Probiotics Support Resilience of the Oral Microbiota during Resolution after Experimental Gingivitis—A Randomized, Double-Blinded, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
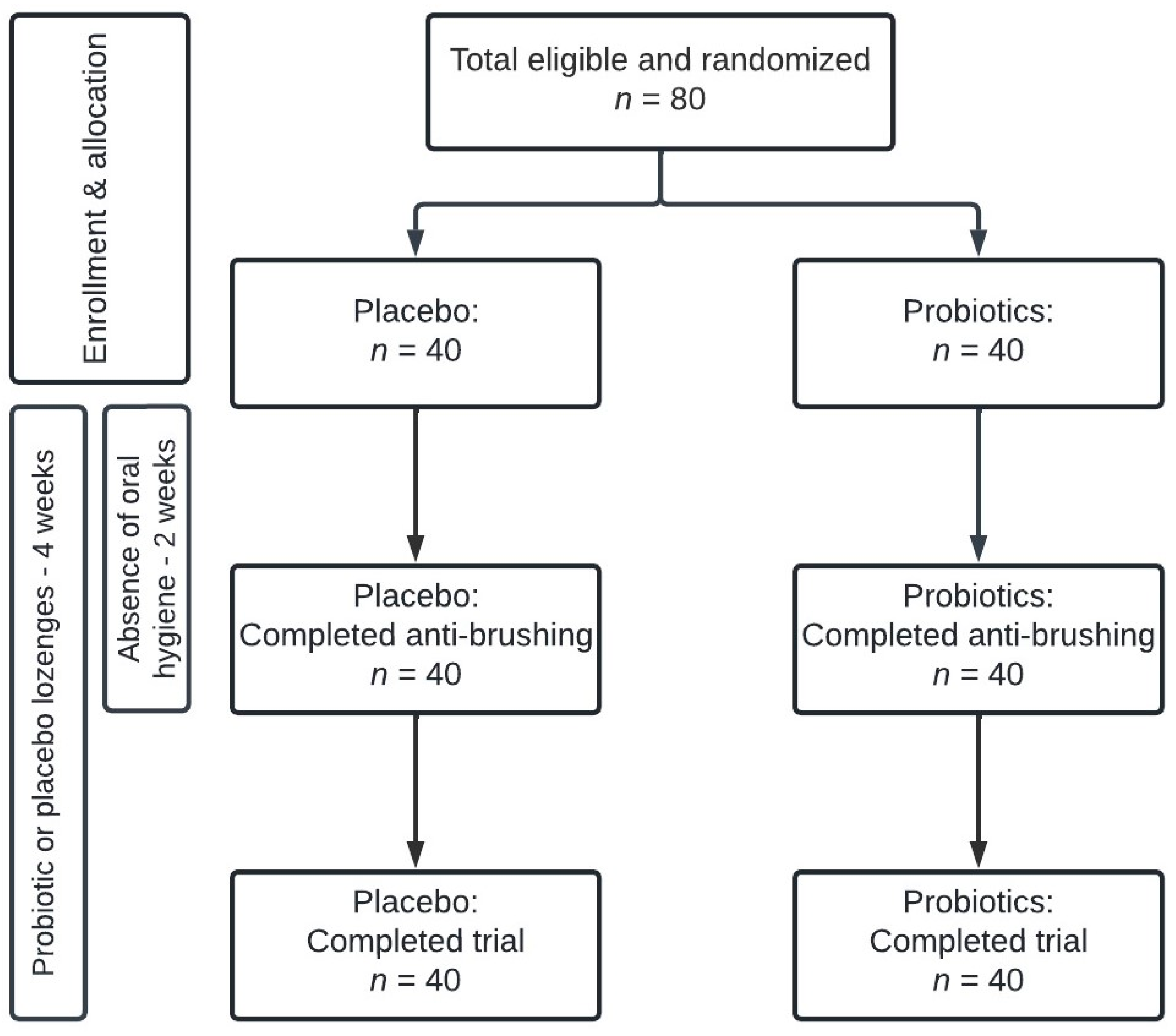
2.1. Study Design
2.2. Study Population
2.3. Collection of Samples
2.4. Clinical Data
2.5. Probiotic and Placebo Lozenges
2.6. DNA Extraction, Library Preparation, and DNA Sequencing
2.7. Cytokine Analysis
2.8. Protein and Enzyme Analysis
2.9. Bioinformatic Processing and Statistics
3. Results
3.1. Background Data
3.2. Sequencing Metadata
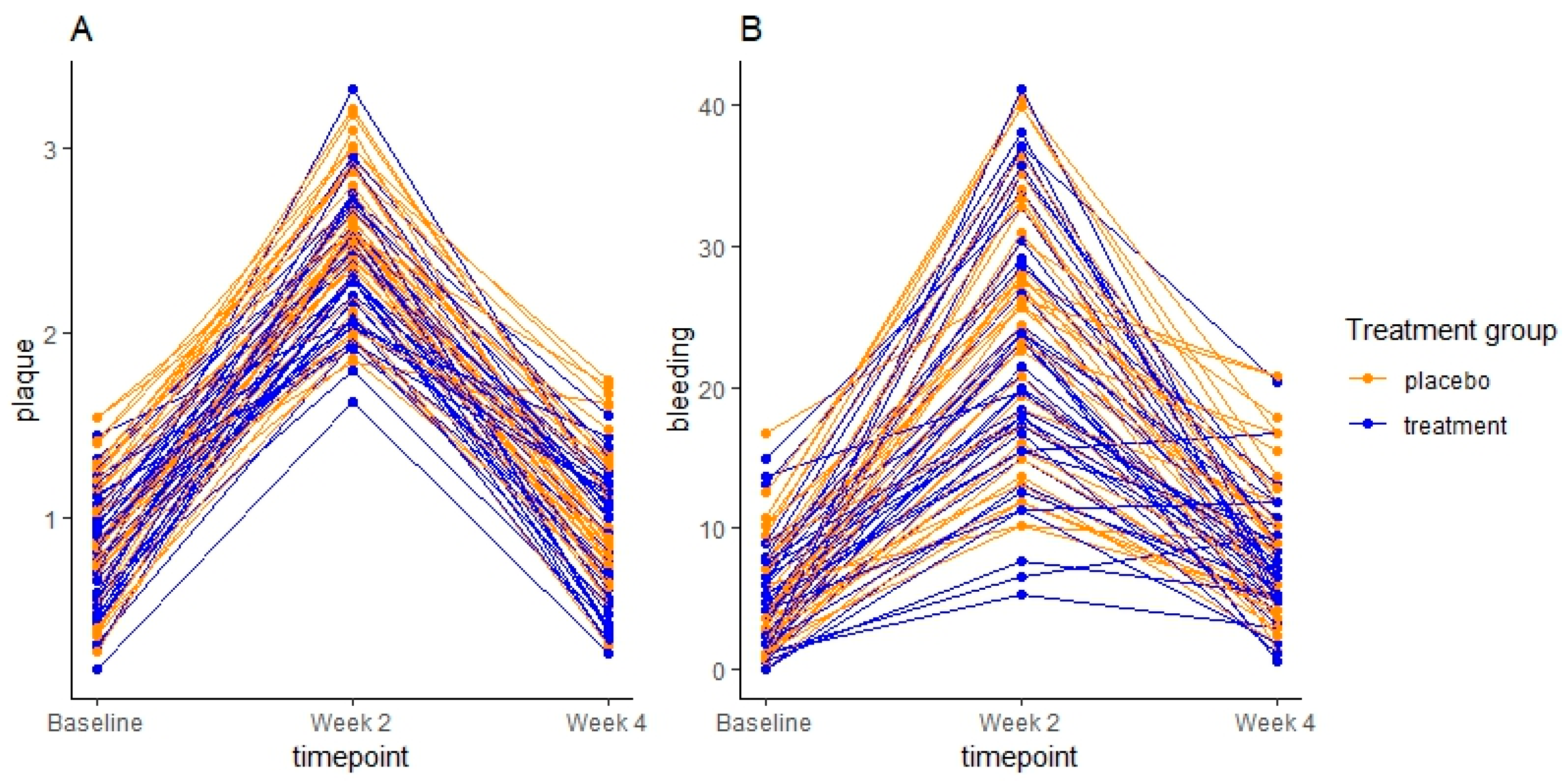
3.3. Impact of Oral Hygiene Neglect on Oral Homeostasis in the Placebo Group
3.4. Clinical Effect of Supplementary Consumption of Probiotics
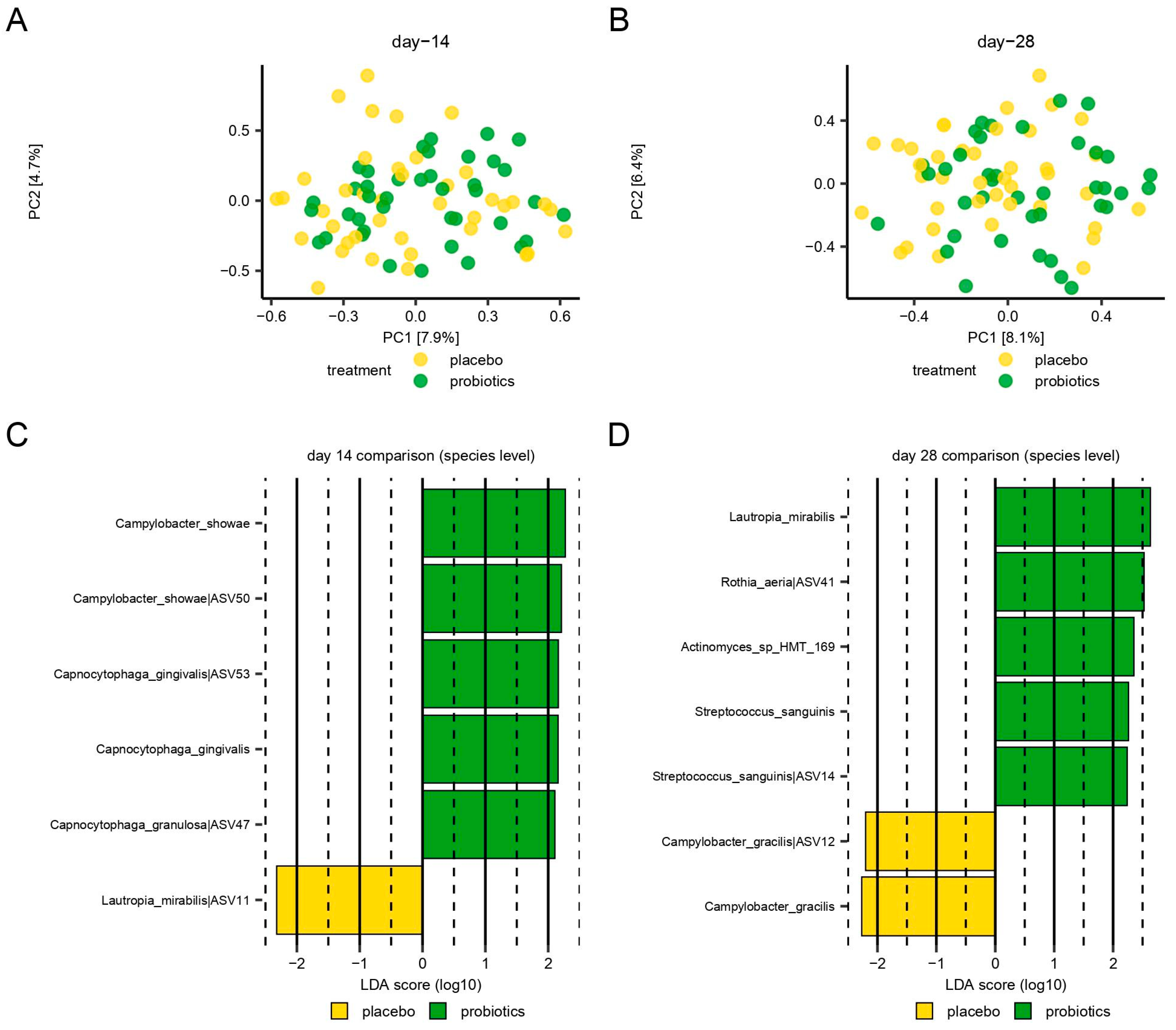
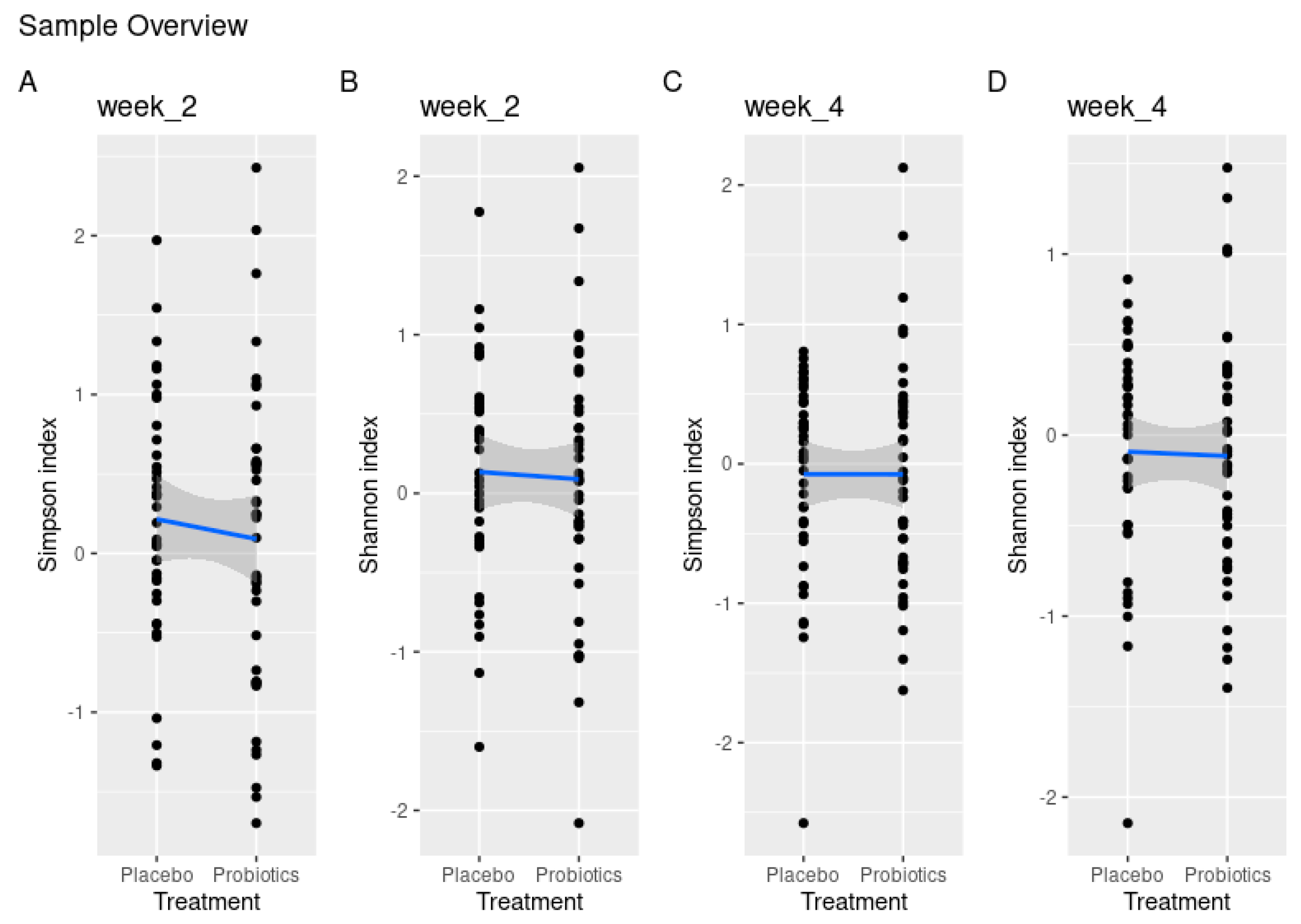
3.5. Microbiological Effect of Supplementary Consumption of Probiotics
3.6. Immunological Effect of Supplementary Consumption of Probiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S46–S73. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Evidence-Based Update on Diagnosis and Management of Gingivitis and Periodontitis. Dent. Clin. N. Am. 2019, 63, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Brody, H.; Lin, G.H.; Rangé, H.; Kuraji, R.; Ye, C.; Kamarajan, P.; Radaic, A.; Gao, L.; Kapila, Y. Probiotics, including nisin-based probiotics, improve clinical and microbial outcomes relevant to oral and systemic diseases. Periodontol. 2000 2020, 82, 173–185. [Google Scholar] [CrossRef]
- Barboza, E.P.; Arriaga, P.C.; Luz, D.P.; Montez, C.; Vianna, K.C. Systematic review of the effect of probiotics on experimental gingivitis in humans. Braz. Oral Res. 2020, 34, e031. [Google Scholar] [CrossRef]
- Akram, Z.; Shafqat, S.S.; Aati, S.; Kujan, O.; Fawzy, A. Clinical efficacy of probiotics in the treatment of gingivitis: A systematic review and meta-analysis. Aust. Dent. J. 2020, 65, 12–20. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Huang, J.; Tao, R. Effect of probiotics on gingival inflammation and oral microbiota: A meta-analysis. Oral Dis. 2022, 28, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Loe, H.; Theilade, E.; Jensen, S.B. Experimental Gingivitis in Man. J. Periodontol. 1965, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lundtorp-Olsen, C.; Enevold, C.; Juel Jensen, C.A.; Stofberg, S.N.; Twetman, S.; Belstrøm, D. Impact of Probiotics on the Salivary Microbiota and Salivary Levels of Inflammation-Related Proteins during Short-Term Sugar Stress: A Randomized Controlled Trial. Pathogens 2021, 10, 392. [Google Scholar] [CrossRef]
- Belstrøm, D.; Sembler-Møller, M.L.; Grande, M.A.; Kirkby, N.; Cotton, S.L.; Paster, B.J.; Twetman, S.; Holmstrup, P. Impact of Oral Hygiene Discontinuation on Supragingival and Salivary Microbiomes. JDR Clin. Transl. Res. 2018, 3, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lundtorp Olsen, C.; Markvart, M.; Vendius, V.F.D.; Damgaard, C.; Belstrøm, D. Short-term sugar stress induces compositional changes and loss of diversity of the supragingival microbiota. J. Oral Microbiol. 2023, 15, 2189770. [Google Scholar] [CrossRef]
- Lundtorp-Olsen, C.; Enevold, C.; Twetman, S.; Belstrøm, D. Probiotics Do Not Alter the Long-Term Stability of the Supragingival Microbiota in Healthy Subjects: A Randomized Controlled Trial. Pathogens 2021, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Bardow, A.; Lykkeaa, J.; Qvist, V.; Ekstrand, K.; Twetman, S.; Fiehn, N.E. Saliva composition in three selected groups with normal stimulated salivary flow rates, but yet major differences in caries experience and dental erosion. Acta Odontol. Scand. 2014, 72, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Lobene, R.R.; Soparkar, P.M.; Newman, M.B. Use of dental floss. Effect on plaque and gingivitis. Clin. Prev. Dent. 1982, 4, 5–8. [Google Scholar]
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, e00187-18. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Demir, E.; Gürsoy, M.; Firatli, E.; Loimaranta, V.; Gürsoy, U.K. Salivary levels of BAFF, TWEAK, and soluble CD163 and salivary arginase activity before and after periodontal treatment. J. Periodontal Res. 2023, 58, 646–654. [Google Scholar] [CrossRef]
- Yilmaz, N.; Polat, R.; Gürsoy, M.; Kaman, W.; Gül Aydin, E.; Fteita, D.; Yilmaz, D.; Bikker, F.; Gürsoy, U.K. Salivary macrophage activation-related chemokines and mitogen-activated kinase kinase (MAPKK)-degrading proteolytic activity in type 1 diabetes mellitus. J. Periodontol. 2023, 94, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.; Brand, H.S.; Ligtenberg, A.J.; Imangaliyev, S.; Tsivtsivadze, E.; van der Weijden, F.; Crielaard, W.; Keijser, B.J.; Veerman, E.C. Interindividual variation, correlations, and sex-related differences in the salivary biochemistry of young healthy adults. Eur. J. Oral Sci. 2015, 123, 149–157. [Google Scholar] [CrossRef]
- Bikker, F.J.; Nascimento, G.G.; Nazmi, K.; Silbereisen, A.; Belibasakis, G.N.; Kaman, W.E.; Lopez, R.; Bostanci, N. Salivary Total Protease Activity Based on a Broad-Spectrum Fluorescence Resonance Energy Transfer Approach to Monitor Induction and Resolution of Gingival Inflammation. Mol. Diagn. Ther. 2019, 23, 667–676. [Google Scholar] [CrossRef]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef]
- Abusleme, L.; Hoare, A.; Hong, B.Y.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Hall, M.W.; Wellappuli, N.C.; Huang, R.C.; Wu, K.; Lam, D.K.; Glogauer, M.; Beiko, R.G.; Senadheera, D.B. Suspension of oral hygiene practices highlights key bacterial shifts in saliva, tongue, and tooth plaque during gingival inflammation and resolution. ISME Commun. 2023, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ciantar, M.; Spratt, D.A.; Newman, H.N.; Wilson, M. Capnocytophaga granulosa and Capnocytophaga haemolytica: Novel species in subgingival plaque. J. Clin. Periodontol. 2001, 28, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar] [CrossRef]
- Greve, D.; Moter, A.; Kleinschmidt, M.C.; Pfäfflin, F.; Stegemann, M.S.; Kursawe, L.; Grubitzsch, H.; Falk, V.; Kikhney, J. Rothia aeria and Rothia dentocariosa as biofilm builders in infective endocarditis. Int. J. Med. Microbiol. 2021, 311, 151478. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Van Steijn, G.J.; Amerongen, A.V.; Veerman, E.C.; Kasanmoentalib, S.; Overdijk, B. Chitinase in whole and glandular human salivas and in whole saliva of patients with periodontal inflammation. Eur. J. Oral Sci. 1999, 107, 328–337. [Google Scholar] [CrossRef]
- Katsiki, P.; Nazmi, K.; Loos, B.G.; Laine, M.L.; Schaap, K.; Hepdenizli, E.; Bikker, F.J.; Brand, H.S.; Veerman, E.C.I.; Nicu, E.A. Comparing periodontitis biomarkers in saliva, oral rinse and gingival crevicular fluid: A pilot study. J. Clin. Periodontol. 2021, 48, 1250–1259. [Google Scholar] [CrossRef]
- Sánchez, G.A.; Miozza, V.; Delgado, A.; Busch, L. Determination of salivary levels of mucin and amylase in chronic periodontitis patients. J. Periodontal Res. 2011, 46, 221–227. [Google Scholar] [CrossRef]
- Belstrøm, D.; Damgaard, C.; Könönen, E.; Gürsoy, M.; Holmstrup, P.; Gürsoy, U.K. Salivary cytokine levels in early gingival inflammation. J. Oral Microbiol. 2017, 9, 1364101. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Petitat, C.; Trepp, S.; Lang, N.P.; Eick, S.; Adam, R.; Ccahuana-Vasquez, R.A.; Barker, M.L.; Timm, H.; Klukowska, M.; et al. Clinical Parameters and Oral Fluid Biomarkers in Gingivitis Subjects using an Electric Toothbrush with Irrigator vs a Manual Toothbrush Alone over 8 Weeks: A Randomised Controlled Clinical Trial. Oral Health Prev. Dent. 2021, 19, 137–147. [Google Scholar] [CrossRef]
- Syndergaard, B.; Al-Sabbagh, M.; Kryscio, R.J.; Xi, J.; Ding, X.; Ebersole, J.L.; Miller, C.S. Salivary biomarkers associated with gingivitis and response to therapy. J. Periodontol. 2014, 85, e295–e303. [Google Scholar] [CrossRef]
- Twetman, S.; Derawi, B.; Keller, M.; Ekstrand, K.; Yucel-Lindberg, T.; Stecksen-Blicks, C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol. Scand. 2009, 67, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kuru, B.E.; Laleman, I.; Yalnızoğlu, T.; Kuru, L.; Teughels, W. The Influence of a Bifidobacterium animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1115–1123. [Google Scholar] [CrossRef]
- de Almeida Silva Levi, Y.L.; Ribeiro, M.C.; Silva, P.H.F.; Silva, G.A.; de Souza Salvador, S.L.; de Souza, S.L.S.; Casarin, R.; Júnior, A.B.N.; Júnior, M.T.; Palioto, D.B.; et al. Effects of oral administration of Bifidobacterium animalis subsp. lactis HN019 on the treatment of plaque-induced generalized gingivitis. Clin. Oral Investig. 2023, 27, 387–398. [Google Scholar] [CrossRef]
- Hallström, H.; Lindgren, S.; Yucel-Lindberg, T.; Dahlén, G.; Renvert, S.; Twetman, S. Effect of probiotic lozenges on inflammatory reactions and oral biofilm during experimental gingivitis. Acta Odontol. Scand. 2013, 71, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.K.; Brandsborg, E.; Holmstrøm, K.; Twetman, S. Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: A randomised controlled trial. Benef. Microbes 2018, 9, 487–494. [Google Scholar] [CrossRef] [PubMed]

| Placebo (n = 40) | Probiotic (n = 40) | p Value [CI] between Groups | |
|---|---|---|---|
| Sex (female/male) | 29/11 | 23/17 | |
| Age | 24 (19–33) | 24 (20–30) | |
| Clinical examination | |||
| Baseline | |||
| Plaque mean (sd) | 0.92 (0.3) | 0.8 (0.3) | |
| BOP % mean (sd) | 5.36 (3.9) | 4.73 (3.7) | |
| Day 14 | |||
| Plaque mean (sd) | 2.55 (0.4) | 2.37 (0.3) | 0.06 [−0.3; 0.01] |
| Baseline vs. day 14 p value [CI] | <0.001 * [1.4; 1.8] | <0.001 * [1.4; 1.8] | |
| BOP % mean (sd) | 24.56 (8.6) | 22.34 (9.2) | 0.39 [−5.2; 2] |
| Baseline vs. day 14 p value [CI] | <0.001 * [15.9; 22.5] | <0.001 * [14.2; 20.9] | |
| Day 28 | |||
| Plaque mean (sd) | 0.88 (0.4) | 0.86 (0.4) | 0.57 [−0.2; 0.1] |
| Day 14 vs. day 28 p value [CI] | <0.001 * [−1.8; −1.4] | <0.001 * [−1.7; −1.3] | |
| Baseline vs. day 28 p value [CI] | 0.7 [−0.1; 0.3] | 0.7 [−0.1; 0.2] | |
| BOP % mean (sd) | 8.77 (5.1) | 7.1 (4.1) | 0.16 [−3.3; 0.5] |
| Day 14 vs. day 28 p value [CI] | <0.001 * [−19.1; −12.5] | <0.001 * [−18.5; −12] | |
| Baseline vs. day 28 p value [CI] | 0.04 * [0.1; 6.7] | 0.2 [−0.9; 5.6] | |
| IL-1β | IL-8 | MCP-1 | MIF | |
|---|---|---|---|---|
| Baseline | ||||
| Placebo mean (sd) | 1.14 (0.51) | 2.25 (0.43) | 1.5 (0.42) | 1.6 (0.81) |
| Probiotics mean (sd) | 1.08 (0.45) | 2.15 (0.38) | 1.38 (0.41) | 1.6 (0.77) |
| Day 14 | ||||
| Placebo mean (sd) | 1.16 (0.45) | 2.23 (0.40) | 1.60 (0.42) | 1.74 (0.79) |
| Baseline vs. day 14 p value [CI] | 0.97 [−0.5; 0.18] | 0.95 [−0.17; 0.13] | 0.31 [−0.06; 0.26] | 0.57 [−0.18; 0.46] |
| Probiotics mean (sd) | 1.09 (0.45) | 2.09 (0.40) | 1.47 (0.40) | 1.49 (0.95) |
| Baseline vs. day 14 p value [CI] | 0.99 [−0.13; 0.15] | 0.44 [−0.19; 0.06] | 0.17 [−0.03; 0.22] | 0.67 [−0.43; 0.2] |
| Between groups p value [CI] | 0.67 [−0.19; 0.12] | 0.24 [−0.22; 0.06] | 0.47 [−0.21; 0.10] | 0.15 [−0.60; 0.10] |
| Day 28 | ||||
| Placebo mean (sd) | 1.12 (0.54) | 2.21 (0.46) | 1.45 (0.40) | 1.68 (0.82) |
| Baseline vs. day 28 p value [CI] | 0.93 [−0.19; 0.14] | 0.77 [−0.19; 0.11] | 0.76 [−0.21; 0.11] | 0.82 [−0.24; 0.4] |
| Probiotics mean (sd) | 1.03 (0.48) | 2.12 (0.48) | 1.42 (0.44) | 1.58 (0.95) |
| Baseline vs. day 28 p value [CI] | 0.65 [−0.19; 0.09] | 0.82 [−0.15; 0.09] | 0.65 [−0.08; 0.17] | 0.98 [−0.34; 0.29] |
| Between groups p value [CI] | 0.60 [−0.22; 0.13] | 0.84 [−0.18; 0.15] | 0.52 [−0.10; 0.20] | 0.52 [−0.42; 0.21] |
| Amylase Activity (Slope/Min) | Total Protease Activity (dF/dT) | Chitinase Activity (Slope/s) | Albumin (µg/mL) | |
|---|---|---|---|---|
| Baseline | ||||
| Placebo mean (sd) | 0.04 (0.03) | 3.12 (0.32) | 0.29 (0.19) | 1.82 (0.31) |
| Probiotics mean (sd) | 0.05 (0.02) | 3.1 (0.22) | 0.34 (0.03) | 1.77 (0.27) |
| Day 14 | ||||
| Placebo mean (sd) | 0.03 (0.01) | 3.10 (0.24) | 0.26 (0.17) | 1.69 (0.25) |
| Baseline vs. day 14 p value [CI] | <0.001 * [−0.03; 0.0006] | 0.84 [−0.14; 0.08] | 0.33 [−0.07; 0.02] | 0.02 * [−0.24; −0.02] |
| Probiotics mean (sd) | 0.04 (0.02) | 3.09 (0.19) | 0.31 (0.19) | 1.70 (0.29) |
| Baseline vs. day 14 p value [CI] | <0.001 * [−0.02; −0.0005] | 0.98 [−0.1; 0.08] | 0.48 [−0.07; 0.02] | 0.26 [−0.17; 0.03] |
|
Between groups p value [CI] | 0.20 [−0.003; 0.01] | 0.99 [−0.09; 0.09] | 0.48 [−0.03; 0.07] | 0.59 [−0.08; 0.15] |
| Day 28 | ||||
| Placebo mean (sd) | 0.04 (0.02) | 3.05 (0.23) | 0.22 (0.16) | 1.60 (0.32) |
| Baseline vs. day 28 p value [CI] | 0.04 * [−0.03; 0.0004] | 0.25 [−0.18; 0.04] | <0.001 * [−0.11; −0.02] | <0.001 * [0.33; −0.11] |
| Probiotics mean (sd) | 0.04 (0.02) | 3.00 (0.23) | 0.25 (0.15) | 1.61 (0.26) |
| Baseline vs. day 14 p value [CI] | 0.009 * [−0.02; −0.002] | 0.03 * [−0.19; −0.007] | <0.001 * [−0.14; −0.04] | 0.001 * [−0.26; −0,05] |
|
Between groups p value [CI] | 0.54 [−0.005; 0.009] | 0.41 [−0.14; 0.06] | 0.96 [−0.04; 0.04] | 0.38 [−0.06; 0.15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundtorp Olsen, C.; Massarenti, L.; Vendius, V.F.D.; Gürsoy, U.K.; Van Splunter, A.; Bikker, F.J.; Gürsoy, M.; Damgaard, C.; Markvart, M.; Belstrøm, D. Probiotics Support Resilience of the Oral Microbiota during Resolution after Experimental Gingivitis—A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2023, 15, 4805. https://doi.org/10.3390/nu15224805
Lundtorp Olsen C, Massarenti L, Vendius VFD, Gürsoy UK, Van Splunter A, Bikker FJ, Gürsoy M, Damgaard C, Markvart M, Belstrøm D. Probiotics Support Resilience of the Oral Microbiota during Resolution after Experimental Gingivitis—A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients. 2023; 15(22):4805. https://doi.org/10.3390/nu15224805
Chicago/Turabian StyleLundtorp Olsen, Christine, Laura Massarenti, Vincent Frederik Dahl Vendius, Ulvi Kahraman Gürsoy, Annina Van Splunter, Floris J. Bikker, Mervi Gürsoy, Christian Damgaard, Merete Markvart, and Daniel Belstrøm. 2023. "Probiotics Support Resilience of the Oral Microbiota during Resolution after Experimental Gingivitis—A Randomized, Double-Blinded, Placebo-Controlled Trial" Nutrients 15, no. 22: 4805. https://doi.org/10.3390/nu15224805
APA StyleLundtorp Olsen, C., Massarenti, L., Vendius, V. F. D., Gürsoy, U. K., Van Splunter, A., Bikker, F. J., Gürsoy, M., Damgaard, C., Markvart, M., & Belstrøm, D. (2023). Probiotics Support Resilience of the Oral Microbiota during Resolution after Experimental Gingivitis—A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients, 15(22), 4805. https://doi.org/10.3390/nu15224805






