Methylliberine Ingestion Improves Various Indices of Affect but Not Cognitive Function in Healthy Men and Women
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Participants
2.3. Neuropsychological Assessments
2.4. Visual-Analog Scales
2.5. Supplement Protocol
2.6. Anthropometric and Other Resting Measures
2.7. Adverse Events (AEs)
2.8. Statistical Analyses
3. Results
3.1. Stroop
3.2. Trail Making Test B (TMT-B)
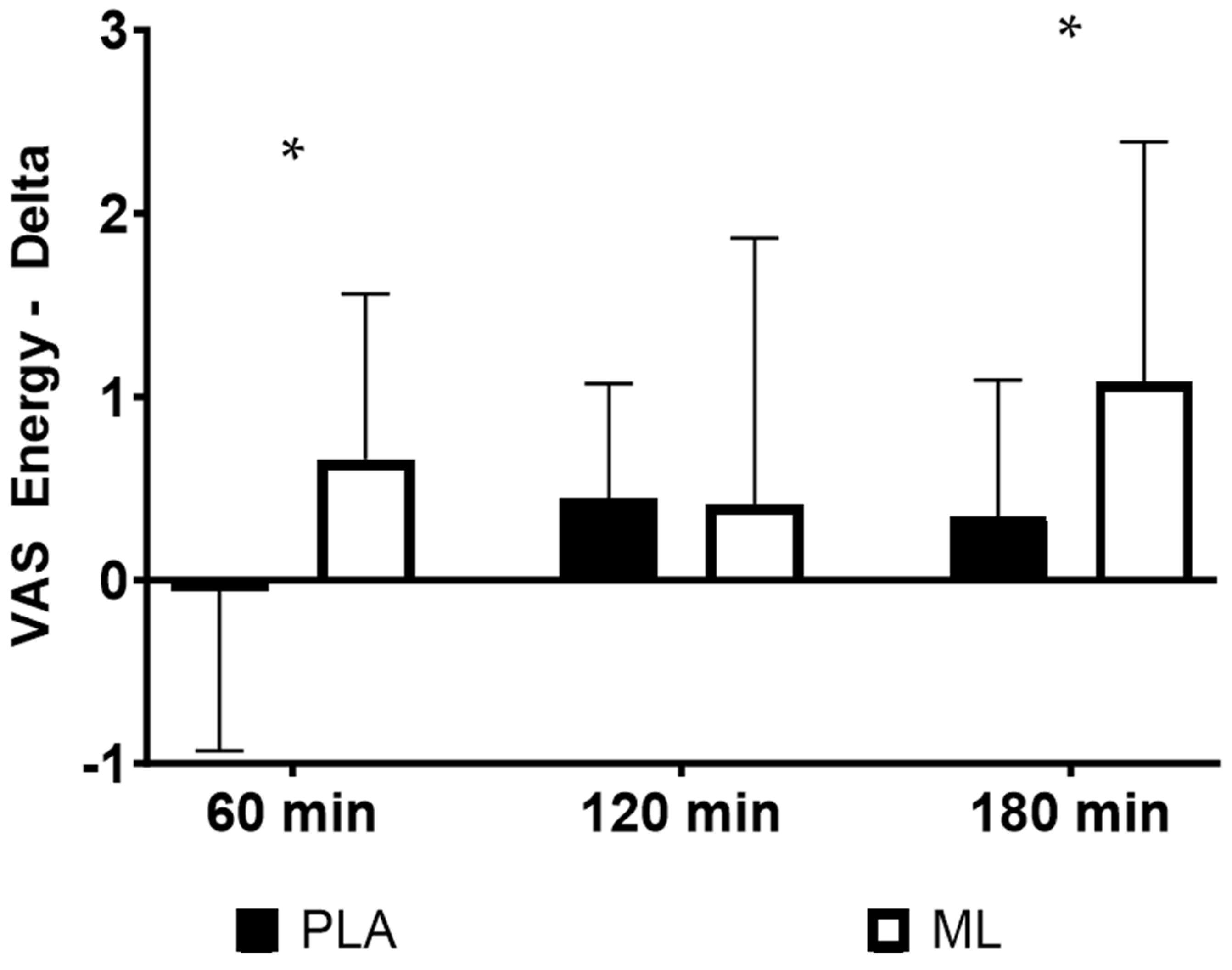
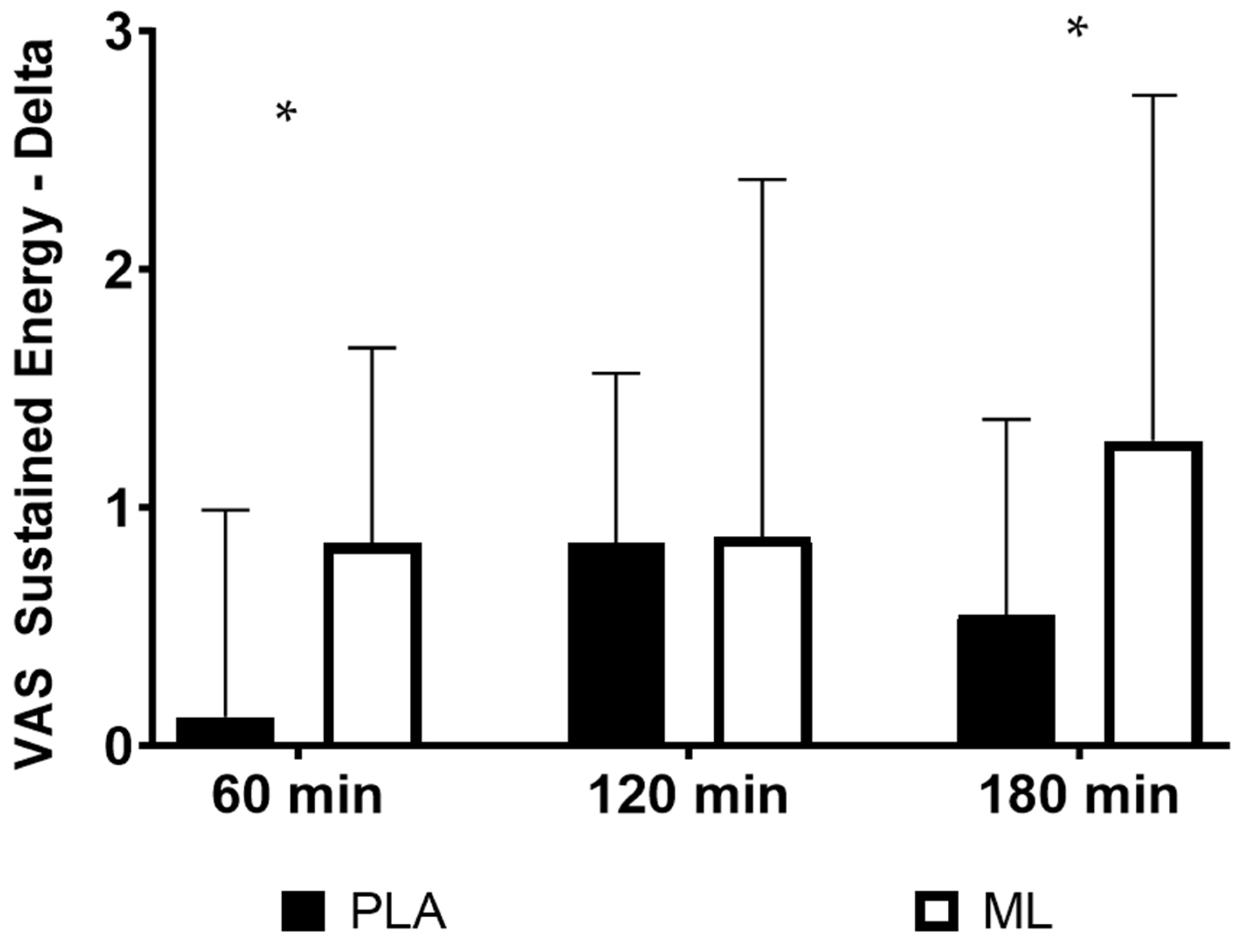
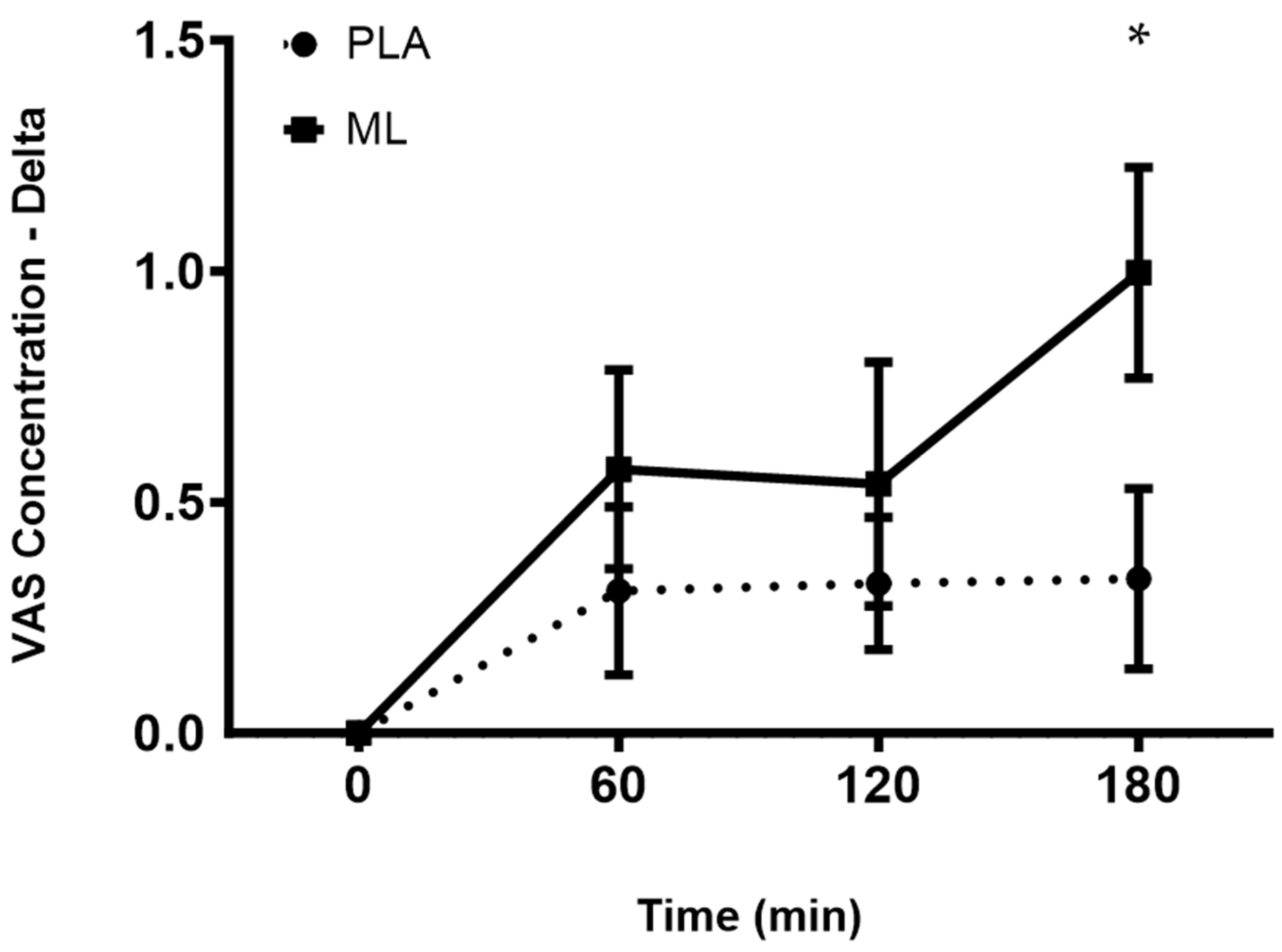
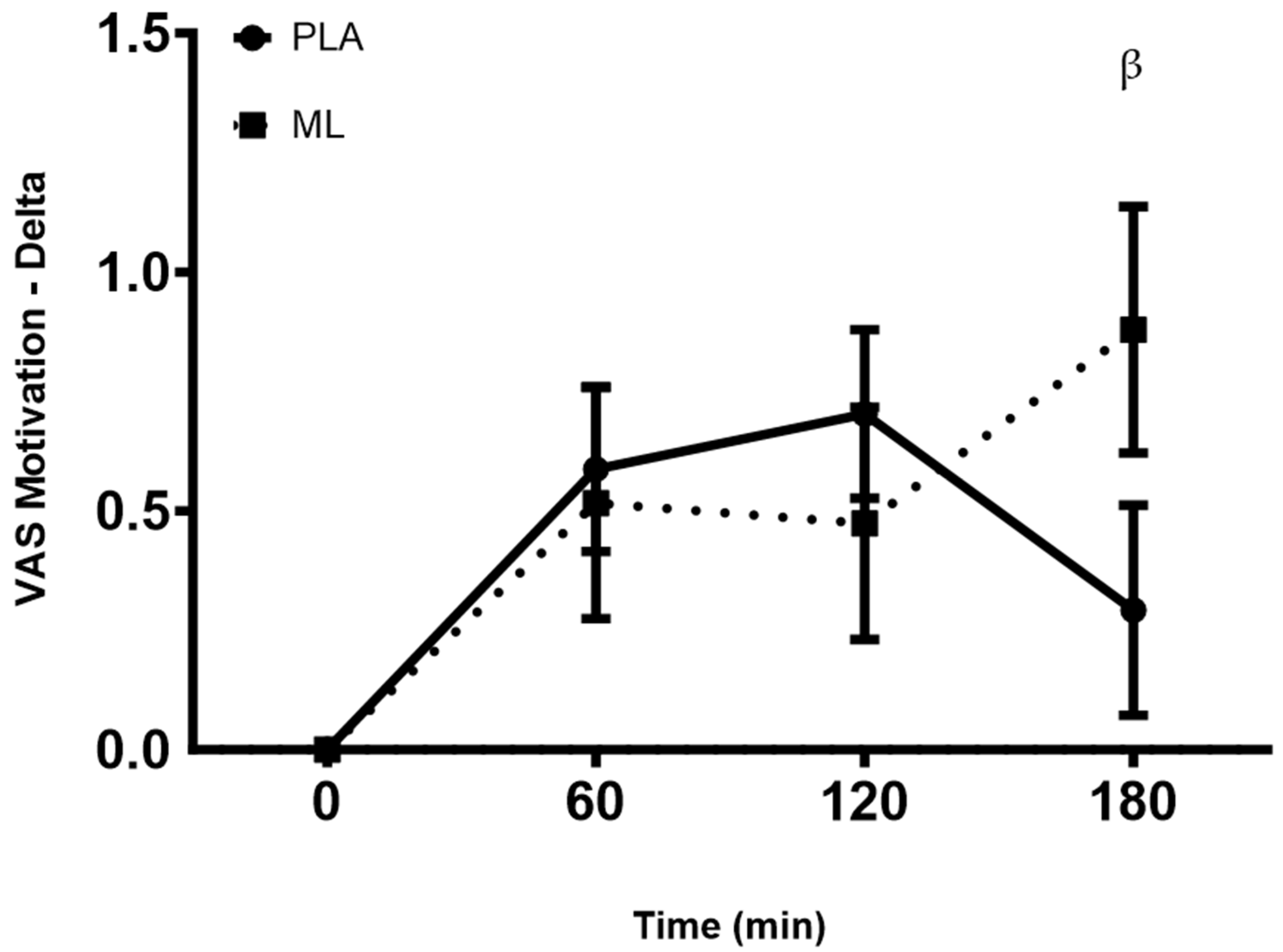
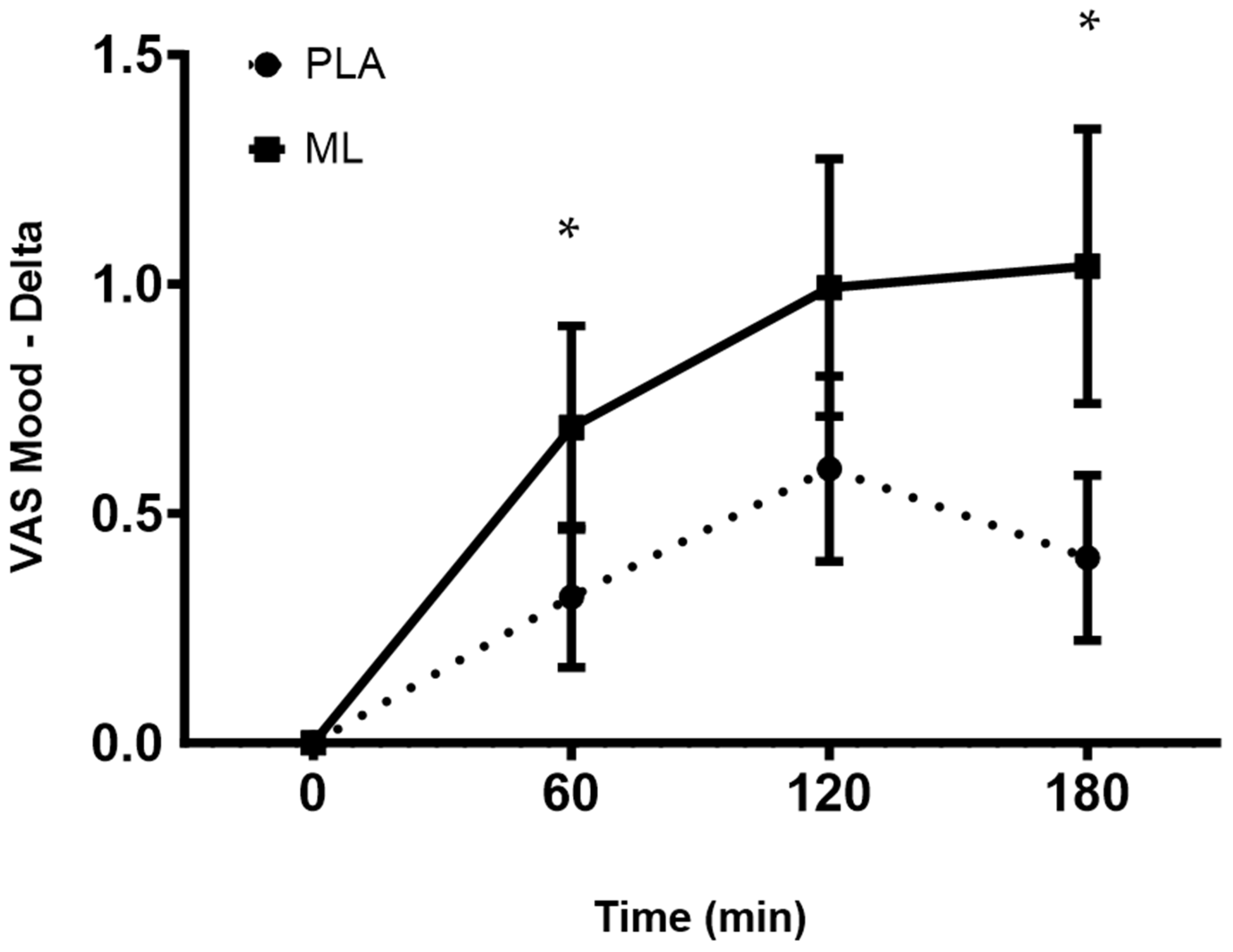
3.3. Visual Analog Scales (VAS)
3.4. Hemodynamics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malík, M.; Tlustoš, P. Nootropics as Cognitive Enhancers: Types, Dosage and Side Effects of Smart Drugs. Nutrients 2022, 14, 3367. [Google Scholar] [CrossRef]
- Pranav, J. A Review on Natural Memory Enhancers (Nootropics). Unique J. Eng. Adv. Sci. 2013, 1, 8–18. [Google Scholar]
- Lorca, C.; Mulet, M.; Arévalo-Caro, C.; Sanchez, M.Á.; Perez, A.; Perrino, M.; Bach-Faig, A.; Aguilar-Martínez, A.; Vilella, E.; Gallart-Palau, X.; et al. Plant-Derived Nootropics and Human Cognition: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 63, 5521–5545. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Cintineo, H.P.; Bello, M.L.; Chandler, A.J.; Cardaci, T.D.; McFadden, B.A.; Arent, S.M. Effects of Caffeine, Methylliberine, and Theacrine on Vigilance, Marksmanship, and Hemodynamic Responses in Tactical Personnel: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2022, 19, 543–564. [Google Scholar] [CrossRef] [PubMed]
- La Monica, M.B.; Listman, J.B.; Donovan, I.; Johnson, T.E.; Heeger, D.J.; Mackey, W.E. Effects of TeaCrine® (Theacrine), DynamineTM (Methylliberine), and Caffeine on Gamer Psychomotor Performance in a First-Person Shooter Video Game Scenario. J. Exerc. Nutr. 2021, 4. [Google Scholar]
- Tartar, J.L.; Banks, J.B.; Marang, M.; Pizzo, F.; Antonio, J.; Tartar, J.L.; Banks, J.B.; Marang, M.; Pizzo, F.; Antonio, J. A Combination of Caffeine, TeaCrine® (Theacrine), and Dynamine® (Methylliberine) Increases Cognitive Performance and Reaction Time Without Interfering With Mood in Adult Male Egamers. Cureus 2021, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.; Butawan, M.; Pence, J. Acute Impact of a Single Dose of Dynamine®, TeaCrine®, Caffeine, and Their Combination on Systemic Hemodynamics and Associated Measures in Men and Women. Med. Res. Arch. 2020, 8, 4. [Google Scholar] [CrossRef]
- VanDusseldorp, T.A.; Stratton, M.T.; Bailly, A.R.; Holmes, A.J.; Alesi, M.G.; Feito, Y.; Mangine, G.T.; Hester, G.M.; Esmat, T.A.; Barcala, M.; et al. Safety of Short-Term Supplementation with Methylliberine (Dynamine®) Alone and in Combination with TeaCrine® in Young Adults. Nutrients 2020, 12, 654. [Google Scholar] [CrossRef]
- Murbach, T.S.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A Toxicological Evaluation of Methylliberine (Dynamine®). J. Toxicol. 2019, 2019, e4981420. [Google Scholar] [CrossRef]
- Harmony, T.; Fernández, T.; Silva, J.; Bernal, J.; Díaz-Comas, L.; Reyes, A.; Marosi, E.; Rodríguez, M.; Rodríguez, M. EEG Delta Activity: An Indicator of Attention to Internal Processing during Performance of Mental Tasks. Int. J. Psychophysiol. 1996, 24, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Nigbur, R.; Ivanova, G.; Stürmer, B. Theta Power as a Marker for Cognitive Interference. Clin. Neurophysiol. 2011, 122, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Mondal, G.; Wang, Y.-H.; Yates, R.; Bloomer, R.; Butawan, M. Caffeine and Methylliberine: A Human Pharmacokinetic Interaction Study: Original Research. J. Exerc. Nutr. 2022, 5. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Mondal, G.; Butawan, M.; Bloomer, R.J.; Yates, C.R. Development of a Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS) Method for Characterizing Caffeine, Methylliberine, and Theacrine Pharmacokinetics in Humans. J. Chromatogr. B 2020, 1155, 122278. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ma, D.; Crone, L.B.; Butawan, M.; Meibohm, B.; Bloomer, R.J.; Yates, C.R. Assessment of the Drug–Drug Interaction Potential Between Theacrine and Caffeine in Humans. J. Caffeine Res. 2017, 7, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Daley; Butts-Lamb, P.; Padgett, W. Subclasses of Adenosine Receptors in the Central Nervous System: Interaction with Caffeine and Related Methylxanthines. Cell. Mol. Neurobiol. 1983, 3, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for Purines and Pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Jensen, A.R.; Rohwer, W.D. The Stroop Color-Word Test: A Review. Acta Psychol. 1966, 25, 36–93. [Google Scholar] [CrossRef]
- Kane, M.J.; Engle, R.W. Working-Memory Capacity and the Control of Attention: The Contributions of Goal Neglect, Response Competition, and Task Set to Stroop Interference. J. Exp. Psychol. Gen. 2003, 132, 47–70. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef]
- Bowie, C.; Harvey, P. Administration and Interpretation of Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Hicks, G.; Nino-Murcia, G. Validity and Reliability of a Scale to Assess Fatigue. Psychiatry Res. 1991, 36, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.L.; Cesareo, K.R.; Raub, B.; Kedia, A.W.; Sandrock, J.E.; Kerksick, C.M.; Ziegenfuss, T.N. Effects of Hemp Extract on Markers of Wellness, Stress Resilience, Recovery and Clinical Biomarkers of Safety in Overweight, But Otherwise Healthy Subjects. J. Diet. Suppl. 2020, 17, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Ziegenfuss, T.N.; Kedia, A.W.; Sandrock, J.E.; Raub, B.J.; Kerksick, C.M.; Lopez, H.L. Effects of an Aqueous Extract of Withania Somnifera on Strength Training Adaptations and Recovery: The STAR Trial. Nutrients 2018, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.L.; Walker, A.J.; McFadden, B.A.; Sanders, D.J.; Arent, S.M. The Effects of TeaCrine® and Caffeine on Endurance and Cognitive Performance during a Simulated Match in High-Level Soccer Players. J. Int. Soc. Sports Nutr. 2019, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, Y.; Dong, C.; Zhou, Z.; Zheng, X. Effects of Various Doses of Caffeine Ingestion on Intermittent Exercise Performance and Cognition. Brain Sci. 2020, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.T.; Holmes, A.J.; Bailly, A.R.; Modjeski, A.S.; Barie, M.; Serafini, P.; Feito, Y.; Mangine, G.T.; Tuggle, K.R.; Esmat, T.A.; et al. A72 Effect of DynamineTM with and without TeaCrine® over Four Weeks of Continuous Use on Cardiovascular Function and Psychometric Parameters: A Pilot Study. In Proceedings of the Fifteenth International Society of Sports Nutrition (ISSN) Conference and Expo, Clearwater Beach, FL, USA, 7–9 June 2018. [Google Scholar]
- Ziegenfuss, T.N.; Habowski, S.M.; Sandrock, J.E.; Kedia, A.W.; Kerksick, C.M.; Lopez, H.L. A Two-Part Approach to Examine the Effects of Theacrine (TeaCrine®) Supplementation on Oxygen Consumption, Hemodynamic Responses, and Subjective Measures of Cognitive and Psychometric Parameters. J. Diet. Suppl. 2017, 14, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Mumford, P.; Roberts, M.; Hayward, S.; Mullins, J.; Urbina, S.; Wilborn, C. Safety of TeaCrine®, a Non-Habituating, Naturally-Occurring Purine Alkaloid over Eight Weeks of Continuous Use. J. Int. Soc. Sports Nutr. 2016, 13, 2. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Marques-Jiménez, D.; Refoyo, I.; Del Coso, J.; León-Guereño, P.; Calleja-González, J. Effect of Caffeine Supplementation on Sports Performance Based on Differences Between Sexes: A Systematic Review. Nutrients 2019, 11, 2313. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Madan, C.R. How Does Caffeine Influence Memory? Drug, Experimental, and Demographic Factors. Neurosci. Biobehav. Rev. 2021, 131, 525–538. [Google Scholar] [CrossRef]
- Sisti, J.S.; Hankinson, S.E.; Caporaso, N.E.; Gu, F.; Tamimi, R.M.; Rosner, B.; Xu, X.; Ziegler, R.; Eliassen, A.H. Caffeine, Coffee, and Tea Intake and Urinary Estrogens and Estrogen Metabolites in Premenopausal Women. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1174–1183. [Google Scholar] [CrossRef]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International Society of Sports Nutrition Position Stand: Caffeine and Exercise Performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- Feduccia, A.A.; Wang, Y.; Simms, J.A.; Yi, H.Y.; Li, R.; Bjeldanes, L.; Ye, C.; Bartlett, S.E. Locomotor Activation by Theacrine, a Purine Alkaloid Structurally Similar to Caffeine: Involvement of Adenosine and Dopamine Receptors. Pharmacol. Biochem. Behav. 2012, 102, 241–248. [Google Scholar] [CrossRef]
- Salamone, J.D.; Farrar, A.M.; Font, L.; Patel, V.; Schlar, D.E.; Nunes, E.J.; Collins, L.E.; Sager, T.N. Differential Actions of Adenosine A1 and A2A Antagonists on the Effort-Related Effects of Dopamine D2 Antagonism. Behav. Brain Res. 2009, 201, 216–222. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Ferrigno, S.; Yang, J.-H.; Rotolo, R.A.; Presby, R.E. The Psychopharmacology of Effort-Related Decision Making: Dopamine, Adenosine, and Insights into the Neurochemistry of Motivation. Pharmacol. Rev. 2018, 70, 747–762. [Google Scholar] [CrossRef] [PubMed]
| Men (N = 12) | Women (N = 13) | |
|---|---|---|
| Age (years) | 33.5 ± 10.7 | 33.5 ± 11.1 |
| Height (cm) | 180.6 ± 7.7 | 170.8 ± 7.2 |
| Weight (kg) | 79.0 ± 8.0 | 68.1 ± 11.1 |
| Body Mass Index (kg/m2) | 24.9 ± 1.4 | 23.9 ± 2.6 |
| Systolic Blood Pressure (mm Hg) | 122.4 ± 8.7 | 113.3 ± 13.5 |
| Diastolic Blood Pressure (mm Hg) | 78.6 ± 9.8 | 76.5 ± 7.8 |
| Resting Heart Rate (bpm) | 66.2 ± 8.7 | 70.0 ± 12.6 |
| Stroop Test | TMT-B | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Score (au) | Accuracy (%) | Average Time per Score (ms) | Time to Completion (s) | |||||
| Time | PLA | ML | PLA | ML | PLA | ML | PLA | ML |
| Day 0 | 125.2 ± 18.4 | 125.0 ± 17.9 | 99.1 ± 1.2 | 99.2 ± 1.1 | 0.99 ± 0.14 | 0.98 ± 0.13 | 22.8 ± 6.1 | 24.3 ± 7.2 |
| 0 min (Day 4) | 124.7 ± 19.2 | 127.4 ± 18.8 | 99.0 ± 1.1 | 99.2 ± 1.3 | 0.99 ± 0.17 | 0.96 ± 0.14 | 21.8 ± 5.5 | 21.6 ± 5.8 ⸸ |
| 60 min (Day 4) | 128.5 ± 17.4 | 131.5 ± 17.5 * | 99.0 ± 1.3 | 99.1 ± 1.8 | 0.95 ± 0.14 | 0.93 ± 0.12 | 21.7 ± 6.0 | 20.4 ± 5.2 |
| 120 min (Day 4) | 131.3 ± 17.0 * | 132.1 ± 16.3 * | 98.8 ± 1.7 | 99.0 ± 1.2 | 0.93 ± 0.12 | 0.92 ± 0.11 * | 21.1 ± 5.4 | 20.2 ± 4.1 |
| 180 min (Day 4) | 131.7 ± 17.7 | 134.8 ± 17.7 * | 98.8 ± 1.3 | 98.9 ± 1.8 | 0.92 ± 0.12 * | 0.91 ± 0.11 * | 19.3 ± 4.0 * | 20.3 ± 3.8 |
| Time | ||||||
|---|---|---|---|---|---|---|
| Group | Day 0 | 0 Min (Day 4) | 60 Min (Day 4) | 120 Min (Day 4) | 180 Min (Day 4) | |
| Energy (cm) | PLA | 5.6 ± 1.9 | 5.6 ± 1.9 | 5.8 ± 2.0 | 6.1 ± 1.8 | 6.1 ± 1.9 * |
| ML | 5.5 ± 2.0 | 5.8 ± 2.1 | 6.2 ± 1.9 | 6.2 ± 2.2 | 6.5 ± 1.9 * | |
| Sustained energy (cm) | PLA | 5.7 ± 1.8 | 5.6 ± 1.9 | 5.8 ± 2.0 | 6.2 ± 1.7 * | 6.1 ± 1.9 * |
| ML | 5.6 ± 2.0 | 5.7 ± 2.1 | 6.2 ± 1.9 * | 6.3 ± 2.2 | 6.5 ± 2.0 * | |
| Mental Stamina (cm) | PLA | 5.8 ± 1.8 | 5.6 ± 2.0 | 6.0 ± 1.9 | 6.1 ± 1.7 * | 6.0 ± 1.9 * |
| ML | 5.7 ± 1.9 | 6.0 ± 1.9 | 6.3 ± 1.9 | 6.3 ± 2.1 | 6.6 ± 1.9 | |
| Focus (cm) | PLA | 5.9 ± 1.9 | 6.0 ± 1.9 | 6.0 ± 2.0 | 6.2 ± 1.8 | 6.2 ± 1.9 |
| ML | 5.9 ± 1.8 | 6.0 ± 2.0 | 6.4 ± 1.8 | 6.5 ± 2.0 | 6.7 ± 2.0 | |
| Drive (cm) | PLA | 5.7 ± 1.9 | 5.7 ± 2.0 | 6.0 ± 1.9 | 6.3 ± 1.7 * | 6.0 ± 1.8 |
| ML | 5.8 ± 1.9 | 5.9 ± 1.9 | 6.1 ± 2.1 | 6.2 ± 2.0 | 6.4 ± 2.1 | |
| Vigor (cm) | PLA | 5.8 ± 1.8 | 5.7 ± 2.0 | 6.1 ± 2.0 | 6.3 ± 1.8 * | 5.9 ± 1.8 |
| ML | 5.8 ± 2.0 | 5.8 ± 2.0 | 6.2 ± 2.0 | 6.3 ± 1.9 | 6.4 ± 2.0 * | |
| Positive Outlook (cm) | PLA | 6.8 ± 1.7 | 6.4 ± 1.9 ⸸ | 6.7 ± 1.8 | 7.0 ± 1.7 * | 6.8 ± 1.9 |
| ML | 6.6 ± 1.9 | 6.4 ± 2.0 | 6.8 ± 1.9 | 7.0 ± 1.7 | 7.0 ± 1.7 * | |
| Well-being (cm) | PLA | 7.0 ± 1.7 | 6.6 ± 1.8 | 6.9 ± 1.8 | 7.0 ± 1.6 | 6.9 ± 1.8 |
| ML | 6.8 ± 2.0 | 6.4 ± 1.7 | 6.8 ± 1.7 | 7.3 ± 1.6 * | 7.2 ± 1.6 * | |
| Ability to Tolerate Stress (cm) | PLA | 6.1 ± 1.9 | 5.8 ± 2.1 | 6.0 ± 2.0 | 6.1 ± 2.0 | 5.8 ± 2.0 |
| ML | 6.1 ± 2.0 | 5.7 ± 2.0 | 6.2 ± 2.1 | 6.4 ± 1.9 * | 6.4 ± 1.9 | |
| Heart Rate (bpm) | SBP (mmHg) | DBP (mmHg) | ||||
|---|---|---|---|---|---|---|
| Time | PLA | ML | PLA | ML | PLA | ML |
| Day 0 | 71.8 ± 13.7 | 71.4 ± 11.9 | 122.0 ± 12.2 | 118.6 ± 12.9 | 77.1 ± 7.1 | 76.6 ± 10.2 |
| 0 min (Day 4) | 71.6 ± 11.4 | 69.8 ± 11.2 | 120.6 ± 12.8 | 121.4 ± 16.0 | 75.3 ± 9.3 | 77.3 ± 10.0 |
| 60 min (Day 4) | 66.5 ± 13.5 | 66.4 ± 11.8 | 118.2 ± 12.5 | 117.9 ± 12.9 | 76.2 ± 9.2 | 76.9 ± 8.8 |
| 120 min (Day 4) | 66.4 ± 13.5 | 64.7 ± 9.9 | 119.2 ± 11.9 | 120.6 ± 13.5 | 79.5 ± 11.7 * | 75.6 ± 9.1 |
| 180 min (Day 4) | 65.7 ± 12.0 | 66.0 ± 10.9 | 121.6 ± 11.7 | 120.0 ± 12.5 | 77.8 ± 9.8 | 77.2 ± 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Monica, M.B.; Raub, B.; Malone, K.; Hartshorn, S.; Grdic, J.; Gustat, A.; Sandrock, J. Methylliberine Ingestion Improves Various Indices of Affect but Not Cognitive Function in Healthy Men and Women. Nutrients 2023, 15, 4509. https://doi.org/10.3390/nu15214509
La Monica MB, Raub B, Malone K, Hartshorn S, Grdic J, Gustat A, Sandrock J. Methylliberine Ingestion Improves Various Indices of Affect but Not Cognitive Function in Healthy Men and Women. Nutrients. 2023; 15(21):4509. https://doi.org/10.3390/nu15214509
Chicago/Turabian StyleLa Monica, Michael B., Betsy Raub, Keeley Malone, Shelley Hartshorn, Jodi Grdic, Ashley Gustat, and Jennifer Sandrock. 2023. "Methylliberine Ingestion Improves Various Indices of Affect but Not Cognitive Function in Healthy Men and Women" Nutrients 15, no. 21: 4509. https://doi.org/10.3390/nu15214509
APA StyleLa Monica, M. B., Raub, B., Malone, K., Hartshorn, S., Grdic, J., Gustat, A., & Sandrock, J. (2023). Methylliberine Ingestion Improves Various Indices of Affect but Not Cognitive Function in Healthy Men and Women. Nutrients, 15(21), 4509. https://doi.org/10.3390/nu15214509





