Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Preparation
2.2. Animal and Experimental Design
2.3. Disease Activity Index (DAI) Evaluation
2.4. Histopathology
2.5. Gut Microbiota Analysis
2.6. Untargeted Metabolomics
2.7. Eukaryotic Transcriptomics
2.8. Cytokine Assay
2.9. Data Analysis
3. Results
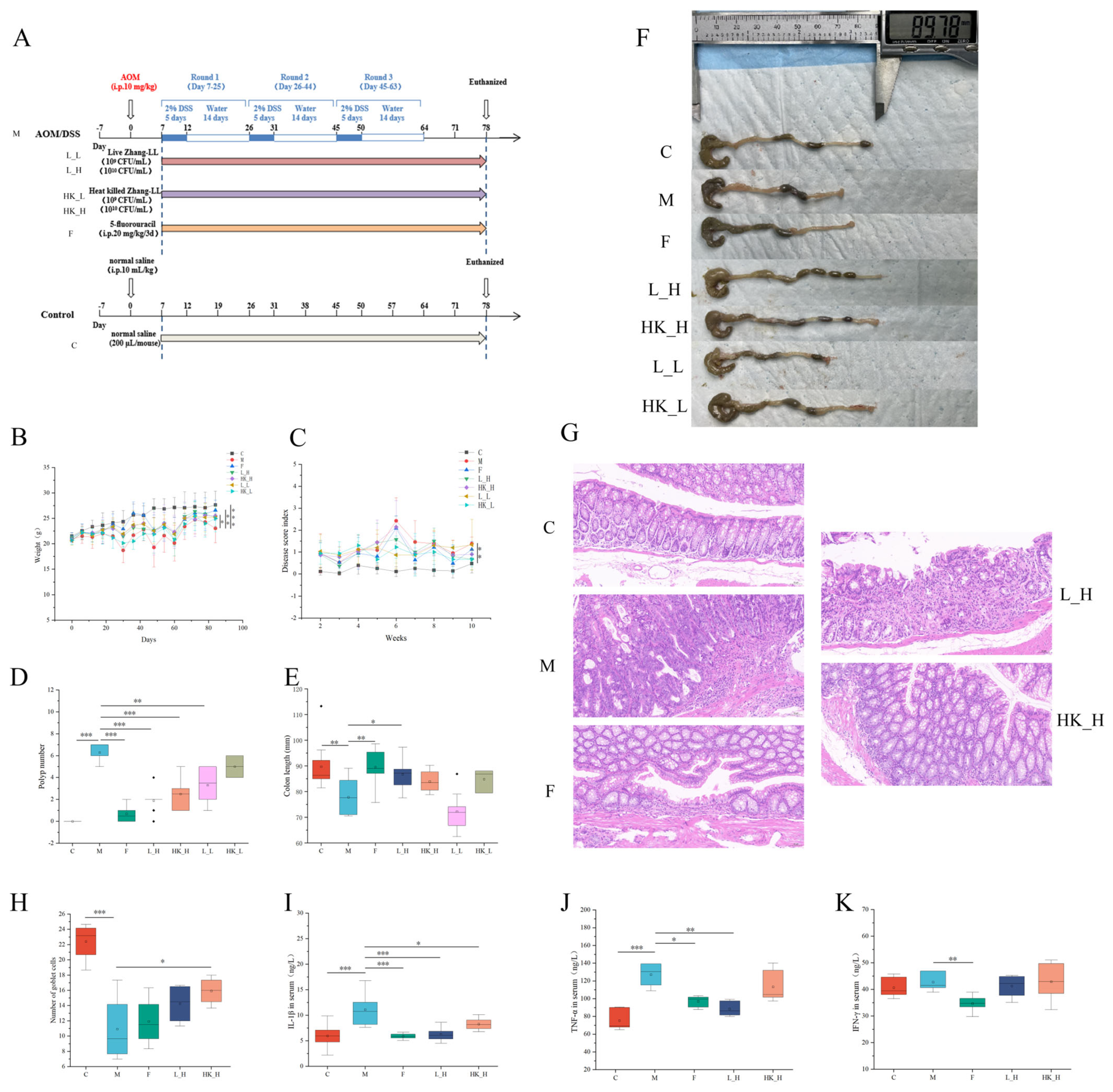
3.1. High Doses of Zhang-LL Bacteria Mitigate CRC Progression in AOM/DSS Mice
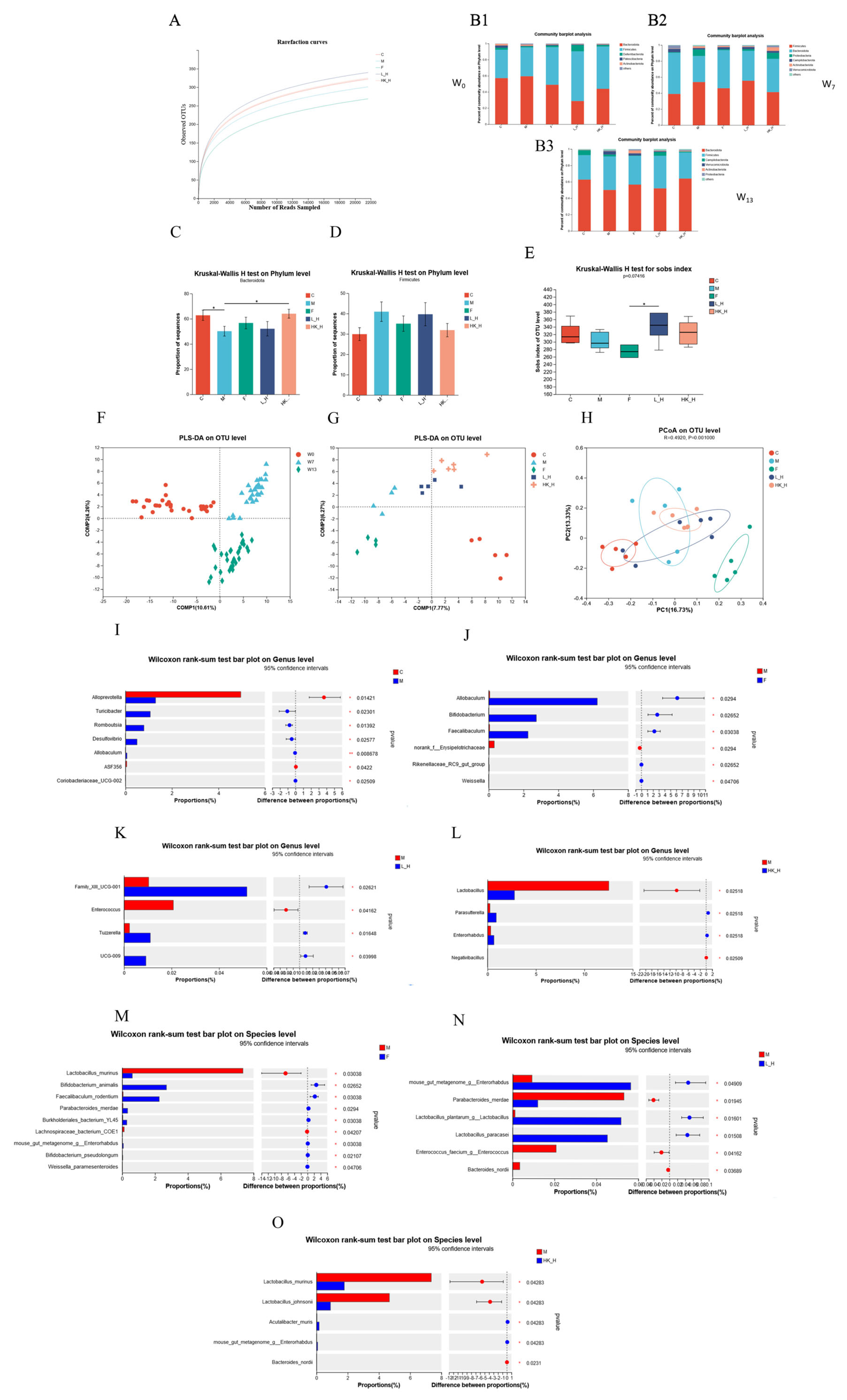
3.2. L_H and HK_H Regulate Gut Microbiota Structure in AOM/DSS Mice
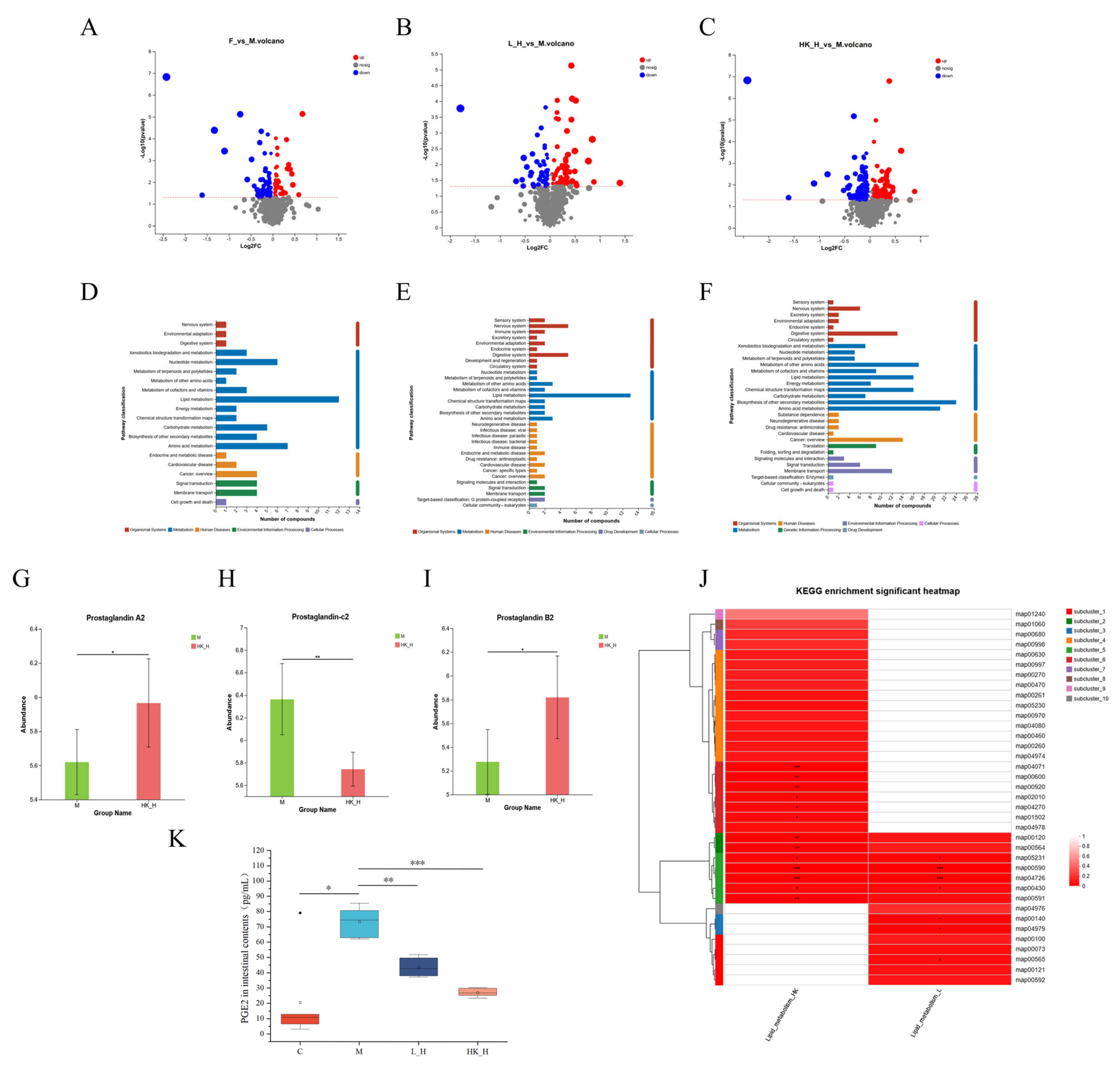
3.3. L_H and HK_H Alter Gut Microbiota-Derived Metabolites in AOM/DSS Mice
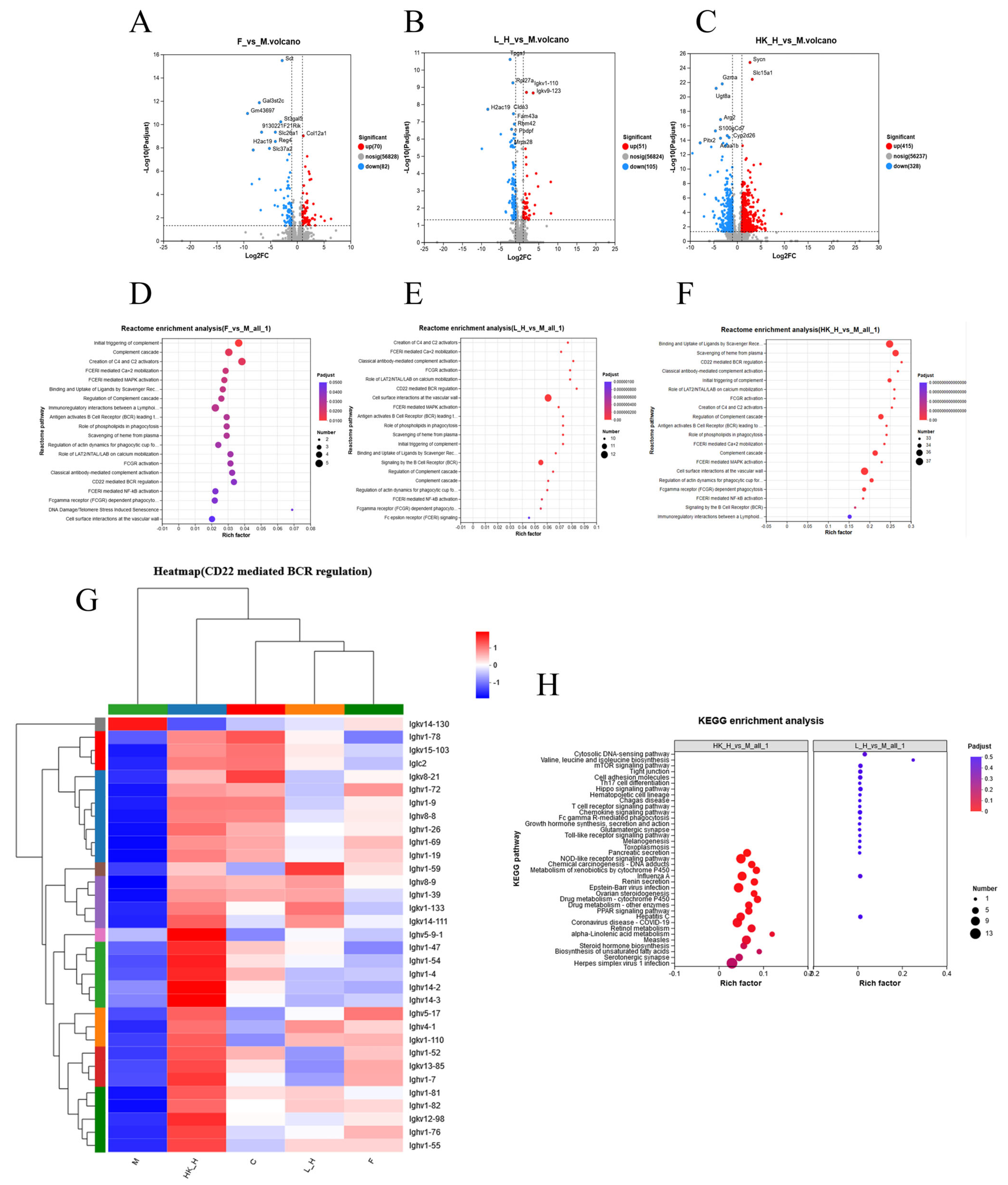
3.4. Effects of L_H and HK_H on Gene Expression in Colon Tissues of AOM/DSS Mice
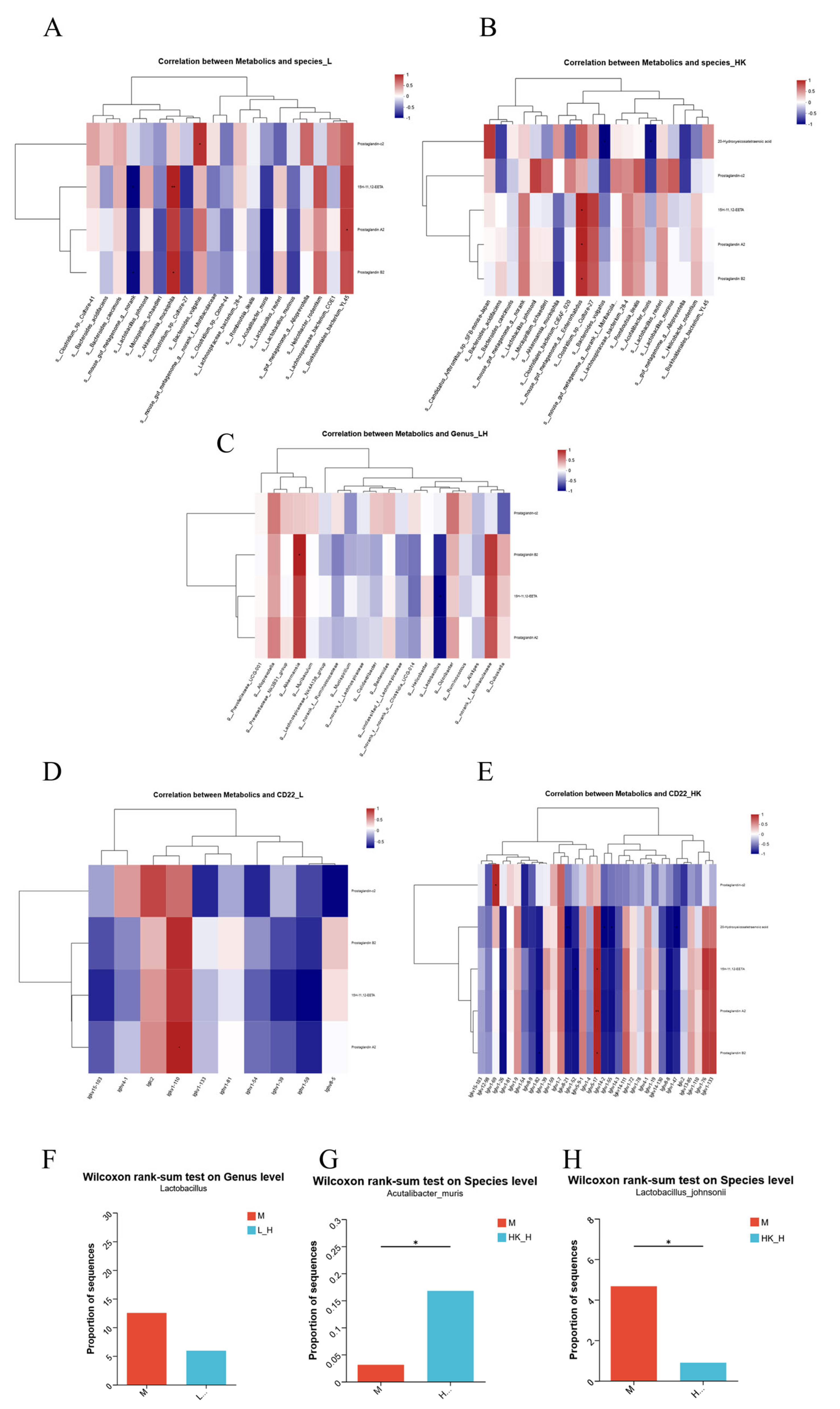
3.5. Multi-Omics Joint Analysis of the Effects of L_H and HK_H on Gut Microbiota, Metabolites, and Gene Expression in AOM/DSS Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Meybodi, M.A.; et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, M.; Wang, K.; Khandpur, N.; Rossato, S.L.; Drouin-Chartier, J.-P.; Steele, E.M.; Giovannucci, E.; Song, M.; Zhang, F.F. Association of ultra-processed food consumption with colorectal cancer risk among men and women: Results from three prospective US cohort studies. BMJ 2022, 378, e068921. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Xiao, J.; Sun, J.; Yu, L.; Zhang, H.; Meng, X.; Yuan, S.; Timofeeva, M.; Law, P.J.; et al. Alcohol consumption, DNA methylation and colorectal cancer risk: Results from pooled cohort studies and Mendelian randomization analysis. Int. J. Cancer 2022, 151, 83–94. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Bai, X.; Wei, H.; Liu, W.; Coker, O.O.; Gou, H.; Liu, C.; Zhao, L.; Li, C.; Zhou, Y.; Wang, G.; et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 2022, 71, 2439–2450. [Google Scholar] [CrossRef]
- Bardou, M.; Rouland, A.; Martel, M.; Loffroy, R.; Barkun, A.N.; Chapelle, N. Review article: Obesity and colorectal cancer. Aliment. Pharmacol. Ther. 2022, 56, 407–418. [Google Scholar] [CrossRef]
- Chen, J.; Chen, N.; Huang, T.; Huang, N.; Zhuang, Z.; Liang, H. Sleep pattern, healthy lifestyle and colorectal cancer incidence. Sci. Rep. 2022, 12, 18317. [Google Scholar] [CrossRef]
- Kastrinos, F.; Samadder, N.J.; Burt, R.W. Use of Family History and Genetic Testing to Determine Risk of Colorectal Cancer. Gastroenterology 2020, 158, 389–403. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, K.C.; Hou, Y.Q.; Guo, M.; Yao, F.; Chen, Z.X. Gut microbiome in tumorigenesis and therapy of colorectal cancer. J. Cell Physiol. 2023, 238, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Gao, F.; Yu, B.; Rao, B.; Sun, Y.; Yu, J.; Wang, D.; Cui, G.; Ren, Z. The effect of the intratumoral microbiome on tumor occurrence, progression, prognosis and treatment. Front. Immunol. 2022, 13, 1051987. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Ge, W.; Shi, C.; Xiao, G.; Wang, X.; Lu, X. The probiotic Companilactobacillus crustorum MN047 alleviates colitis-associated tumorigenesis via modulating the intestinal microenvironment. Food Funct. 2021, 12, 11331–11342. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, H.; Teng, C.; Yang, X.; Qin, P.; Richel, A.; Zhang, L.; Blecker, C.; Ren, G. Supplementation of quinoa peptides alleviates colorectal cancer and restores gut microbiota in AOM/DSS-treated mice. Food Chem. 2023, 408, 135196. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Lian, W.S.; Wang, F.S.; Yang, C.H.; Huang, W.T.; Yang, J.W.; Chen, I.Y.; Yang, M.Y. Presume Why Probiotics May Not Provide Protection in Inflammatory Bowel Disease through an Azoxymethane and Dextran Sodium Sulfate Murine Model. Int. J. Mol. Sci. 2022, 23, 9689. [Google Scholar] [CrossRef]
- Pineiro, M.; Stanton, C. Probiotic bacteria: Legislative framework—Requirements to evidence basis. J. Nutr. 2007, 137, 850S–853S. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Nowak, A.; Zaklos-Szyda, M.; Rosicka-Kaczmarek, J.; Motyl, I. Anticancer Potential of Post-Fermentation Media and Cell Extracts of Probiotic Strains: An In Vitro Study. Cancers 2022, 14, 1853. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Bender, M.J.; McPherson, A.C.; Phelps, C.M.; Pandey, S.P.; Laughlin, C.R.; Shapira, J.H.; Medina Sanchez, L.; Rana, M.; Richie, T.G.; Mims, T.S.; et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 2023, 186, 1846–1862.e26. [Google Scholar] [CrossRef]
- Sun, M.; Liu, W.; Song, Y.; Tuo, Y.; Mu, G.; Ma, F. The Effects of Lactobacillus plantarum-12 Crude Exopolysaccharides on the Cell Proliferation and Apoptosis of Human Colon Cancer (HT-29) Cells. Probiotics Antimicrob. Proteins 2021, 13, 413–421. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Blasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Hao, Y.; Zhong, S.; Liu, H.; Han, T.; Xie, Y. Isolation and partial characterization of a bacteriocin produced by Lactobacillus plantarum BM-1 isolated from a traditionally fermented Chinese meat product. Microbiol. Immunol. 2013, 57, 746–755. [Google Scholar] [CrossRef]
- Jin, J.; Wu, S.; Xie, Y.; Liu, H.; Gao, X.; Zhang, H. Live and heat-killed cells of Lactobacillus plantarum Zhang-LL ease symptoms of chronic ulcerative colitis induced by dextran sulfate sodium in rats. J. Funct. Foods 2020, 71, 103994. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J.; Pang, X.; Bian, Z.; Zhu, J.; Hao, Y.; Zhang, H.; Xie, Y. Plantaricin BM-1 decreases viability of SW480 human colorectal cancer cells by inducing caspase-dependent apoptosis. Front. Microbiol. 2022, 13, 1103600. [Google Scholar] [CrossRef]
- Han, B.; Zhai, Y.; Li, X.; Zhao, H.; Sun, C.; Zeng, Y.; Zhang, W.; Lu, J.; Kai, G. Total flavonoids of Tetrastigma hemsleyanum Diels et Gilg inhibits colorectal tumor growth by modulating gut microbiota and metabolites. Food Chem. 2023, 410, 135361. [Google Scholar] [CrossRef]
- Du, C.; Li, Z.; Zhang, J.; Yin, N.; Tang, L.; Li, J.; Sun, J.; Yu, X.; Chen, W.; Xiao, H.; et al. The protective effect of carnosic acid on dextran sulfate sodium-induced colitis based on metabolomics and gut microbiota analysis. Food Sci. Hum. Wellness 2023, 12, 1212–1223. [Google Scholar] [CrossRef]
- Liu, C.; Huang, S.; Wu, Z.; Li, T.; Li, N.; Zhang, B.; Han, D.; Wang, S.; Zhao, J.; Wang, J. Cohousing-mediated microbiota transfer from milk bioactive components-dosed mice ameliorate colitis by remodeling colonic mucus barrier and lamina propria macrophages. Gut Microbes 2021, 13, 1903826. [Google Scholar] [CrossRef]
- GlobalSurg Collaborative; NIHR Global Health Unit on Global Surgery. Impact of malnutrition on early outcomes after cancer surgery: An international, multicentre, prospective cohort study. Lancet Glob. Health 2023, 11, e341–e349. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Enos, R.T.; Velazquez, K.T.; Bader, J.E.; Carson, M.; Chatzistamou, I.; Walla, M.; Pena, M.M.; Kubinak, J.L.; et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav. Immun. 2019, 80, 44–55. [Google Scholar] [CrossRef]
- Doublier, S.; Cirrincione, S.; Scardaci, R.; Botta, C.; Lamberti, C.; Giuseppe, F.D.; Angelucci, S.; Rantsiou, K.; Cocolin, L.; Pessione, E. Putative probiotics decrease cell viability and enhance chemotherapy effectiveness in human cancer cells: Role of butyrate and secreted proteins. Microbiol. Res. 2022, 260, 127012. [Google Scholar] [CrossRef]
- Kvakova, M.; Kamlarova, A.; Stofilova, J.; Benetinova, V.; Bertkova, I. Probiotics and postbiotics in colorectal cancer: Prevention and complementary therapy. World J. Gastroenterol. 2022, 28, 3370–3382. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, Y.; Yang, B.; Xie, S.; Shen, W.; Wu, Y.; Sui, Z.; Cai, J.; Ni, C.; Ye, J. High-Fat Diet Enhances the Liver Metastasis Potential of Colorectal Cancer through Microbiota Dysbiosis. Cancers 2022, 14, 2573. [Google Scholar] [CrossRef]
- Lee, P.S.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Inhibitory Effect of Garcinol on Obesity-Exacerbated, Colitis-Mediated Colon Carcinogenesis. Mol. Nutr. Food Res. 2021, 65, e2100410. [Google Scholar] [CrossRef]
- Liu, J.; Qi, M.; Qiu, C.; Wang, F.; Xie, S.; Zhao, J.; Wu, J.; Song, X. Integrative analysis of the mouse fecal microbiome and metabolome reveal dynamic phenotypes in the development of colorectal cancer. Front. Microbiol. 2022, 13, 1021325. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Liu, H.M.; Leu, Y.L.; Hsu, C.H.; Lee, T.Y. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, G.; Jeon, B.N.; Fang, S.; Park, H. Bifidobacterium Strain-Specific Enhances the Efficacy of Cancer Therapeutics in Tumor-Bearing Mice. Cancers 2021, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Pan, H.; Li, X.; Gao, Y.; Liu, G.; Xia, L.C. Identifying Gut Microbiota Associated with Colorectal Cancer Using a Zero-Inflated Lognormal Model. Front. Microbiol. 2019, 10, 826. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Deng, M.; Chen, X.; Jiang, L.; Zhang, J.; Tao, L.; Yu, W.; Qiu, Y. Enterococcus faecalis promotes the progression of colorectal cancer via its metabolite: Biliverdin. J. Transl. Med. 2023, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, K.; Liu, P.; Xu, X.; Zhou, Y.; Gan, L.; Yao, L.; Li, B.; Chen, T.; Fang, N. Improvement Effect of Bifidobacterium animalis subsp. lactis MH-02 in Patients Receiving Resection of Colorectal Polyps: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Immunol. 2022, 13, 940500. [Google Scholar] [CrossRef]
- Ghorbani, E.; Avan, A.; Ryzhikov, M.; Ferns, G.; Khazaei, M.; Soleimanpour, S. Role of lactobacillus strains in the management of colorectal cancer: An overview of recent advances. Nutrition 2022, 103–104, 111828. [Google Scholar] [CrossRef]
- Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Tsela, D.; Tiptiri-Kourpeti, A.; Anestopoulos, I.; Kotsianidis, I.; Bezirtzoglou, E.; Pappa, A.; Galanis, A. Lactobacillus paracasei K5 displays adhesion, anti-proliferative activity and apoptotic effects in human colon cancer cells. Benef. Microbes 2018, 9, 975–983. [Google Scholar] [CrossRef]
- Chai, X.; Wen, L.; Song, Y.; He, X.; Yue, J.; Wu, J.; Chen, X.; Cai, Z.; Qi, Z. DEHP exposure elevated cardiovascular risk in obese mice by disturbing the arachidonic acid metabolism of gut microbiota. Sci. Total Environ. 2023, 875, 162615. [Google Scholar] [CrossRef]
- ElKhatib, M.A.W.; Isse, F.A.; El-Kadi, A.O.S. Effect of inflammation on cytochrome P450-mediated arachidonic acid metabolism and the consequences on cardiac hypertrophy. Drug Metab. Rev. 2022, 55, 50–74. [Google Scholar] [CrossRef]
- Ye, Z.; Shen, Y.; Jin, K.; Qiu, J.; Hu, B.; Jadhav, R.R.; Sheth, K.; Weyand, C.M.; Goronzy, J.J. Arachidonic acid-regulated calcium signaling in T cells from patients with rheumatoid arthritis promotes synovial inflammation. Nat. Commun. 2021, 12, 907. [Google Scholar] [CrossRef]
- Sztolsztener, K.; Chabowski, A.; Harasim-Symbor, E.; Bielawiec, P.; Konstantynowicz-Nowicka, K. Arachidonic Acid as an Early Indicator of Inflammation during Non-Alcoholic Fatty Liver Disease Development. Biomolecules 2020, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Gu, L.; Hu, L.; Jiang, C.; Li, Q.; Sun, L.; Zhou, H.; Liu, Y.; Xue, H.; Li, J.; et al. FADS1-arachidonic acid axis enhances arachidonic acid metabolism by altering intestinal microecology in colorectal cancer. Nat. Commun. 2023, 14, 2042. [Google Scholar] [CrossRef]
- Zhang, Z.; Ghosh, A.; Connolly, P.J.; King, P.; Wilde, T.; Wang, J.; Dong, Y.; Li, X.; Liao, D.; Chen, H.; et al. Gut-Restricted Selective Cyclooxygenase-2 (COX-2) Inhibitors for Chemoprevention of Colorectal Cancer. J. Med. Chem. 2021, 64, 11570–11596. [Google Scholar] [CrossRef]
- Lars Nitschke, T.T. Molecular interactions regulate BCR signal inhibition by CD22 and CD72. Trends Immunol. 2004, 25, 543–550. [Google Scholar] [CrossRef]
- Kawasaki, N.; Rademacher, C.; Paulson, J.C. CD22 regulates adaptive and innate immune responses of B cells. J. Innate Immun. 2011, 3, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Fleming, B.D.; Ho, M. Development of Glypican-3 Targeting Immunotoxins for the Treatment of Liver Cancer: An Update. Biomolecules 2020, 10, 934. [Google Scholar] [CrossRef]
- Zaib, T.; Cheng, K.; Liu, T.; Mei, R.; Liu, Q.; Zhou, X.; He, L.; Rashid, H.; Xie, Q.; Khan, H.; et al. Expression of CD22 in Triple-Negative Breast Cancer: A Novel Prognostic Biomarker and Potential Target for CAR Therapy. Int. J. Mol. Sci. 2023, 24, 2152. [Google Scholar] [CrossRef]
- Chen, L.; Xu, B.; Long, X.; Gu, J.; Lou, Y.; Wang, D.; Cao, Y.; Wang, N.; Li, C.; Wang, G.; et al. CAR T-cell therapy for a relapsed/refractory acute B-cell lymphoblastic lymphoma patient in the context of Li-Fraumeni syndrome. J. Immunother. Cancer 2019, 8, e000364. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.A.D.; Akatsu, C.; Abdu-Allah, H.H.M.; Suganuma, Y.; Imamura, A.; Ando, H.; Takematsu, H.; Ishida, H.; Tsubata, T. The Protein Tyrosine Phosphatase SHP-1 (PTPN6) but Not CD45 (PTPRC) Is Essential for the Ligand-Mediated Regulation of CD22 in BCR-Ligated B Cells. J. Immunol. 2021, 206, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-C.; Teng, H.-W.; Shiau, C.-W.; Lin, H.; Hung, M.-H.; Chen, Y.-L.; Huang, J.-W.; Tai, W.-T.; Yu, H.-C.; Chen, K.-F. SHP-1 is a target of regorafenib in colorectal cancer. Oncotarget 2014, 5, 6243–6251. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Liu, H.; Zhao, G.; Chen, A.; Jiang, Y.; Hu, Y.; Liu, D.; Xie, N.; Liang, W.; Chen, X.; et al. LncGMDS-AS1 promotes the tumorigenesis of colorectal cancer through HuR-STAT3/Wnt axis. Cell Death Dis. 2023, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kuai, Y.; Lin, S.; Li, L.; Gu, Q.; Zhang, X.; Li, X.; He, Y.; Chen, S.; Xia, X.; et al. NF-κB Activator 1 downregulation in macrophages activates STAT3 to promote adenoma-adenocarcinoma transition and immunosuppression in colorectal cancer. BMC Med. 2023, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fatima, S.; Chen, M.; Xu, K.; Huang, C.; Gong, R.-H.; Su, T.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Toll-like receptor 4 is a master regulator for colorectal cancer growth under high-fat diet by programming cancer metabolism. Cell Death Dis. 2021, 12, 791. [Google Scholar] [CrossRef]
- Ohashi, K.; Wang, Z.; Yang, Y.M.; Billet, S.; Tu, W.; Pimienta, M.; Cassel, S.L.; Pandol, S.J.; Lu, S.C.; Sutterwala, F.S.; et al. NOD-like receptor C4 Inflammasome Regulates the Growth of Colon Cancer Liver Metastasis in NAFLD. Hepatology 2019, 70, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Ford-Roshon, D.; Russo, M.; O’brien, C.; Xiong, X.; Gurjao, C.; Grandclaudon, M.; Raghavan, S.; Corsello, S.M.; Carr, S.A.; et al. RNF43 G659fs is an oncogenic colorectal cancer mutation and sensitizes tumor cells to PI3K/mTOR inhibition. Nat. Commun. 2022, 13, 3181. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, D.; Wang, T.; Ji, J.; Jin, C.; Peng, C.; Tan, Y.; Zhou, J.; Wang, L.; Feng, Y.; et al. CAF-derived exosomal WEE2-AS1 facilitates colorectal cancer progression via promoting degradation of MOB1A to inhibit the Hippo pathway. Cell Death Dis. 2022, 13, 796. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Hupp, T.; Duchnowska, R.; Marek-Trzonkowska, N.; Połom, K. Next-generation probiotics—Do they open new therapeutic strategies for cancer patients? Gut Microbes 2022, 14, 2035659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Jin, T.; Qin, N.; Ren, X.; Xia, X. Live and pasteurized Akkermansia muciniphila attenuate hyperuricemia in mice through modulating uric acid metabolism, inflammation, and gut microbiota. Food Funct. 2022, 13, 12412–12425. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 2021, 13, 1927633. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, J.; Wu, H.; Yu, D.; Fang, X. Akkermansia muciniphila Aspartic Protease Amuc_1434* Inhibits Human Colorectal Cancer LS174T Cell Viability via TRAIL-Mediated Apoptosis Pathway. Int. J. Mol. Sci. 2020, 21, 3385. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, W.; Wang, Q.; Yang, L.; Bian, X.; Jiang, X.; Lv, L.; Yan, R.; Xia, J.; Han, S.; et al. The negative effect of Akkermansia muciniphila-mediated post-antibiotic reconstitution of the gut microbiota on the development of colitis-associated colorectal cancer in mice. Front. Microbiol. 2022, 13, 932047. [Google Scholar] [CrossRef]
- Garzetti, D.; Jochum, L.; Stecher, B. Acutalibacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Willey: Hoboken, NJ, USA, 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; White, J.R.; Godoy-Vitorino, F.; Rodríguez-Hilario, A.; Navarro, K.; González, H.; Michailidi, C.; Jedlicka, A.; Canapp, S.; Bondy, J.; et al. High-resolution microbiome profiling uncovers Fusobacterium nucleatum, Lactobacillus gasseri/johnsonii, and Lactobacillus vaginalis associated to oral and oropharyngeal cancer in saliva from HPV positive and HPV negative patients treated with surgery and chemo-radiation. Oncotarget 2017, 8, 110931–110948. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gong, Z.; Sun, Z.; Li, J.; Xu, N.; Thorne, R.F.; Zhang, X.D.; Liu, X.; Liu, G. Microbiome and metabolic features of tissues and feces reveal diagnostic biomarkers for colorectal cancer. Front. Microbiol. 2023, 14, 1034325. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Liu, W.; Bian, Z.; Ma, Y.; Kang, Z.; Jin, J.; Li, X.; Ge, S.; Hao, Y.; Zhang, H.; et al. Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation. Nutrients 2023, 15, 4512. https://doi.org/10.3390/nu15214512
Zhu J, Liu W, Bian Z, Ma Y, Kang Z, Jin J, Li X, Ge S, Hao Y, Zhang H, et al. Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation. Nutrients. 2023; 15(21):4512. https://doi.org/10.3390/nu15214512
Chicago/Turabian StyleZhu, Jingxin, Wenbo Liu, Zheng Bian, Yumeng Ma, Zixin Kang, Junhua Jin, Xiangyang Li, Shaoyang Ge, Yanling Hao, Hongxing Zhang, and et al. 2023. "Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation" Nutrients 15, no. 21: 4512. https://doi.org/10.3390/nu15214512
APA StyleZhu, J., Liu, W., Bian, Z., Ma, Y., Kang, Z., Jin, J., Li, X., Ge, S., Hao, Y., Zhang, H., & Xie, Y. (2023). Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation. Nutrients, 15(21), 4512. https://doi.org/10.3390/nu15214512





