.H.; Hart, L.C.; Manning-Courtney, P.; Murray, D.S.; Bing, N.M.; Summer, S. Food Variety as a Predictor of Nutritional Status Among Children with Autism. J. Autism Dev. Disord. 2011, 42, 549–556. [Google Scholar] [CrossRef]
- Pivina, L.; Semenova, Y.; Doşa, M.D.; Dauletyarova, M.; Bjørklund, G. Iron Deficiency, Cognitive Functions, and Neurobehavioral Disorders in Children. J. Mol. Neurosci. 2019, 68, 1–10. [Google Scholar] [CrossRef]
- Bener, A.; Khattab, A.O.; Bhugra, D.; Hoffmann, G.F. Iron and Vitamin D Levels among Autism Spectrum Disorders Children. Ann. Afr. Med. 2017, 16, 186–191. [Google Scholar] [CrossRef]
- Barnhill, K.; Gutierrez, A.; Ghossainy, M.; Marediya, Z.; Devlin, M.; Sachdev, P.; Marti, C.N.; Hewitson, L. Dietary status and nutrient intake of children with autism spectrum disorder: A case-control study. Res. Autism Spectr. Disord. 2018, 50, 51–59. [Google Scholar] [CrossRef]
- Siddiqi, S.; Urooj, A.; D’souza, M.J. Dietary Patterns and Anthropometric Measures of Indian Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 49, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, M.; Yang, T.; Lai, X.; Tang, T.; Chen, J.; Li, L.; Li, T. Nutritional Status and Symptoms in Preschool Children with Autism Spectrum Disorder: A Two-Center Comparative Study in Chongqing and Hainan Province, China. Front. Pediatr. 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Abushaikha, A.; Alnaser, K.; Obeidat, M.D.; Al-Shami, I. Nutritional Status of Pre-school Children and Determinant Factors of Autism: A Case-Control Study. Front. Nutr. 2021, 8, 627011. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Angley, M.T.; Gerber, J.P.; Sorich, M.J. A review of candidate urinary biomarkers for autism spectrum disorder. Biomarkers 2011, 16, 537–552. [Google Scholar] [CrossRef]
- Reichelt, K.; Knivsberg, A.M. Can the Pathophysiology of Autism be Explained by the Nature of the Discovered Urine Peptides? Nutr. Neurosci. 2003, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tveiten, D.; Finvold, A.; Andersson, M.; Reichelt, K.L. Peptides and Exorphins in the Autism Spectrum. Open J. Psychiatry 2014, 4, 275–287. [Google Scholar] [CrossRef][Green Version]
- Doenyas, C. Dietary interventions for autism spectrum disorder: New perspectives from the gut-brain axis. Physiol. Behav. 2018, 194, 577–582. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Baspinar, B.; Yardimci, H. Gluten-Free Casein-Free Diet for Autism Spectrum Disorders: Can It Be Effective in Solving Behavioural and Gastrointestinal Problems? Eurasian J. Med. 2020, 52, 292–297. [Google Scholar] [CrossRef]
- Reissmann, A. Gluten-free and casein-free diets in the management of autism spectrum disorder: A systematic literature review. Mov. Nutr. Health Dis. 2020, 4, 21–38. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.L.; Mitchell, J.; Ingram, J.; Hellmers, R.; Laake, D.; et al. Comprehensive Nutritional and Dietary Intervention for Autism Spectrum Disorder—A Randomized, Controlled 12-Month Trial. Nutrients 2018, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Hafid, A.; Ahami, A.O.T. The Efficacy of the Gluten-Free Casein-Free Diet for Moroccan Autistic Children. Curr. Res. Nutr. Food Sci. J. 2018, 6, 734–741. [Google Scholar] [CrossRef]
- Lee, R.W.Y.; Corley, M.J.; Pang, A.; Arakaki, G.; Abbott, L.; Nishimoto, M.; Miyamoto, R.; Lee, E.; Yamamoto, S.; Maunakea, A.K.; et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol. Behav. 2018, 188, 205–211. [Google Scholar] [CrossRef] [PubMed]
- González-Domenech, P.J.; Díaz Atienza, F.; García Pablos, C.; Serrano Nieto, S.; Herreros Rodríguez, Ó.; Gutiérrez-Rojas, L.; Martínez-Ortega, J.M. Influence of a Gluten-free, Casein-free Diet on Behavioral Disturbances in Children and Adolescents Diagnosed with Autism Spectrum Disorder: A 3-month Follow-up Pilot Study. J. Ment. Health Res. Intellect. Disabil. 2019, 12, 256–272. [Google Scholar] [CrossRef]
- González-Domenech, P.J.; Díaz Atienza, F.; García Pablos, C.; Fernández Soto, M.L.; Martínez-Ortega, J.M.; Gutiérrez-Rojas, L. Influence of a Combined Gluten-Free and Casein-Free Diet on Behavior Disorders in Children and Adolescents Diagnosed with Autism Spectrum Disorder: A 12-Month Follow-Up Clinical Trial. J. Autism Dev. Disord. 2020, 50, 935–948. [Google Scholar] [CrossRef]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-Free Diet in Children with Autism Spectrum Disorders: A Randomized, Controlled, Single-Blinded Trial. J. Autism Dev. Disord. 2020, 50, 482–490. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Herz, J. High-Fat Diet Changes Hippocampal Apolipoprotein E (ApoE) in a Genotype- and Carbohydrate-Dependent Manner in Mice. PLoS ONE 2016, 11, e0148099. [Google Scholar] [CrossRef]
- Gogou, M.; Kolios, G. Are therapeutic diets an emerging additional choice in autism spectrum disorder management? World J. Pediatr. 2018, 14, 215–223. [Google Scholar] [CrossRef]
- Verrotti, A.; Iapadre, G.; Pisano, S.; Coppola, G. Ketogenic diet and childhood neurological disorders other than epilepsy: An overview. Expert Rev. Neurother. 2016, 17, 461–473. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, Y.; Tomi, M.; Shin, B.-C.; Thamotharan, S.; Mazarati, A.; Sankar, R.; Wang, E.A.; Cepeda, C.; Levine, M.S.; et al. Sex-Specific Life Course Changes in the Neuro-Metabolic Phenotype of Glut3 Null Heterozygous Mice: Ketogenic Diet Ameliorates Electroencephalographic Seizures and Improves Sociability. Endocrinology 2017, 158, 936–949. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef] [PubMed]
- Verpeut, J.L.; DiCicco-Bloom, E.; Bello, N.T. Ketogenic diet exposure during the juvenile period increases social behaviors and forebrain neural activation in adult Engrailed 2 null mice. Physiol. Behav. 2016, 161, 90–98. [Google Scholar] [CrossRef]
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: A case-control study. Metab. Brain Dis. 2017, 32, 1935–1941. [Google Scholar] [CrossRef]
- Spilioti, M.; Evangeliou, A.E.; Tramma, D.; Theodoridou, Z.; Metaxas, S.; Michailidi, E.; Bonti, E.; Frysira, H.; Haidopoulou, A.; Asprangathou, D.; et al. Evidence for treatable inborn errors of metabolism in a cohort of 187 Greek patients with autism spectrum disorder (ASD). Front. Hum. Neurosci. 2013, 7, 858. [Google Scholar] [CrossRef] [PubMed]
- Żarnowska, I.; Chrapko, B.; Gwizda, G.; Nocuń, A.; Mitosek-Szewczyk, K.; Gasior, M. Therapeutic use of carbohydrate-restricted diets in an autistic child; A case report of clinical and 18FDG PET findings. Metab. Brain Dis. 2018, 33, 1187–1192. [Google Scholar] [CrossRef]
- Napoli, E.; Dueñas, N.; Giulivi, C. Potential Therapeutic Use of the Ketogenic Diet in Autism Spectrum Disorders. Front. Pediatr. 2014, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.; Kraja, G.; Eller, D.; Eck, K.; McBrian, D.; Bain, J.M. Assessing the feasibility of using the ketogenic diet in autism spectrum disorder. J. Hum. Nutr. Diet. 2023, 36, 1303–1315. [Google Scholar] [CrossRef]
- Li, Q.; Liang, J.; Fu, N.; Han, Y.; Qin, J. A Ketogenic Diet and the Treatment of Autism Spectrum Disorder. Front. Pediatr. 2021, 9, 650624. [Google Scholar] [CrossRef]
- Mayes, S.D.; Zickgraf, H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Res. Autism Spectr. Disord. 2019, 64, 76–83. [Google Scholar] [CrossRef]
- Balasco, L.; Provenzano, G.; Bozzi, Y. Sensory Abnormalities in Autism Spectrum Disorders: A Focus on the Tactile Domain, From Genetic Mouse Models to the Clinic. Front. Psychiatry 2020, 10, 1016. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Spulber, G.; Spulber, S.; Hagenäs, L.; Åmark, P.; Dahlin, M. Growth dependence on insulin-like growth factor-1 during the ketogenic diet. Epilepsia 2009, 50, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Newmaster, K.; Zhu, Z.; Bolt, E.; Chang, R.J.; Day, C.; Mhanna, A.; Paudel, S.; Farooq, O.; Swaminathan, A.; Acharya, P.; et al. A Review of the Multi-Systemic Complications of a Ketogenic Diet in Children and Infants with Epilepsy. Children 2022, 9, 1372. [Google Scholar] [CrossRef]
- Feingold, B. Why Your Child Is Hyperactive; Random House: New York, NY, USA, 1985. [Google Scholar]
- Neggers, Y.H. Dietary Interventions in Autism. In Autism Spectrum Disorders; Williams, T., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Lipton, M.A.; Mayo, J.P. Diet and hyperkinesis—An update. J. Am. Diet. Assoc. 1983, 83, 132–134. [Google Scholar]
- Abdallah, H.; Soliman, S.; El-kader, A. Language Training Program Coupled with Feingold Diet for Caregivers to Develop Autisticreceptive Language Skills. Mansoura Nurs. J. 2019, 6, 107–125. [Google Scholar] [CrossRef]
- Mattes, J.A. The Feingold diet: A current reappraisal. J. Learn. Disabil. 1983, 16, 319–323. [Google Scholar] [CrossRef]
- Wender, E.H. The food additive-free diet in the treatment of behavior disorders: A review. J. Dev. Pediatr. Behav. Pediatr. 1986, 7, 35–42. [Google Scholar] [CrossRef][Green Version]
- Williams, J.I.; Cram, D.M. Diet in the management of hyperkinesis: A review of the tests of the Feingold hypotheses. Can. Psychiatr. Assoc. J. 1978, 23, 241–248. [Google Scholar] [CrossRef]
- Kavale, K.A.; Forness, S.R. Hyperactivity and diet treatment: A meta-analysis of the Feingold hypothesis. J. Learn. Disabil. 1983, 16, 324–330. [Google Scholar] [CrossRef]
- Schab, D.W.; Trinh, N.T. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. Dev. Behav. Pediatr. 2004, 25, 423–434. [Google Scholar] [CrossRef]
- Pagan, C.; Benabou, M.; Leblond, C.; Cliquet, F.; Mathieu, A.; Lemière, N.; Goubran-Botros, H.; Delorme, R.; Leboyer, M.; Callebert, J.; et al. Decreased phenol sulfotransferase activities associated with hyperserotonemia in autism spectrum disorders. Transl. Psychiatry 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Doreswamy, S.; Bashir, A.; Guarecuco, J.E.; Lahori, S.; Baig, A.; Narra, L.R.; Patel, P.; Heindl, S.E. Effects of diet, nutrition, and exercise in children with autism and autism spectrum disorder: A literature review. Cureus 2020, 12, 10. [Google Scholar] [CrossRef]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 2016, 182, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Emam, A.M.; Mamdouh, E.; Abdelrahim, S. Candida albicans infection in autism. J. Am. Sci. 2012, 8, 739–744. [Google Scholar]
- Patel, J.; Kang, D.W.; Adams, J.; Krajmalnik-Brown, R. Analysis of Yeast and Fungi in Children with ASD vs. Neurotypical Controls 2018. Undergraduate Thesis, Honors College at Arizona State University, Tempe, AZ, USA, 2018. [Google Scholar]
- Jendraszak, M.; Gałęcka, M.; Kotwicka, M.; Regdos, A.; Pazgrat-Patan, M.; Andrusiewicz, M. Could selected gut microorganisms be diagnostic biomarkers for autism spectrum disorders? Study based on a commercial microbiota test. Res. Sq. 2021, 1–19. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism—Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Abd El Halim, R.; Amer, M.; Salem, G.; Abdelhamid, D.; El Hossiny, R. Quantitative Stool Culture of Candida in Egyptian Children with Autism Spectrum Disorder. Egypt. J. Hosp. Med. 2023, 90, 1592–1596. [Google Scholar] [CrossRef]
- Lasheras, I.; Seral, P.; Latorre, E.; Barroso, E.; Gracia-García, P.; Santabárbara, J. Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian J. Psychiatry 2019, 47, 101874. [Google Scholar] [CrossRef]
- Kantarcioglu, A.S.; Kiraz, N.; Aydin, A. Microbiota–Gut–Brain Axis: Yeast Species Isolated from Stool Samples of Children with Suspected or Diagnosed Autism Spectrum Disorders and In Vitro Susceptibility Against Nystatin and Fluconazole. Mycopathologia 2015, 181, 1–7. [Google Scholar] [CrossRef]
- Crook, W.G. Yeast can Affect Behavior and Learning. Acad. Ther. 1984, 19, 517–526. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Curtin, C.; Phillips, S.; Anderson, S.E.; Maslin, M.; Must, A. Changes in Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 439–446. [Google Scholar] [CrossRef]
- Vissoker, R.E.; Latzer, Y.; Gal, E. Eating and feeding problems and gastrointestinal dysfunction in Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2015, 12, 10–21. [Google Scholar] [CrossRef]
- Gutierrez, D.; Weinstock, A.; Antharam, V.C.; Gu, H.; Jasbi, P.; Shi, X.; Dirks, B.; Krajmalnik-Brown, R.; Maldonado, J.; Guinan, J.; et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol. Ecol. 2019, 96, 187. [Google Scholar] [CrossRef]
- Lam, S.; Zuo, T.; Ho, M.; Chan, F.K.L.; Chan, P.K.S.; Ng, S.C. Review article: Fungal alterations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2019, 50, 1159–1171. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE 2013, 8, e66019. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? J. Clin. Med. 2022, 11, 442. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 2019, 9, 43. [Google Scholar] [CrossRef]
- MacAlpine, J.; Daniel-Ivad, M.; Liu, Z.; Yano, J.; Revie, N.M.; Todd, R.T.; Stogios, P.J.; Sanchez, H.; O’meara, T.R.; Tompkins, T.A.; et al. A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 2021, 12, 6151. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, J.J.; Klukowski, M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks—A possible new overlap syndrome. Pediatr. Health Med. Ther. 2015, 6, 153–166. [Google Scholar] [CrossRef]
- Melmed, R.; Schneider, C.; Fabes, R.; Phillips, J.; Reichelt, T.K. Metabolic markers and gastrointestinal symptoms in children with autism and related disorders. J. Pediatr. Gastroenterol. Nutr. 2000, 31, S31–S32. [Google Scholar]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Paik, M.C.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef]
- Barnhill, K.; Devlin, M.; Moreno, H.T.; Potts, A.; Richardson, W.; Schutte, C.; Hewitson, L. Brief Report: Implementation of a Specific Carbohydrate Diet for a Child with Autism Spectrum Disorder and Fragile X Syndrome. J. Autism Dev. Disord. 2018, 50, 1800–1808. [Google Scholar] [CrossRef]
- Alibek, K.; Niyazmetova, L.; Farmer, S.; Isakov, T. Persistent Inflammation Initiated by TORCH Infections and Dysbiotic Microbiome in Autism Spectrum Disorders: A Prospect for Future Interventions. Res. Ideas Outcomes 2022, 8, e91179. [Google Scholar] [CrossRef]
- Knight-Sepulveda, K.; Kais, S.; Santaolalla, R.; Abreu, M.T. Diet and Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2015, 11, 511–520. [Google Scholar]
- The Specific Carbohydrate Diet. Available online: https://med.stanford.edu/content/dam/sm/gastroenterology/documents/IBD/CarbDiet%20PDF%20final.pdf (accessed on 18 July 2023).
- Vatanparast, H.; Bailey, D.A.; Baxter-Jones, A.D.G.; Whiting, S.J. The Effects of Dietary Protein on Bone Mineral Mass in Young Adults May Be Modulated by Adolescent Calcium Intake. J. Nutr. 2007, 137, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Neumeyer, A.M.; Sokoloff, N.C.; McDonnell, E.I.; Macklin, E.A.; McDougle, C.J.; Holmes, T.M.; Hubbard, J.L.; Misra, M. Nutrition and Bone Density in Boys with Autism Spectrum Disorder. J. Acad. Nutr. Diet. 2018, 118, 865–877. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Flores-Rojas, K.; de la Torre-Aguilar, M.J.; Gomez-Fernández, A.R.; Martín-Borreguero, P.; Perez-Navero, J.L.; Gil, A.; Gil-Campos, M. Dietary Patterns, Eating Behavior, and Nutrient Intakes of Spanish Preschool Children with Autism Spectrum Disorders. Nutrients 2021, 13, 3551. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Liu, S.; Zhang, Q.; Wang, P.; Cao, Y.; Wu, L. Supplementation with selenium attenuates autism-like behaviors and improves oxidative stress, inflammation and related gene expression in an autism disease model. J. Nutr. Biochem. 2022, 107, 109034. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-Van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Khan, F.; Rahman, S.; Akhter, S.; Momen, A.B.I.; Raihan, S.G. Vitamin B6 and Magnesium on Neurobehavioral Status of Autism Spectrum Disorder: A Randomized, Double-Blind, Placebo Controlled Study. Bangladesh J. Med. 2021, 32, 12–18. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Connolly, H.V.; Katz, T.; Goldman, S.E.; Weiss, S.K.; Halbower, A.C.; Shui, A.M.; Macklin, E.A.; Hyman, S.L.; Malow, B.A. Randomized, Placebo-Controlled Trial of Ferrous Sulfate to Treat Insomnia in Children with Autism Spectrum Disorders. Pediatr. Neurol. 2019, 104, 30–39. [Google Scholar] [CrossRef] [PubMed]
- DelRosso, L.M.; Reuter-Yuill, L.M.; Cho, Y.; Ferri, R.; Mogavero, M.P.; Picchietti, D.L. Clinical efficacy and safety of intravenous ferric carboxymaltose treatment for restless legs symptoms and low serum ferritin in children with autism spectrum disorder. Sleep Med. 2022, 100, 488–493. [Google Scholar] [CrossRef]
- Triana, N.; Sulchan, M.; Maxitalia, M.; Suryani, M. Effect of Selenium on Stress Oxidative in ASD Children. J. Pharm. Negat. Results 2023, 14, 389–392. [Google Scholar] [CrossRef]
- Meguid, N.A.; Bjørklund, G.; Gebril, O.H.; Doşa, M.D.; Anwar, M.; Elsaeid, A.; Gaber, A.; Chirumbolo, S. The role of zinc supplementation on the metallothionein system in children with autism spectrum disorder. Acta Neurol. Belg. 2019, 119, 577–583. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Xiong, X.-Q.; Yang, T.; Cui, T.; Hou, N.-L.; Lai, X.; Liu, S.; Guo, M.; Liang, X.-H.; et al. Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders—A pilot study. BMC Microbiol. 2017, 17, 204. [Google Scholar] [CrossRef]
- GGuo, M.; Zhu, J.; Yang, T.; Lai, X.; Liu, X.; Liu, J.; Chen, J.; Li, T. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): A pilot study. Brain Res. Bull. 2018, 137, 35–40. [Google Scholar] [CrossRef]
- Obara, T.; Ishikuro, M.; Tamiya, G.; Ueki, M.; Yamanaka, C.; Mizuno, S.; Kikuya, M.; Metoki, H.; Matsubara, H.; Nagai, M.; et al. Potential identification of vitamin B6 responsiveness in autism spectrum disorder utilizing phenotype variables and machine learning methods. Sci. Rep. 2018, 8, 14840. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J.; Chirumbolo, S.; Bjørklund, G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab. Brain Dis. 2017, 32, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Hendren, R.L.; James, S.J.; Widjaja, F.; Lawton, B.; Rosenblatt, A.; Bent, S. Randomized, Placebo-Controlled Trial of Methyl B12 for Children with Autism. J. Child Adolesc. Psychopharmacol. 2016, 26, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Nashabat, M.; Maegawa, G.; Nissen, P.H.; Nexo, E.; Al-Shamrani, H.; Al-Owain, M.; Alfadhel, M. Long-term Outcome of 4 Patients with Transcobalamin Deficiency Caused by 2 Novel TCN2 Mutations. J. Pediatr. Hematol. 2017, 39, e430–e436. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zou, M.; Zhao, D.; Xia, W.; Wu, L. Efficacy of Folic Acid Supplementation in Autistic Children Participating in Structured Teaching: An Open-Label Trial. Nutrients 2016, 8, 337. [Google Scholar] [CrossRef]
- Moradi, H.; Sohrabi, M.; Taheri, H.; Khodashenas, E.; Movahedi, A. Comparison of the effects of perceptual-motor exercises, vitamin D supplementation and the combination of these interventions on decreasing stereotypical behavior in children with autism disorder. Int. J. Dev. Disabil. 2018, 66, 122–132. [Google Scholar] [CrossRef]
- Javadfar, Z.; Abdollahzad, H.; Moludi, J.; Rezaeian, S.; Amirian, H.; Foroughi, A.A.; Nachvak, S.M.; Goharmehr, N.; Mostafai, R. Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: A randomized clinical trial. Nutrition 2020, 79–80, 110986. [Google Scholar] [CrossRef]
- Mazahery, H.; Stonehouse, W.; Delshad, M.; Kruger, M.C.; Conlon, C.A.; Beck, K.L.; Von Hurst, P.R. Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials. Nutrients 2017, 9, 155. [Google Scholar] [CrossRef]
- Feng, J.; Shan, L.; Du, L.; Wang, B.; Li, H.; Wang, W.; Wang, T.; Dong, H.; Yue, X.; Xu, Z.; et al. Clinical improvement following vitamin D3 supplementation in Autism Spectrum Disorder. Nutr. Neurosci. 2016, 20, 284–290. [Google Scholar] [CrossRef]
- Kerley, C.P.; Power, C.; Gallagher, L.; Coghlan, D. Lack of effect of vitamin D3 supplementation in autism: A 20-week, placebo-controlled RCT. Arch. Dis. Child. 2017, 102, 1030–1036. [Google Scholar] [CrossRef]
- Boone, K.M.; Klebanoff, M.A.; Rogers, L.K.; Rausch, J.; Coury, D.L.; Keim, S.A. Effects of Omega-3-6-9 fatty acid supplementation on behavior and sleep in preterm toddlers with autism symptomatology: Secondary analysis of a randomized clinical trial. Early Hum. Dev. 2022, 169, 105588. [Google Scholar] [CrossRef]
- Keim, S.A.; Gracious, B.; Boone, K.M.; Klebanoff, M.A.; Rogers, L.K.; Rausch, J.; Coury, D.L.; Sheppard, K.W.; Husk, J.; Rhoda, D.A. ω-3 and ω-6 Fatty Acid Supplementation May Reduce Autism Symptoms Based on Parent Report in Preterm Toddlers. J. Nutr. 2018, 148, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Doaei, S.; Bourbour, F.; Teymoori, Z.; Jafari, F.; Kalantari, N.; Torki, S.A.; Ashoori, N.; Gorgani, S.N.; Gholamalizadeh, M. The effect of omega-3 fatty acids supplementation on social and behavioral disorders of children with autism: A randomized clinical trial. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 12–18. [Google Scholar] [CrossRef]
- McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P.; Eliassen, J.C.; Alfieri, D.; Weber, W.; Jarvis, K.; DelBello, M.P.; et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 2010, 91, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Siafis, S.; Çıray, O.; Wu, H.; Schneider-Thoma, J.; Bighelli, I.; Krause, M.; Rodolico, A.; Ceraso, A.; Deste, G.; Huhn, M.; et al. Pharmacological and dietary-supplement treatments for autism spectrum disorder: A systematic review and network meta-analysis. Mol. Autism 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Łukasik, J.; Szajewska, H. ω-3 Fatty Acid Supplementation Does Not Affect Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 367–376. [Google Scholar] [CrossRef]
- Liu, J.; Wan, G.-B.; Huang, M.-S.; Agyapong, G.; Zou, T.-L.; Zhang, X.-Y.; Liu, Y.-W.; Song, Y.-Q.; Tsai, Y.-C.; Kong, X.-J. Probiotic Therapy for Treating Behavioral and Gastrointestinal Symptoms in Autism Spectrum Disorder: A Systematic Review of Clinical Trials. Curr. Med. Sci. 2019, 39, 173–184. [Google Scholar] [CrossRef]
- Coretti, L.; Cristiano, C.; Florio, E.; Scala, G.; Lama, A.; Keller, S.; Cuomo, M.; Russo, R.; Pero, R.; Paciello, O.; et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep. 2017, 7, 45356. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Tuladhar, S.; Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Essa, M.M.; Bishir, M.; Bolla, S.R.; Nanjaiah, N.D.; Guillemin, G.J.; et al. Autism and Gut–Brain Axis: Role of Probiotics. Adv. Neurobiol. 2020, 24, 587–600. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Navarro, F.; Liu, Y.; Rhoads, J.M. Can probiotics benefit children with autism spectrum disorders? World J. Gastroenterol. 2016, 22, 10093–10102. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Gibson, E.L.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Kung, J.Y.; Tun, H.M.; Wine, E.; Madsen, K.L.; Zwaigenbaum, L.; Haqq, A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res. 2021, 14, 1820–1836. [Google Scholar] [CrossRef] [PubMed]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef]
- Nordahl, C.W.; Andrews, D.S.; Dwyer, P.; Waizbard-Bartov, E.; Restrepo, B.; Lee, J.K.; Heath, B.; Saron, C.; Rivera, S.M.; Solomon, M.; et al. The Autism Phenome Project: Toward Identifying Clinically Meaningful Subgroups of Autism. Front. Neurosci. 2022, 15, 786220. [Google Scholar] [CrossRef]
| Tendency | Brief Characteristics | |
|---|---|---|
| Group 1 (sensory) | Foods are selected based on sensory factors, such as texture, smell, taste, temperature, and color | Severe intellectual disability (the sensory motor stage in the theory of mental development) • Oral hypersensitivity |
| Group 2 (visual) | Foods are judged at a glance based on color, shape, or cooking method | Moderate intellectual disability • Severely restricted imagination |
| Group 3 (familiarity) | Selection of familiar foods | Visual performance is dominant • Developmental age for cognition >2 years • Difficulty in social imagination (prediction) |
| Group 4 (environmental stimulation) | Food choices are affected by environmental factors: place, plate, cup, people, and room temperature | Attention deficit disorder • Each developmental age is variable |
| Nutrient | Intake | Subjects | Source |
|---|---|---|---|
| Protein Other Macronutrients, Vitamin A Vitamin B1, B2, B3, B6 Folic Acid Vitamin B12 Calcium Iron Zinc Selenium | Lower in ASD No difference Lower in ASD Lower in ASD Lower in ASD Lower in ASD Lower in ASD Lower in ASD Lower in ASD Higher in ASD | 86 children with ASD aged 2–8 years and 57 age-matched peers without ASD | Barnhill et al. [41] |
| Energy Fats Potassium Copper Folate Iron Vitamin C | No difference No difference Lower in ASD Lower in ASD Lower in ASD Lower in ASD Lower in ASD | 63 ASD children in the age range of 4 to 10 years and 50 typically developing children matched on age and socio-economic status to the ASD children | Malhi et al. [30] |
| Protein Calcium Iron Zinc Vitamin B2 | Adequate (RDA) Lower than RDA Lower than RDA Lower than RDA Lower than RDA | 53 children with ASD (45 boys and 8 girls) in the age group of 2–13 years | Siddiqi et al. [42] |
| Fiber Calcium Vitamin E Vitamin A Vitamin C Folic Acid Zinc | Lower than DRI Lower than DRI Lower than DRI Lower than DRI Lower than DRI Lower than DRI Lower than DRI | 70 children with ASD and severe food selectivity | Sharp et al. [29] |
| Folate Zinc Vitamin B12 Vitamin D | Lower in ASD Lower in ASD Lower in ASD Lower in ASD | 738 ASD children and 302 typically developing children (TD) age 2–6 | Zhu et al. [43] |
| Vitamin E Vitamin K Vitamin B2 Vitamin B6 Vitamin A Vitamin D Vitamin B12 Folate Magnesium Phosphorus Zinc Selenium | Lower in ASD Lower in ASD Lower in ASD Lower in ASD Lower than DRI in ASD & TD Lower than DRI in ASD & TD Lower than DRI in ASD &TD Lower than DRI in ASD & TD Lower than DRI in ASD & TD Lower than DRI in ASD & TD Lower than DRI in ASD & TD Lower than DRI in ASD &TD | 52 ASD cases (37 boys and 15 girls), 51 TD children (26 boys and 25 girls) aged 3–6 | Alkhalidy et al. [44] |
| Study Group | Scientific Evidence | References |
|---|---|---|
| Animal Studies | ||
| The study analyzed the protective effects of a KD on sociability, spatial learning, memory, and electroencephalogram seizures in glut3 heterozygous null (glut3+/−) mice exhibiting features relevant to ASD. | The study reported on a KD-related partial restoration of social features and alleviation of seizure events in male subjects. The KD also exerted neuroprotective effects in female subjects due to higher circulating and cerebrospinal fluid ketone concentrations and/or lower brain Glut3 concentrations. | Dai et al. [61] |
| The study examined mutant EL mice with comorbid epilepsy and ASD symptoms. | A clear sex-related difference was reported in response to the KD. The KD improved multiple measures of sociability and reduced repetitive behavior in female mice, but had limited effects in males. | Ruskin et al. [62] |
| The experiments involved En2 knockout mice exposed to a KD from postnatal day 21 to 60. | The early timing of a dietary intervention was recognized as an important factor in diet-dependent brain reorganization and maturation. Although monoamine levels in the forebrain regions were not affected in two null mice (En2(−/−)), increased social contact and reduced grooming behavior were evident in response to KD intervention. | Verpeut et al. [63] |
| The study analyzed sociability and social behavior in male and female MIA mice | Male MIA offspring were significantly asocial in the three-chamber sociability test, while female mice displayed normal and social behavior. After 3–4 weeks of KD treatment, the lack of sociability in male offspring was completely reversed and MIA-induced, self-directed, repetitive behavior was reduced. | Ruskin et al. [62] |
| Human studies | ||
| 45 children aged 3–8 years | A KD reduced autistic manifestations in the Autism Treatment Evaluation Test (ATEC) and the Childhood Autism Rating Scale (CARS), in particular by improving sociability. | El-Rashidy et al. [64] |
| 15 children aged 2–17 years | A modified ketogenic gluten-free diet supplemented using medium-chain triglycerides (MCTs) improved the social affect subdomain and scores in the total autism diagnostic observation schedule, 2nd edition (ADOS-2) scores, but it had no effect on restricted and repetitive behavior scores. | Lee et al. [54] |
| Six ASD (aged 4–14 years old) patients with a pathological increase in beta-hydroxybutyrate | A KD improved social communication in one of the six ASD patients and reduced the prevalence of comorbidities in patients, including attention deficit hyperactivity disorder (ADHD), compulsive behavior, preoccupation with parts of objects, and abnormal sleep. | Spilioti et al. [65] |
| 6-year-old child | In a case study of a child with ASD, a KD improved behavior and intellect, and decreased the 18F-FDG uptake in the whole cortex. | Żarnowska et al. [66] |
| Most Common Side Effects | References |
|---|---|
| The KD can be difficult to maintain, especially in children with limited food preferences. It is important to have a plan in place to ensure that the child is able to stick to the diet. ASD patients also consume fewer foods and exhibit more feeding problems and diverse eating behaviors (selective intake, food refusal, food aversion, and atypical eating). Some foods are refused due to presentation or the need to use certain utensils. In a study by Albers et al. [68] 73% of the respondents rated adherence to the KD as more difficult, compared with age-matched controls, whereas only 26% of the subjects did not report such difficulties. These results confirm that the administration of a KD to ASD children is difficult. | Li et al. [69] Mayes & Zickgraf [70] |
| The sensory abnormalities commonly associated with ASD can influence the administration of and adherence to the KD. Parents reported that children with ASD were significantly more averse to food textures (p < 0.0001), in particular foods with a slimy and creamy texture. According to the authors, taste preferences and consistent food routines are important or very important determinants of the successful implementation of the KD. | Albers et al. [68] Balasco et al. [71] Cermak et al. [72] |
| Taste, smell, and texture hypersensitivities/aversions were regarded as the key difficulties in the implementation of a KD | Albers et al. [68] |
| Nutrient Deficiencies: The KD is very restrictive, and it may not provide growing children with the necessary nutrients. In children, the KD may suppress physical development and cause height deceleration. Parents should work with healthcare providers or dietitians to eliminate the risk of nutritional deficits. | Spulber et al. [73] |
| Possible Side-Effects: Some children may experience side effects from the KD, such as constipation, nausea, and vomiting. During the initial phase of the diet, common side effects also include hypoglycemia, metabolic acidosis, and refusal to eat. | Neal et al. [74] Newmaster et al. [75] |
| Supplement | Intervention | Subject | Main Results | References |
|---|---|---|---|---|
| Mg+B6 | Mg: 50 mg for children aged 2–3 years, 100 mg for children aged 4–8 years, 200 mg for children aged 9–12 years. Vitamin B6: 25 mg for children aged 2–3 years, 50 mg for children aged 4–8 years and 100 mg for children aged 9–12 years. Duration of intervention: 3 months | 70 children with ASD | The improvement in the overall score of the treated group was statistically significant relative to the placebo group and the intervention group. The improvement in the cognition and emotion score was statistically significant. The improvement in the social, communication and sensory deficiency score was not statistically significant. | Khan et al. [119]. A randomized, double-blind, placebo controlled study |
| Fe | 3 mg/kg/day of liquid ferrous sulfate for 3 months | 20 children with ASD | An improvement in iron levels and a reduction in the overall severity score on the Sleep Clinical Global Impression Scale were observed. Actigraphy measurements did not reveal significant improvements in the primary outcome measure, i.e., sleep onset latency and wake time after sleep onset. | Reynolds et al. [120]. A randomized placebo-controlled trial |
| Fe | Infusion of ferrous carboxymaltose (FCM) at 15 mg/kg up to a maximum dose of 750 mg | 19 children with ASD (age: 4–11 years) | In most children (84.2%) exhibiting ASD, symptoms of restless legs, and serum ferritin levels below 30 μg/L experienced clinical amelioration and notably enhanced serum iron parameters following a sole intravenous ferric carboxymaltose (FCM) infusion. | DelRosso et al. [121]. Retrospective study |
| Se | Intervention group 1 received powdered selenium supplement at 1 × 20 g/day; intervention group 2 received a functional food product with a high content of selenium (bovine heart extract) at 50 g/day | 65 children with ASD (age: 2–6 years) | Supplementation did not induce significant differences in total glutathione peroxidase levels. | Triana et al. [122] Randomized controlled trial |
| Zn | A dietary nutraceutical formula containing Zn was administered for 12 weeks. The daily Zn dose was adjusted to the participants’ body weight in kg plus 15–20 mg. | 30 children with ASD (age: 3–8 years) | Zn supplementation markedly reduced CARS scores in children with ASD. Serum Zn and metallothionein levels increased significantly after Zn supplementation. | Meguid et al. [123] |
| Vitamin A | Participants with low plasma retinol levels (<1.05 μmol/L) received a single oral dose of vitamin A at 200,000 IU and completed a 6-month follow-up study | 64 children (aged 1–8 years) with ASD | Vitamin A induced changes in the composition of gut microbiota and improved selected ASD-related biomarkers such as CD38 and RORA. In children with ASD, significant taxonomic associations with vitamin A were identified in the Bacteroidetes/Bacteroidales group. | Liu et al. [124] Single-blinded, non-randomized intervention pilot study |
| Vitamin A | 200,000 IU for 6 months | 33 ASD patients (mean age: 5.14 ± 1.33 years) | Vitamin A decreased serum levels of 5-hydroxytryptamine (5-HT). Differences in CARS scores were noted before and after the administration of vitamin A. Improvements were observed in social interactions, emotional responses, motor skills, adaptability, sensory sensitivity (including taste, smell, and touch), anxiety levels, and verbal and non-verbal communication. Moreover, the general impression and the overall score increased significantly after vitamin A supplementation. Furthermore, excluding restricted interests, all symptoms related to the neurodevelopmental deficits reported by parents improved substantially after vitamin A administration. | Guo et al. [125] |
| Vitamin B6 | 5 mg of vitamin B6/kg body weight per day for 2 weeks, followed by 10 mg of vitamin B6/kg body weight per day for 2 weeks. Total duration of treatment: 4 weeks | 17 children with ASD (mean age: 8.8 years) | Five clusters were identified. Cluster 1 consisted of all persons who responded to vitamin B6. Persons with ASD may be highly heterogeneous. | Obara et al. [126] Single-arm intervention |
| Vitamin B6 | No data | 236 children with ASD (age: 3–16 years) | Supplementation with vitamin B and magnesium induced significant changes in tryptophan levels. | Kałużna-Czaplińska, et al. [127]. |
| Vitamin B12 | 75 μg/kg every third day for 8 weeks | 57 children with ASD (age: 3–7 years) | Vitamin B12 improved the CGI-I score, but not ABC or SRS. The supplement increased plasma methionine levels, decreased SAH levels, and improved the SAM-to-SAH ratio. | Hendren et al. [128]. Randomized controlled trial |
| Vitamin cB12 replaced with mB12; hB12 | Four children with ASD | Anemia and metabolic acidosis showed improvement when cyanocobalamin (cB12) was replaced with methylcobalamin (mB12). Furthermore, homocysteine levels returned to normal after oral administration of 10 mg of hydroxocobalamin (hB12) in a single pediatric case. | Nashabat et al. [129]. | |
| Folic acid | 400 μg, twice daily for 3 months | 66 children with ASD (age: 4.5 ± 1.1 years) | Folic acid improved social engagement, cognitive language and preverbal abilities, receptive communication skills, emotional expression, and interactions and communication in children with ASD. In addition, positive changes in folic acid and homocysteine levels stabilized the glutathione-dependent redox balance. | Sun et al. [130]. Open-label trial |
| Folic acid | 600 μg twice daily for 3 months Additional supplements (omega-3 and omega-6 fatty acids, carnitine) were administered for 12 months | 67 children and adults with ASD (age: 3–58 years) | In a clinical assessment with blinding, folic acid improved non-verbal cognitive aptitude relative to the control (untreated) group. In a semi-blinded trial, the treated cohort demonstrated greater progress in ASD symptomatology and developmental progress than the control group. The concentrations of EPA, DHA, carnitine, vitamins A, B2, B5, B6, and B12, folic acid, and coenzyme Q10 increased markedly in the treated group and differed significantly from the control. | Adams et al. [52]. Single-blinded study |
| Vitamin D | 300 IU/kg/day (max. 5000 IU/day) for 3 months | 100 children with ASD (age: 6–9 years) | Vitamin D supplementation significantly alleviated the clinical symptoms of ASD measured on the CARS and ATEC scales. Supplementation did not induce significant changes in the serum levels of serotonin and IL-6 on the ABC-C scale. | Moradi et al. [131]. Randomized controlled trial |
| Vitamin D | 300 IU/kg/day (max. 6000 IU/day) for 15 weeks | A total of 43 children, including 22 children with ASD (age: 3–13 years) | Vitamin D supplementation induced a significant decrease in irritability and hyperactivity on ABC-C subscales. Supplementation decreased lethargy/social withdrawal, inappropriate speech, and stereotypic behavior. | Javadfar et al. [132]. Randomized, double-blind, placebo-controlled, parallel-group trial |
| Vitamin D3 | 2000 IU/day | 117 children with ASD (age: 2.5–8.0 years) | The rate of positive response (at least a 25% reduction in the ABC-hyperactivity score and the ABC-irritability score) was 68% and 63%, respectively. | Mazahery et al. [133]. 12-month randomized double-blind, placebo-controlled study |
| Vitamin D3 | Administered IM at 150,000 IU/month (a total of three injections) and orally at 400 IU per day for 3 months | 215 children with ASD (mean age: 4.76 ± 0.95 years (37 autistic children received vitamin D3) | Serum levels of 25(OH) D were negatively correlated with total ABC scores and language subscale scores. Vitamin D3 supplementation significantly decreased CARS and ABC symptom scores. The treatment effects were more pronounced in younger children with ASD (≤3 years). | Feng et al. [134]. |
| Vitamin D3 | 2000 IU for 20 weeks | 42 children with ASD | The primary endpoint (stereotypical behavior subscale of the ABC) did not show any observable effect. The self-care score in the DD-CGAS improved in the D3 group (p = 0.02). There was a tendency toward reduced inappropriate speech in the placebo group compared to the D3 group (p = 0.08), although the difference was not significant. | Kerley et al. [135]. Parallel, randomized, double-blind, placebo-controlled trial involving two visits to a clinic |
| Omega -3–6-9 | 706 mg of omega-3 fatty acids (including 338 mg of EPA and 225 mg of DHA), 280 mg of omega-6 fatty acids (including 83 mg of GLA), and 306 mg of omega-9 fatty acids | 31 children aged 18–38 months | The magnitude of clinical benefit was moderate for anxious and depressed behaviors (p = 0.049) and internalizing behaviors (p = 0.05), and large for adaptive behaviors in interpersonal relationships (p = 0.01). The remaining behaviors and sleep were not affected. | Boone et al. [136]. 90-day randomized (1:1), double-blinded, placebo-controlled trial |
| Omega-3 | 706 mg of omega-3 fatty acids (including 338 mg of EPA and 225 mg of DHA), 280 mg of omega-6 fatty acids (including 83 mg of GLA), and 306 mg of omega-9 fatty acids | 31 children (age: 18–38 months) | Greater reduction in ASD symptoms on the Brief Infant Toddler Social Emotional Assessment ASD scale. No other outcome measure reflected a similar magnitude or a significant effect. | Keim et al. [137]. 90-day randomized, fully blinded, placebo-controlled trial |
| Omega-3 | 1000 mg of omega-3 daily in the experimental group and 1000 mg of medium-chain triglycerides (placebo) daily in the control group for 8 weeks | 54 children, (age: 5–15 years) | Significant improvement in stereotyped behaviors (p = 0.02), social communication (p = 0.02), and the GARS score (p = 0.001). No significant change in scores on the social interaction subscale. | Doaei et al. [138]. A double-blind, randomized clinical trial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Önal, S.; Sachadyn-Król, M.; Kostecka, M. A Review of the Nutritional Approach and the Role of Dietary Components in Children with Autism Spectrum Disorders in Light of the Latest Scientific Research. Nutrients 2023, 15, 4852. https://doi.org/10.3390/nu15234852
Önal S, Sachadyn-Król M, Kostecka M. A Review of the Nutritional Approach and the Role of Dietary Components in Children with Autism Spectrum Disorders in Light of the Latest Scientific Research. Nutrients. 2023; 15(23):4852. https://doi.org/10.3390/nu15234852
Chicago/Turabian StyleÖnal, Seda, Monika Sachadyn-Król, and Małgorzata Kostecka. 2023. "A Review of the Nutritional Approach and the Role of Dietary Components in Children with Autism Spectrum Disorders in Light of the Latest Scientific Research" Nutrients 15, no. 23: 4852. https://doi.org/10.3390/nu15234852
APA StyleÖnal, S., Sachadyn-Król, M., & Kostecka, M. (2023). A Review of the Nutritional Approach and the Role of Dietary Components in Children with Autism Spectrum Disorders in Light of the Latest Scientific Research. Nutrients, 15(23), 4852. https://doi.org/10.3390/nu15234852






