The Associations of Breastfeeding Status at 6 Months with Anthropometry, Body Composition, and Cardiometabolic Markers at 5 Years in the Ethiopian Infant Anthropometry and Body Composition Birth Cohort
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Setting and Participants
2.2. Data Collection
2.2.1. Exposure: Breastfeeding Questionnaires at 6 Months
2.2.2. Outcomes: Anthropometry, Body Composition, and Cardiometabolic Markers at 5 Years
2.2.3. Covariates
2.3. Ethical Consideration
2.4. Statistical Analysis
3. Results
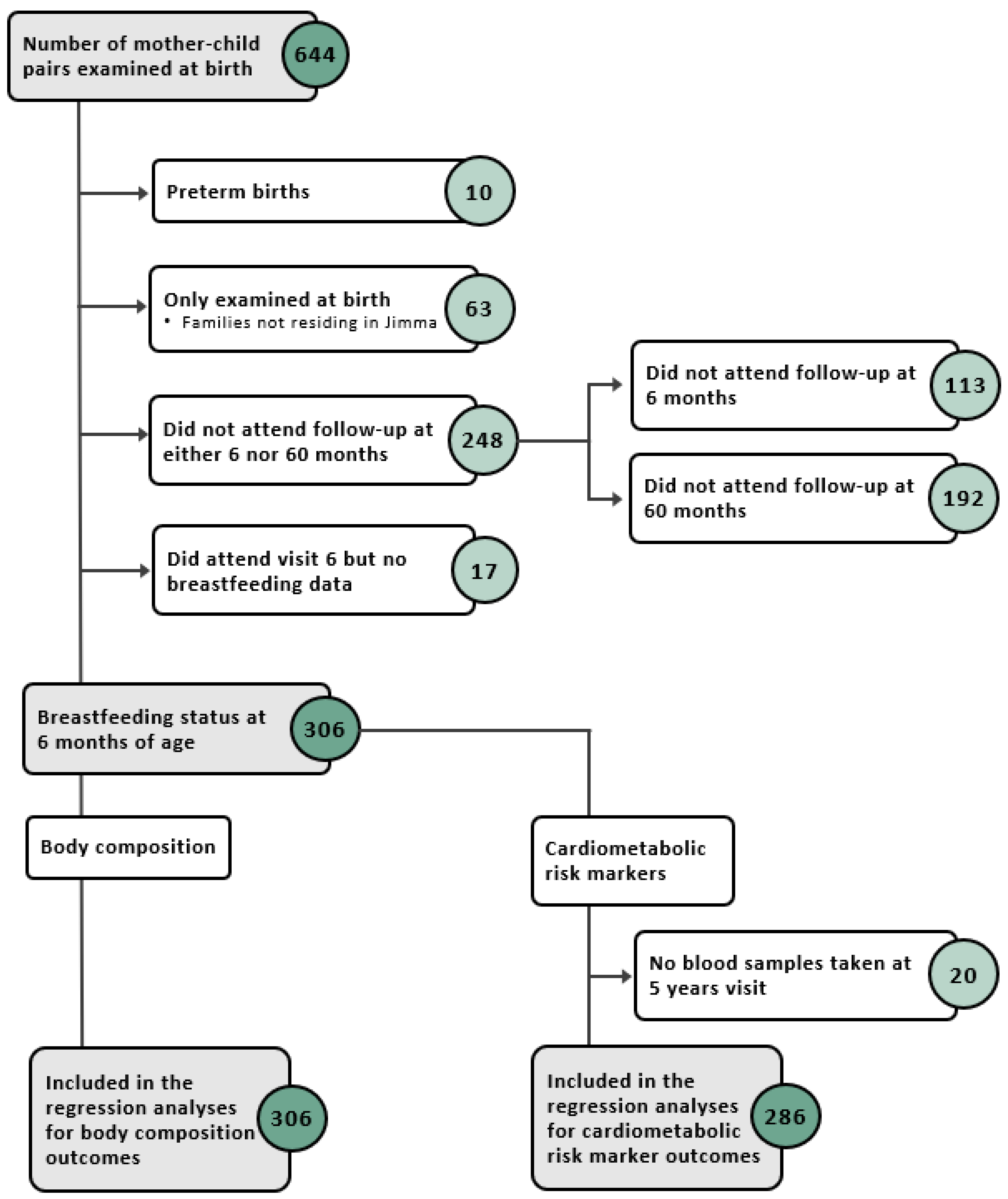
3.1. Study Population
3.2. The Associations of BF Status at 6 Months with Outcomes at 5 Years
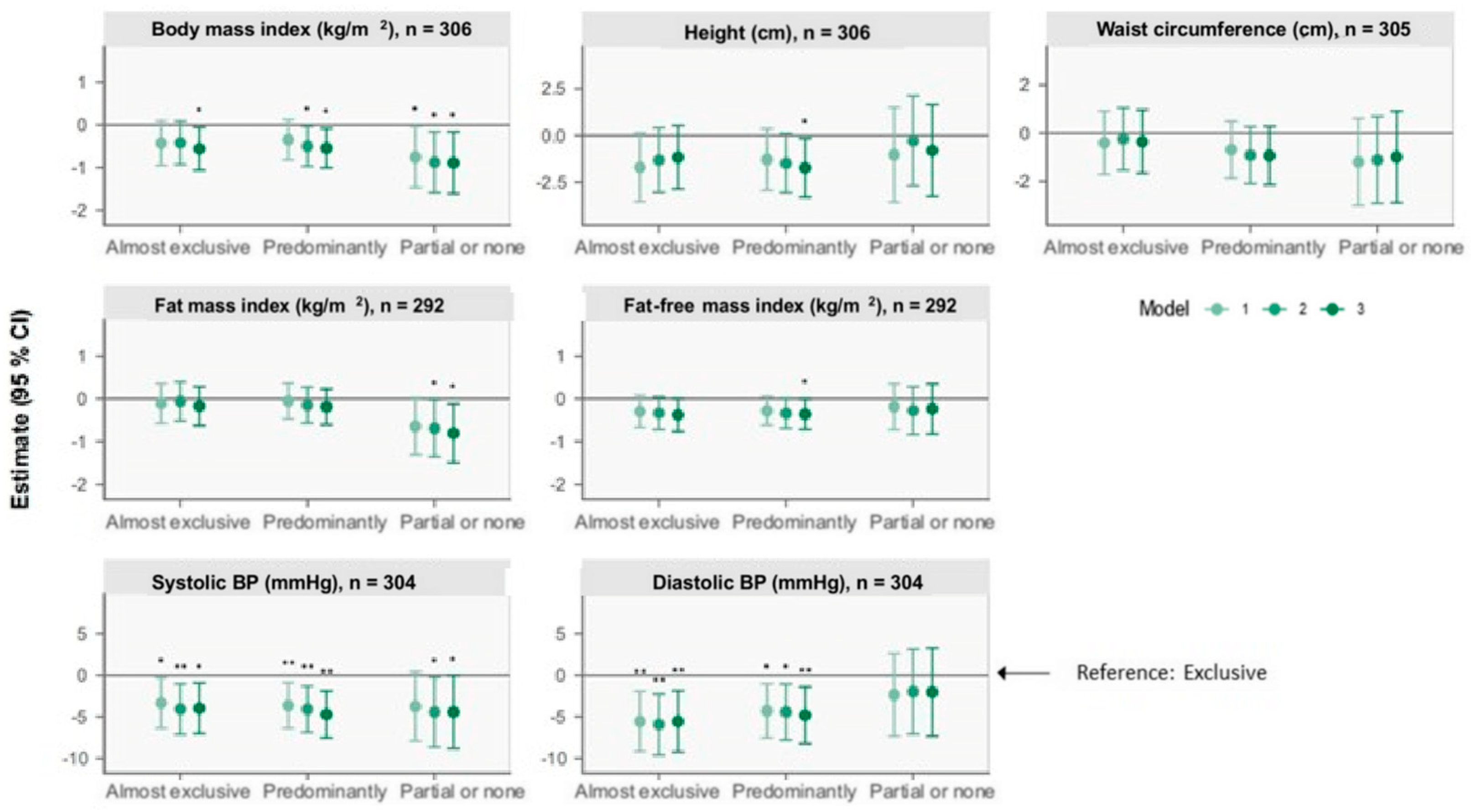
3.2.1. Anthropometry
3.2.2. Body Composition
3.2.3. Blood Pressure
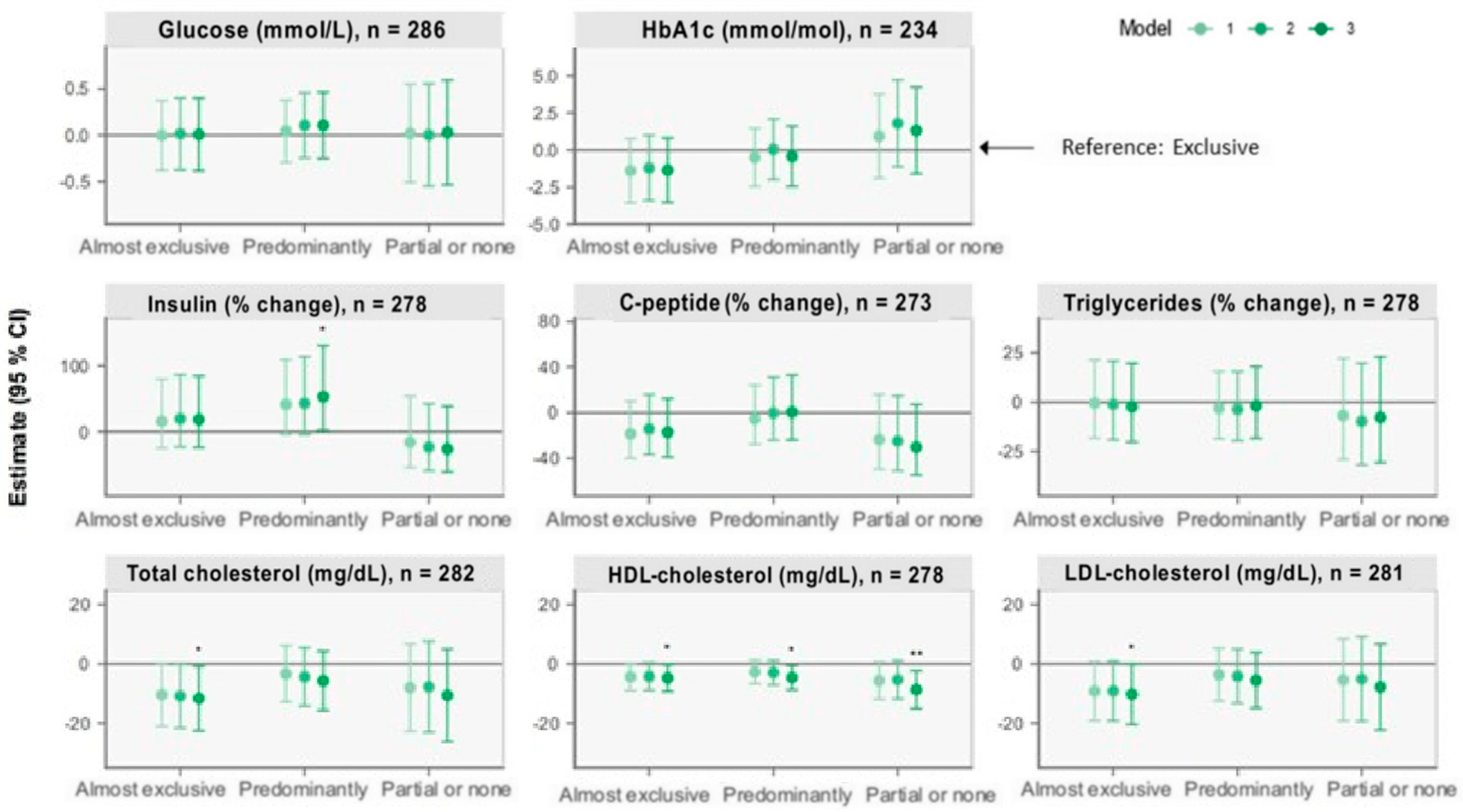
3.2.4. Other Cardiometabolic Markers
4. Discussion
4.1. Anthropometry and Body Composition
4.2. Cardiometabolic Markers and Blood Pressure
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Anthropometry | Body Composition | Blood Pressure | |||||
|---|---|---|---|---|---|---|---|
| Body Mass Index (kg/m2) | Height (cm) | Waist Circumference (cm) | Fat Mass Index (kg/m2) | Fat-Free Mass Index (kg/m2) | Systolic Blood Pressure (mmHg) | Diastolic Blood Pressure (mmHg) | |
| Model 1 | n = 306 | n = 306 | n = 305 | n = 292 | n = 292 | n = 304 | n = 304 |
| Exclusive | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Almost exclusive | −0.43 (−0.95, 0.09) | −1.7 (−3.5, 0.1) | −0.4 (−1.7, 0.9) | −0.11 (−0.57, 0.36) | −0.30 (−0.67, 0.08) | −3 (−6, −0.3) | −6 (−9, −2) |
| Predominantly | −0.35 (−0.82, 0.12) | −1.3 (−2.9, 0.4) | −0.7 (−1.9, 0.5) | −0.06 (−0.47, 0.36) | −0.28 (−0.62, 0.06) | −4 (−6, −0.9) | −4 (−8, −1) |
| Partial or none | −0.76 (−1.47, −0.04) | −1.0 (−3.6, 1.5) | −1.2 (−3.0, 0.6) | −0.65 (−1.31, 0.02) | −0.19 (−0.72, 0.35) | −4 (−8, 0.4) | −2 (−7, 3) |
| Model 2 | n = 300 | n = 300 | n = 299 | n = 286 | n = 286 | n = 298 | n = 298 |
| Exclusive | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Almost exclusive | −0.42 (−0.93, 0.09) | −1.3 (−3.0, 0.4) | −0.2 (−1.5, 1.0) | −0.06 (−0.52, 0.40) | −0.33 (−0.71, 0.05) | −4 (−7, −1) | −6 (−10, −2) |
| Predominantly | −0.50 (−0.97, −0.04) | −1.5 (−3.1, 0.1) | −0.9 (−2.1, 0.3) | −0.15 (−0.57, 0.27) | −0.34 (−0.69, 0.009) | −4 (−7, −1) | −4 (−8, −1) |
| Partial or none | −0.88 (−1.59, −0.17) | −0.3 (−2.7, 2.1) | −1.1 (−2.9, 0.7) | −0.69 (−1.36, −0.02) | −0.27 (−0.83, 0.28) | −4 (−9, −0.2) | −2 (−7, 3) |
| Model 3 | n = 298 | n = 298 | n = 297 | n = 284 | n = 284 | n = 296 | n = 296 |
| Exclusive | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Almost exclusive | −0.56 (−1.06, −0.06) | −1.2 (−2.9, 0.5) | −0.4 (−1.7, 1.0) | −0.17 (−0.63, 0.28) | −0.38 (−0.76, 0.007) | −4 (−7, −0.9) | −6 (−9, −2) |
| Predominantly | −0.55 (−1.01, −0.08) | −1.7 (−3.3, −0.2) | −0.9 (−2.1, 0.3) | −0.19 (−0.60, 0.23) | −0.36 (−0.71, −0.005) | −5 (−8, −2) | −5 (−8, −1) |
| Partial or none | −0.89 (−1.61, −0.17) | −0.8 (−3.3, 1.6) | −1.0 (−2.9, 0.9) | −0.81 (−1.50, −0.12) | −0.23 (−0.82, 0.35) | −4 (−9, −0.06) | −2 (−7, 3) |
| Glucose (mmol/L) | HbA1c (mmol/mol) | Insulin (% Change) | C-Peptide (% Change) | Triglycerides (% Change) | Total Cholesterol (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | n = 286 | n = 234 | n = 278 | n = 273 | n = 278 | n = 282 | n = 278 | n = 281 |
| Exclusive | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Almost exclusive | −0.001 (−0.4, 0.4) | −1.4 (−3.5, 0.8) | 16.03 (−25.05, 79.61) | −18.60 (−39.88, 10.22) | −0.4 (−18.2, 21.2) | −10.4 (−21.0, 0.2) | −4.4 (−8.9, 0.2) | −9.2 (−19.1, 0.8) |
| Predominantly | 0.05 (−0.3, 0.4) | −0.5 (−2.4, 1.5) | 41.56 (−4.19, 109.15) | −5.29 (−27.66, 24.00) | −3.0 (−18.6, 15.5) | −3.3 (−12.8, 6.2) | −2.7 (−6.7, 1.4) | −3.6 (−12.5, 5.3) |
| Partial or none | 0.02 (−0.5, 0.6) | 0.9 (−1.9, 3.8) | −15.91 (−54.08, 53.97) | −23.46 (−49.57, 16.16) | −6.9 (−29.0, 22.1) | −8.0 (−22.7, 6.8) | −5.6 (−11.9, 0.6) | −5.4 (−19.2, 8.4) |
| Model 2 | n = 280 | n = 229 | n = 273 | n = 268 | n = 273 | n = 277 | n = 273 | n = 276 |
| Exclusive | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Almost exclusive | 0.02 (−0.4, 0.4) | −1.2 (−3.4, 1.0) | 19.77 (−23.13, 86.61) | −14.23 (−36.61, 16.06) | −1.1 (−19.0, 20.8) | −10.8 (−21.6, 0.04) | −4.2 (−8.9, 0.4) | −9.1 (−19.2, 1.0) |
| Predominantly | 0.1 (−0.2, 0.5) | 0.06 (−2.0, 2.1) | 43.18 (−4.22, 114.03) | −0.14 (−24.01, 31.24) | −3.6 (−19.5, 15.4) | −4.4 (−14.2, 5.5) | −2.9 (−7.1, 1.2) | −4.1 (−13.3, 5.0) |
| Partial or none | 0.003 (−0.5, 0.6) | 1.8 (−1.1, 4.7) | −23.48 (−59.00, 42.81) | −24.80 (−50.76, 14.83) | −9.6 (−31.7, 19.5) | −7.7 (−23.0, 7.6) | −5.3 (−11.8, 1.2) | −5.1 (−19.3, 9.2) |
| Model 3 | n = 279 | n = 228 | n = 272 | n = 267 | n = 272 | n = 276 | n = 272 | n = 275 |
| Exclusive | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Almost exclusive | 0.009 (−0.4, 0.4) | −1.4 (−3.5, 0.8) | 18.44 (−24.10, 84.83) | −17.06 (−38.90, 12.59) | −2.4 (−20.2, 19.5) | −11.7 (−22.5, −0.8) | −4.7 (−9.3, −0.2) | −10.2 (−20.4, −0.1) |
| Predominantly | 0.1 (−0.3, 0.5) | −0.4 (−2.4, 1.6) | 53.34 (2.01, 130.49) | 0.68 (−23.81, 33.04) | −1.8 (−18.3, 18.0) | −5.7 (−15.7, 4.3) | −4.6 (−8.7, −0.5) | −5.5 (−14.9, 3.8) |
| Partial or none | 0.03 (−0.5, 0.6) | 1.3 (−1.6, 4.2) | −26.16 (−60.80, 39.09) | −30.24 (−54.71, 7.45) | −7.7 (−30.7, 22.9) | −10.6 (−26.2, 4.9) | −8.7 (−15.1, −2.3) | −7.8 (−22.3, 6.7) |
- Text A1. Construction of breastfeeding groups
| Question 1 | Question 2 | Question 3 | |
|---|---|---|---|
| Exclusive | Yes | No | No |
| Almost exclusive | Yes | Yes | No |
| Predominantly | Yes | - | Yes |
| Partial or none | No | - | - |
References
- WHO. Noncommunicable Diseases. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 24 March 2022).
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Double Burden of Malnutrition: Policy Brief; World Health Organization: Geneva, Switzerland, 2016; pp. 1–12. [Google Scholar]
- WHO. More than One in Three Low- and Middle-Income Countries Face Both Extremes of Malnutrition. 2019. Available online: https://www.who.int/news/item/16-12-2019-morethan-one-in-three-low--and-middle-income-countries-face-both-extremes-of-malnutrition (accessed on 21 December 2020).
- Hoffman, D.; Arts, M.; Bégin, F. The “First 1,000 Days+” as Key Contributor to the Double Burden of Malnutrition. Ann. Nutr. Metab. 2019, 75, 99–102. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Prescott, S.L. Developmental origins of health and disease: The role of human milk in preventing disease in the 21st century. J. Hum. Lact. 2013, 29, 123–127. [Google Scholar] [CrossRef]
- Owen, C.G.; Whincup, P.H.; Cook, D.G. Symposium II: Infant and childhood nutrition and disease: Breast-feeding and cardiovascular risk factors and outcomes in later life: Evidence from epidemiological studies. Proc. Nutr. Soc. 2011, 70, 478–484. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Larnkjaer, A.; Molgaard, C. Early diet, insulin-like growth factor-1, growth and later obesity. World Rev. Nutr. Diet. 2013, 106, 113–118. [Google Scholar] [CrossRef]
- Thompson, A.L. Developmental origins of obesity: Early feeding environments, infant growth, and the intestinal microbiome. Am. J. Hum. Biol. 2012, 24, 350–360. [Google Scholar] [CrossRef]
- Przyrembel, H. Timing of introduction of complementary food: Short- and long-term health consequences. Ann. Nutr. Metab. 2012, 60 (Suppl. 2), 8–20. [Google Scholar] [CrossRef] [PubMed]
- AsghariHanjani, N.; Vafa, M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med. J. Islam. Repub. Iran 2019, 33, 56. [Google Scholar] [CrossRef]
- Martin, R.M.; Gunnell, D.; Smith, G.D. Breastfeeding in infancy and blood pressure in later life: Systematic review and meta-analysis. Am. J. Epidemiol. 2005, 161, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Whincup, P.H.; Kaye, S.J.; Martin, R.M.; Smith, G.D.; Cook, D.G.; Bergstrom, E.; Black, S.; Wadsworth, M.E.J.; Fall, C.H.; et al. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am. J. Clin. Nutr. 2008, 88, 305–314. [Google Scholar] [CrossRef]
- Yang, Z.; Huffman, S.L. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern. Child Nutr. 2013, 9 (Suppl. 1), 105–119. [Google Scholar] [CrossRef] [PubMed]
- WHO. Infant and Young Child Feeding. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 21 December 2020).
- Woldeamanuel, B. Trends and factors associated to early initiation of breastfeeding, exclusive breastfeeding and duration of breastfeeding in Ethiopia: Evidence from the Ethiopia Demographic and Health Survey 2016. Int. Breastfeed J. 2020, 15, 3. [Google Scholar] [CrossRef]
- Pretorius, C.E.; Asare, H.; Kruger, H.S.; Genuneit, J.; Siziba, L.P.; Ricci, C. Exclusive breastfeeding, child mortality, and economic cost in Sub-Saharan Africa. Pediatrics 2021, 147, 3. [Google Scholar] [CrossRef]
- Camhi, S.M.; Katzmarzyk, P.T. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int. J. Pediatr. Obes. 2010, 5, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Davey Smith, G.; Cook, D.G. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am. J. Clin. Nutr. 2006, 84, 1043–1054, Erratum in Am. J. Clin. Nutr. 2012, 95, 779. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.H.D.; Borja, J.B.; Osmond, C.; Richter, L.; Bhargava, S.K.; Martorell, R.; Stein, A.D.; Barros, F.C.; Victora, C.G. Infant-feeding patterns and cardiovascular risk factors in young adulthood: Data from five cohorts in low- and middle-income countries. Int. J. Epidemiol. 2011, 40, 47–62. [Google Scholar] [CrossRef]
- Andersen, G.S.; Girma, T.; Wells JC, K.; Kastel, P.; Michaelsen, K.F.; Friis, H. Fat and fat-free mass at birth: Air Displacement plethysmography measurements on 350 ethiopian newborns. Pediatr. Res. 2011, 70, 501–506. [Google Scholar] [CrossRef]
- Wibaek, R.; Vistisen, D.; Girma, T.; Admassu, B.; Abera, M.; Abdissa, A.; Mudie, K.; Kæstel, P.; Jørgensen, M.E.; Wells, J.C.; et al. Body mass index trajectories in early childhood in relation to cardiometabolic risk profile and body composition at 5 years of age. Am. J. Clin. Nutr. 2019, 110, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Bekuma, T.T.; Firrisa, B.; Negero, M.G.; Kejela, G.; Bikila, H. Factors Affecting Choice of Childbirth Place among Childbearing Age Women in Western Ethiopia: A Community-Based Cross-Sectional Study. Int. J. Reprod. Med. 2020, 2020, 4371513. [Google Scholar] [CrossRef]
- Tarekegn, S.M.; Lieberman, L.S.; Giedraitis, V. Determinants of maternal health service utilization in Ethiopia Analysis of the 2011 Ethiopian Demographic and Health Survey. BMC Pregnancy Childbirth 2014, 14, 161. [Google Scholar] [CrossRef]
- The World Bank. GDP per Capita (Current US$)-Ethiopia. 2019. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=ET&most_recent_value_desc=false (accessed on 7 May 2021).
- The World Bank. GDP (Current US$)-Ethiopia. 2019. Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?locations=ET&most_recent_value_desc=false (accessed on 7 May 2021).
- Global Data. GDL Area Database (4.0). 2021. Available online: https://globaldatalab.org/areadata/iwi/ETH/?levels=1%2B2%2B3%2B5%2B4&interpolation=1&extrapolation=1&extrapolation_years=3&nearest_real=0 (accessed on 2 May 2021).
- Labbok, M.; Krasovec, K. Toward Consistency in Breastfeeding Definitions. Stud. Fam. Plan. 1990, 21, 226–230. [Google Scholar] [CrossRef]
- Fields, D.A.; Allison, D.B. Air-displacement plethysmography pediatric option in 2–6 years old using the four-compartment model as a criterion method. Obesity 2012, 20, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.L.; Novak, K.K.; Driver, M. A simplified score for assessment of fetal maturation of newly born infants. J. Pediatr. 1979, 95, 769–774. [Google Scholar] [CrossRef]
- Global Data. International Wealth Index (IWI). 2021. Available online: https://globaldatalab.org/iwi/ (accessed on 2 May 2021).
- Durmus, B.; Heppe, D.H.; Gishti, O.; Manniesing, R.; Abrahamse-Berkeveld, M.; Van Der Beek, E.M.; Hofma, A.; Duijts, L.; Gaillard, R.; Jaddoe, V.W. General and abdominal fat outcomes in school-age children associated with infant breastfeeding patterns. Am. J. Clin. Nutr. 2014, 99, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Falkner, B.; Daniels, S.R. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension 2004, 44, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Janney, C.A.; Zhang, D.; Sowers, M. Lactation and weight retention. Am. J. Clin. Nutr. 1997, 66, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J.; Albertsson-Wikland, K. Growth in full-term small-for-gestational-age infants: From birth to final height. Pediatr. Res. 1995, 38, 733–739. [Google Scholar] [CrossRef]
- Meas, T.; Deghmoun, S.; Armoogum, P.; Alberti, C.; Levy-Marchal, C. Consequences of being born small for gestational age on body composition: An 8-year follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 3804–3809. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N.; Eneh, C.I.; Ndu, I.K. Relationship Between Exclusive Breastfeeding and Lower Risk of Childhood Obesity: A Narrative Review of Published Evidence. Clin. Med. Insights Pediatr. 2017, 11, 1179556517690196. [Google Scholar] [CrossRef] [PubMed]
- WHO. BMI-for-Age (5–19 Years). 2007. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age?fbclid=IwAR3cvF2RXzGFfK5DFbyV15ccDCBNAMUO52cOsoXOC6TrRJKV1wx6PPVOa5Y (accessed on 4 April 2022).
- WHO. Length/Height-for-Age. 2022. Available online: https://www.who.int/tools/child-growth-standards/standards/length-height-for-age (accessed on 4 April 2022).
- Wells, J.C.; Williams, J.E.; Chomtho, S.; Darch, T.; Grijalva-Eternod, C.; Kennedy, K.; Haroun, D.; Wilson, C.; Cole, T.J.; Fewtrell, M.S. Body-composition reference data for simple and reference techniques and a 4-component model: A new UK reference child. Am. J. Clin. Nutr. 2012, 96, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Patel, R.; Kramer, M.S.; Guthrie, L.; Vilchuck, K.; Bogdanovich, N.; Sergeichick, N.; Gusina, N.; Foo, Y.; Palmer, T.; et al. Effects of Promoting Longer-term and Exclusive Breastfeeding on Adiposity and Insulin-like Growth Factor-I at Age 11.5 Years: A Randomized Trial. JAMA 2013, 309, 1005–1013. [Google Scholar] [CrossRef]
- Demerath, E.W.; Fields, D.A. Body composition assessment in the infant. Am. J. Hum. Biol. 2014, 26, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.P.; Hourihane JO, B.; Kenny, L.C.; Irvine, A.D.; Kiely, M.; Murray, D.M. Gender- and gestational age-specific body fat percentage at birth. Pediatrics 2011, 128, e645–e651. [Google Scholar] [CrossRef]
- Harit, D.; Faridi MM, A.; Aggarwal, A.; Sharma, S.B. Lipid profile of term infants on exclusive breastfeeding and mixed feeding: A comparative study. Eur. J. Clin. Nutr. 2008, 62, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Cole, T.J.; Fewtrell, M.; Lucas, A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: Follow-up of a prospective randomised study. Lancet 2004, 363, 1571–1578. [Google Scholar] [CrossRef]
- Stavnsbo, M.; Resaland, G.K.; Anderssen, S.A.; Steene-Johannessen, J.; Domazet, S.L.; Skrede, T.; Sardinha, L.B.; Kriemler, S.; Ekelund, U.; Andersen, L.B.; et al. Reference values for cardiometabolic risk scores in children and adolescents: Suggesting a common standard. Atherosclerosis 2018, 278, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Stanford Children’s Health: Children and Cholesterol. 2021. Available online: https://www.stanfordchildrens.org/en/topic/default?id=cholesterol-ldl-hdl-and-triglycerides-in-children-and-teens-90-P01593&fbclid=IwAR0GSAyVWjcBktuTmYrwMypO4MZ2fFIVquSc0vOQ_O0F2fAA4j1pQBgwxw (accessed on 5 September 2022).
- Balder, J.W.; Lansberg, P.J.; Hof, M.H.; Wiegman, A.; Hutten, B.A.; Kuivenhoven, J.A. Pediatric lipid reference values in the general population: The Dutch lifelines cohort study. J. Clin. Lipidol. 2018, 12, 1208–1216. [Google Scholar] [CrossRef]
- Singhal, A.; Cole, T.J.; Lucas, A. Early nutrition in preterm infants and later blood pressure: Two cohorts after randomised trials. Lancet 2001, 357, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Grijalva-Eternod, C.S.; Lawlor, D.A.; Wells, J.C.K. Testing a Capacity-Load Model for Hypertension: Disentangling Early and Late Growth Effects on Childhood Blood Pressure in a Prospective Birth Cohort. PLoS ONE 2013, 8, e56078. [Google Scholar] [CrossRef] [PubMed]
- Banker, A.; Bell, C.; Gupta-Malhotra, M.; Samuels, J. Blood pressure percentile charts to identify high or low blood pressure in children. BMC Pediatr. 2016, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.L.; Kwok, M.K.; Nelson, E.A.S.; Lee, S.L.; Leung, G.M.; Schooling, C.M. The association of breastfeeding with insulin resistance at 17 years: Prospective observations from Hong Kong’s “Children of 1997” birth cohort. Matern. Child Nutr. 2018, 14, e12490. [Google Scholar] [CrossRef]
- Mengesha, S.D.; Teklu, K.T.; Weldetinsae, A.; Serte, M.G.; Kenea, M.A.; Dinssa, D.A.; Woldegabriel, M.G.; Alemayehu, T.A.; Belay, W.M. Tobacco use prevalence and its determinate factor in Ethiopia- finding of the 2016 Ethiopian GATS. BMC Public Health 2022, 22, 555. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, J.M.; Heisey, D.M. The abuse of power: The pervasive fallacy of power calculations for data analysis. Am. Stat. 2001, 55, 19–24. [Google Scholar] [CrossRef]
| Child Characteristics | |
|---|---|
| Gestational age (weeks), n = 306 | 39.0 (±1.0) |
| Weight (kg), n = 306 | 3.05 (±0.40) |
| Fat mass (%), n = 304 | 7.0 (±4.7) |
| Maternal Characteristics | |
| Age (y), n = 306 | 24.5 (±4.7) |
| Height (cm), n = 306 | 157.4 (±6.1) |
| BMI 2 (kg/m2), n = 300 | 21.95 (±3.63) |
| Education level (%) | |
| No school, n = 22 | 7.2 |
| Some primary school, n = 138 | 45.1 |
| Completed primary school, n = 47 | 15.4 |
| Secondary school, n = 59 | 19.3 |
| Higher education, n = 40 | 13.1 |
| Occupation (%) | |
| Working, n = 92 | 30.3 |
| Housewife, n = 183 | 60.2 |
| Student, n = 25 | 8.2 |
| Other, n = 4 | 1.3 |
| International Wealth Index 3, n = 306 | 45 (±17) |
| Ethnicity (%) | |
| Oromo, n = 150 | 49.3 |
| Amhara, n = 52 | 17.1 |
| Other, n = 102 | 33.6 |
| Anthropometry | |
|---|---|
| BMI (kg/m2), n = 306 | 14.99 (±1.19) |
| Height (cm), n = 306 | 104.3 (±4.4) |
| Waist circumference (cm), n = 305 | 51.4 (±3.0) |
| Body Composition (BOD POD) | |
| Fat mass index (kg/m2), n = 292 | 3.86 (±1.06) |
| Fat-free mass index (kg/m2), n = 292 | 11.15 (±0.85) |
| Blood Pressure, mmHg | |
| Systolic, n = 304 | 88 (±7) |
| Diastolic, n = 304 | 54 (±8) |
| Cardiometabolic Markers | |
| Glucose (mmol/L), n = 286 | 5.9 (±0.8) |
| HbA1c (mmol/mol), n = 234 | 37.6 (±4.3) |
| Insulin (µU/mL), n = 278 | 5.89 (3.27–11.35) |
| C-peptide (ng/mL), n = 273 | 1.06 (0.67–1.54) |
| Triglycerides (mg/dL), n = 278 | 85.5 (64.3–114.8) |
| Total cholesterol (mg/dL), n = 282 | 132.0 (±23.3) |
| HDL (mg/dL), n = 278 | 30.0 (±9.9) |
| LDL (mg/dL), n = 281 | 63.7 (±21.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heltbech, M.S.; Jensen, C.L.; Girma, T.; Abera, M.; Admassu, B.; Kæstel, P.; Wells, J.C.K.; Michaelsen, K.F.; Friis, H.; Andersen, G.S.; et al. The Associations of Breastfeeding Status at 6 Months with Anthropometry, Body Composition, and Cardiometabolic Markers at 5 Years in the Ethiopian Infant Anthropometry and Body Composition Birth Cohort. Nutrients 2023, 15, 4595. https://doi.org/10.3390/nu15214595
Heltbech MS, Jensen CL, Girma T, Abera M, Admassu B, Kæstel P, Wells JCK, Michaelsen KF, Friis H, Andersen GS, et al. The Associations of Breastfeeding Status at 6 Months with Anthropometry, Body Composition, and Cardiometabolic Markers at 5 Years in the Ethiopian Infant Anthropometry and Body Composition Birth Cohort. Nutrients. 2023; 15(21):4595. https://doi.org/10.3390/nu15214595
Chicago/Turabian StyleHeltbech, Mathilde S., Cecilie L. Jensen, Tsinuel Girma, Mubarek Abera, Bitiya Admassu, Pernille Kæstel, Jonathan C. K. Wells, Kim F. Michaelsen, Henrik Friis, Gregers S. Andersen, and et al. 2023. "The Associations of Breastfeeding Status at 6 Months with Anthropometry, Body Composition, and Cardiometabolic Markers at 5 Years in the Ethiopian Infant Anthropometry and Body Composition Birth Cohort" Nutrients 15, no. 21: 4595. https://doi.org/10.3390/nu15214595
APA StyleHeltbech, M. S., Jensen, C. L., Girma, T., Abera, M., Admassu, B., Kæstel, P., Wells, J. C. K., Michaelsen, K. F., Friis, H., Andersen, G. S., & Wibæk, R. (2023). The Associations of Breastfeeding Status at 6 Months with Anthropometry, Body Composition, and Cardiometabolic Markers at 5 Years in the Ethiopian Infant Anthropometry and Body Composition Birth Cohort. Nutrients, 15(21), 4595. https://doi.org/10.3390/nu15214595





