Sleep-Body Composition Relationship: Roles of Sleep Behaviors in General and Abdominal Obesity in Chinese Adolescents Aged 17–22 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. Sleep Characteristics
2.4. Body Composition Assessment
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. General Characteristics Classified by Gender
3.2. Sleep Characteristics Including Social Jetlag, etc. Classified by Gender
3.3. Body Composition Parameters Classified by Gender
3.4. Multiple Linear Regression Analyses between Sleep Characteristics and Body Composition Indicators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, C.; Yucheng, T.; Shu, L.; Yu, Z. Effects of school-based high-intensity interval training on body composition, cardiorespiratory fitness and cardiometabolic markers in adolescent boys with obesity: A randomized controlled trial. BMC Pediatr. 2022, 22, 112. [Google Scholar] [CrossRef]
- Sun, M.; Feng, W.; Wang, F.; Li, P.; Li, Z.; Li, M.; Tse, G.; Vlaanderen, J.; Vermeulen, R.; Tse, L.A. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 2018, 19, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Sharma, S. Abdominal Obesity, Adipokines and Non-communicable Diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Xie, Q.; Wang, R.; Hu, C.; Zhong, M.; Zou, Y. Waist-to-height ratio and non-alcoholic fatty liver disease in adults. BMC Gastroenterol. 2021, 21, 239. [Google Scholar] [CrossRef]
- Ezzatvar, Y.; Izquierdo, M.; Ramirez-Velez, R.; Del Pozo Cruz, B.; Garcia-Hermoso, A. Accuracy of different cutoffs of the waist-to-height ratio as a screening tool for cardiometabolic risk in children and adolescents: A systematic review and meta-analysis of diagnostic test accuracy studies. Obes. Rev. 2022, 23, e13375. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Liu, Y.; Wang, Y. Effects of different exercise programs on cardiorespiratory fitness and body composition in college students. J. Exerc. Sci. Fit. 2022, 20, 62–69. [Google Scholar] [CrossRef]
- Gotzinger, F.; Santiago-Garcia, B.; Noguera-Julian, A.; Lanaspa, M.; Lancella, L.; Calo Carducci, F.I.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child. Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef]
- Nogueira-de-Almeida, C.A.; Del Ciampo, L.A.; Ferraz, I.S.; Del Ciampo, I.R.L.; Contini, A.A.; Ued, F.D.V. COVID-19 and obesity in childhood and adolescence: A clinical review. J. Pediatr. (Rio. J.) 2020, 96, 546–558. [Google Scholar] [CrossRef]
- Dos Santos, R.R.G.; Forte, G.C.; Mundstock, E.; Amaral, M.A.; da Silveira, C.G.; Amantea, F.C.; Variani, J.F.; Booij, L.; Mattiello, R. Body composition parameters can better predict body size dissatisfaction than body mass index in children and adolescents. Eat. Weight Disord. 2020, 25, 1197–1203. [Google Scholar] [CrossRef]
- Xu, Y.X.; Zhang, A.H.; Yu, Y.; Wan, Y.H.; Tao, F.B.; Sun, Y. Sex-specific association of exposure to bedroom light at night with general and abdominal adiposity in young adults. Ecotoxicol. Environ. Saf. 2021, 223, 112561. [Google Scholar] [CrossRef]
- Gao, M.; Wei, Y.X.; Lyu, J.; Yu, C.Q.; Guo, Y.; Bian, Z.; Pei, P.; Du, H.D.; Chen, J.S.; Chen, Z.M.; et al. The cut-off points of body mass index and waist circumference for predicting metabolic risk factors in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi 2019, 40, 1533–1540. [Google Scholar] [PubMed]
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Stich, F.M.; Huwiler, S.; D’Hulst, G.; Lustenberger, C. The Potential Role of Sleep in Promoting a Healthy Body Composition: Underlying Mechanisms Determining Muscle, Fat, and Bone Mass and Their Association with Sleep. Neuroendocrinology 2022, 112, 673–701. [Google Scholar] [CrossRef]
- Bojanic, D.; Ljubojevic, M.; Krivokapic, D.; Gontarev, S. Waist circumference, waist-to-hip ratio, and waist-to-height ratio reference percentiles for abdominal obesity among Macedonian adolescents. Nutr. Hosp. 2020, 37, 786–793. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Schafer, A.A.; Domingues, M.R.; Dahly, D.L.; Meller, F.O.; Goncalves, H.; Wehrmeister, F.C.; Assuncao, M.C. Sleep Duration Trajectories and Body Composition in Adolescents: Prospective Birth Cohort Study. PLoS ONE 2016, 11, e0152348. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bangdiwala, S.I.; Rangarajan, S.; Lear, S.A.; AlHabib, K.F.; Mohan, V.; Teo, K.; Poirier, P.; Tse, L.A.; Liu, Z.; et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: A study of 116,632 people from 21 countries. Eur. Heart J. 2019, 40, 1620–1629. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Catarozoli, C. Sleep During the Pandemic. Sleep Med. Clin. 2023, 18, 219–224. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alhaj, O.A.; Humood, A.M.; Alenezi, A.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Saif, Z.Q.; Bragazzi, N.L.; Pandi-Perumal, S.R.; BaHammam, A.S.; et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 2022, 62, 101591. [Google Scholar] [CrossRef]
- Vernia, F.; Di Ruscio, M.; Ciccone, A.; Viscido, A.; Frieri, G.; Stefanelli, G.; Latella, G. Sleep disorders related to nutrition and digestive diseases: A neglected clinical condition. Int. J. Med. Sci. 2021, 18, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yang, Y.; Zhang, J.; Liu, Y. The relationship between daytime napping and obesity: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 12124. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Montaruli, A.; Castelli, L.; Mule, A.; Scurati, R.; Esposito, F.; Galasso, L.; Roveda, E. Biological Rhythm and Chronotype: New Perspectives in Health. Biomolecules 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, N.; Schmitting, S.; Kruger, B.; Nothlings, U.; Buyken, A.; Alexy, U. Changes in chronotype and social jetlag during adolescence and their association with concurrent changes in BMI-SDS and body composition, in the DONALD Study. Eur. J. Clin. Nutr. 2022, 76, 765–771. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.M.; Burch, J.B.; Youngstedt, S.D.; Wirth, M.D.; Hardin, J.W.; Hurley, T.G.; Blair, S.N.; Hand, G.A.; Shook, R.P.; Drenowatz, C.; et al. Relationships between chronotype, social jetlag, sleep, obesity and blood pressure in healthy young adults. Chronobiol. Int. 2019, 36, 493–509. [Google Scholar] [CrossRef]
- Lavados-Romo, P.; Andrade-Mayorga, O.; Morales, D.G.; Munoz, D.S.; Balboa-Castillo, D.T. Association of screen time and physical activity with health-related quality of life in college students. J. Am. Coll. Health 2023, 71, 1504–1509. [Google Scholar] [CrossRef]
- Robinson, T.N.; Banda, J.A.; Hale, L.; Lu, A.S.; Fleming-Milici, F.; Calvert, S.L.; Wartella, E. Screen Media Exposure and Obesity in Children and Adolescents. Pediatrics 2017, 140 (Suppl. 2), S97–S101. [Google Scholar] [CrossRef]
- Hjetland, G.J.; Skogen, J.C.; Hysing, M.; Sivertsen, B. The Association Between Self-Reported Screen Time, Social Media Addiction, and Sleep Among Norwegian University Students. Front. Public Health 2021, 9, 794307. [Google Scholar] [CrossRef]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la, O.A.; Dote-Montero, M.; Gutierrez, A.; Castillo, M.J. Association between Sleep Quality and Body Composition in Sedentary Middle-Aged Adults. Medicina 2018, 54, 91. [Google Scholar] [CrossRef]
- Seo, S.H.; Shim, Y.S. Association of Sleep Duration with Obesity and Cardiometabolic Risk Factors in Children and Adolescents: A Population-Based Study. Sci. Rep. 2019, 9, 9463. [Google Scholar] [CrossRef] [PubMed]
- Mongrain, V.; Lavoie, S.; Selmaoui, B.; Paquet, J.; Dumont, M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in Morningness-Eveningness. J. Biol. Rhythms 2004, 19, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Faubel, R.; Leon-Munoz, L.; Zuluaga, M.C.; Banegas, J.R.; Rodriguez-Artalejo, F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am. J. Clin. Nutr. 2008, 87, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pensuksan, W.C.; Lohsoonthorn, V.; Lertmaharit, S.; Gelaye, B.; Williams, M.A. Obstructive Sleep Apnea and Multiple Anthropometric Indices of General Obesity and Abdominal Obesity among Young Adults. Int. J. Soc. Sci. Stud. 2014, 2, 89–99. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, H.; Qian, Y.; Liu, Y.; Zou, J.; Yi, H.; Guan, J.; Yin, S. Abdominal Obesity Is More Strongly Correlated with Obstructive Sleep Apnea than General Obesity in China: Results from Two Separated Observational and Longitudinal Studies. Obes. Surg. 2019, 29, 2535–2547. [Google Scholar] [CrossRef]
- Kail, R. Developmental change in speed of processing during childhood and adolescence. Psychol. Bull. 1991, 109, 490–501. [Google Scholar] [CrossRef]
- Healthy China Action (2019–2030). 2019. Available online: http://www.gov.cn/xinwen/2019-07/15/content_5409694.htm (accessed on 8 August 2023).
- Reed, D.L.; Sacco, W.P. Measuring Sleep Efficiency: What Should the Denominator Be? J. Clin. Sleep. Med. 2016, 12, 263–266. [Google Scholar] [CrossRef]
- Higgins, S.; Stoner, L.; Black, K.; Wong, J.E.; Quigg, R.; Meredith-Jones, K.; Skidmore, P.M. Social jetlag is associated with obesity-related outcomes in 9-11-year-old children, independent of other sleep characteristics. Sleep. Med. 2021, 84, 294–302. [Google Scholar] [CrossRef]
- Suikki, T.; Maukonen, M.; Partonen, T.; Jousilahti, P.; Kanerva, N.; Mannisto, S. Association between social jet lag, quality of diet and obesity by diurnal preference in Finnish adult population. Chronobiol. Int. 2021, 38, 720–731. [Google Scholar] [CrossRef]
- Shuai, M.; Zuo, L.S.; Miao, Z.; Gou, W.; Xu, F.; Jiang, Z.; Ling, C.W.; Fu, Y.; Xiong, F.; Chen, Y.M.; et al. Multi-omics analyses reveal relationships among dairy consumption, gut microbiota and cardiometabolic health. EBioMedicine 2021, 66, 103284. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, L.; Wang, Y.; Li, C.; Zhang, B.; He, J.; Guo, P.; Qi, X.; Zhang, M.; Guo, C.; et al. Gender differences in the association between sleep duration and body mass index, percentage of body fat and visceral fat area among Chinese adults: A cross-sectional study. BMC Endocr. Disord. 2021, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, D.; Partida-Gaytan, A.; Wells, J.C.; Reyes-Delpech, P.; Avila-Rosano, F.; Ortiz-Obregon, M.; Gomez-Mendoza, F.; Diaz-Escobar, L.; Clark, P. Obesogenic Lifestyle and Its Influence on Adiposity in Children and Adolescents, Evidence from Mexico. Nutrients 2020, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Foster, K.E. Ghrelin-leptin tango in body-weight regulation. Gastroenterology 2003, 124, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Capers, P.L.; Fobian, A.D.; Kaiser, K.A.; Borah, R.; Allison, D.B. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes. Rev. 2015, 16, 771–782. [Google Scholar] [CrossRef]
- Patel, S.R.; Malhotra, A.; White, D.P.; Gottlieb, D.J.; Hu, F.B. Association between reduced sleep and weight gain in women. Am. J. Epidemiol. 2006, 164, 947–954. [Google Scholar] [CrossRef]
- Bonsignore, M.R. Obesity and Obstructive Sleep Apnea. Handb. Exp. Pharmacol. 2022, 274, 181–201. [Google Scholar]
- Leinum, C.J.; Dopp, J.M.; Morgan, B.J. Sleep-disordered breathing and obesity: Pathophysiology, complications, and treatment. Nutr. Clin. Pract. 2009, 24, 675–687. [Google Scholar] [CrossRef]
- Miller, M.A.; Kruisbrink, M.; Wallace, J.; Ji, C.; Cappuccio, F.P. Sleep duration and incidence of obesity in infants, children, and adolescents: A systematic review and meta-analysis of prospective studies. Sleep 2018, 41, zsy018. [Google Scholar] [CrossRef]
- Mota, M.C.; Silva, C.M.; Balieiro, L.C.T.; Goncalves, B.F.; Fahmy, W.M.; Crispim, C.A. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLoS ONE 2019, 14, e0212126. [Google Scholar] [CrossRef]
- Bray, M.S.; Young, M.E. Circadian rhythms in the development of obesity: Potential role for the circadian clock within the adipocyte. Obes. Rev. 2007, 8, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.P.; Crowley, S.J.; Alfano, C.A.; Hannay, K.M.; Thompson, D.; Baranowski, T. Potential circadian and circannual rhythm contributions to the obesity epidemic in elementary school age children. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 25. [Google Scholar] [CrossRef]
- McHill, A.W.; Wright, K.P., Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 2017, 18 (Suppl. S1), 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.M.; Hale, L.; Chang, A.M. Social jetlag, eating behaviours and BMI among adolescents in the USA. Br. J. Nutr. 2020, 124, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Zeron-Rugerio, M.F.; Cambras, T.; Izquierdo-Pulido, M. Social Jet Lag Associates Negatively with the Adherence to the Mediterranean Diet and Body Mass Index among Young Adults. Nutrients 2019, 11, 1756. [Google Scholar] [CrossRef]
- Murillo-Castillo, K.D.; Frongillo, E.A.; Corella-Madueno, M.A.; Quizan-Plata, T. Food Insecurity Was Associated with Lower Fruits and Vegetables Consumption but Not with Overweight and Obesity in Children from Mexican Fishing Communities. Ecol. Food Nutr. 2020, 59, 420–435. [Google Scholar] [CrossRef]
- Santos, D.C.D.; Oliveira Filho, J.G.; Sousa, T.L.; Ribeiro, C.B.; Egea, M.B. Ameliorating effects of metabolic syndrome with the consumption of rich-bioactive compounds fruits from Brazilian Cerrado: A narrative review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7632–7649. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Liang, Y.; Xue, H.; Gong, Y.; Xiong, J.; He, F.; Yang, Y.; Cheng, G. Associations between nocturnal sleep duration, midday nap duration and body composition among adults in Southwest China. PLoS ONE 2019, 14, e0223665. [Google Scholar] [CrossRef]
- Yazdanpanah, M.H.; Farjam, M.; Naghizadeh, M.M.; Jedi, F.; Mohebi, K.; Homayounfar, R. Sleep duration and anthropometric indices in an Iranian population: The Fasa PERSIAN cohort study. Sci. Rep. 2021, 11, 16249. [Google Scholar] [CrossRef]
- Loredo, J.S.; Weng, J.; Ramos, A.R.; Sotres-Alvarez, D.; Simonelli, G.; Talavera, G.A.; Patel, S.R. Sleep Patterns and Obesity: Hispanic Community Health Study/Study of Latinos Sueno Ancillar Study. Chest 2019, 156, 348–356. [Google Scholar] [CrossRef]
- Troisi, R.J.; Weiss, S.T.; Parker, D.R.; Sparrow, D.; Young, J.B.; Landsberg, L. Relation of obesity and diet to sympathetic nervous system activity. Hypertension 1991, 17, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zou, J.; Fang, S.; Zhou, J. Association between daytime napping and obesity in Chinese middle-aged and older adults. J. Glob. Health 2020, 10, 020804. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Sanz, J.A.; Tapia-Ayuga, C.E. Blue light emission spectra of popular mobile devices: The extent of user protection against melatonin suppression by built-in screen technology and light filtering software systems. Chronobiol. Int. 2020, 37, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Soltero, E.G.; Navabi, N.; Vander Wyst, K.B.; Hernandez, E.; Castro, F.G.; Ayers, S.L.; Mendez, J.; Shaibi, G.Q. Examining 24-Hour Activity and Sleep Behaviors and Related Determinants in Latino Adolescents and Young Adults with Obesity. Health Educ. Behav. 2022, 49, 291–303. [Google Scholar] [CrossRef]
- Moitra, P.; Madan, J.; Verma, P. Independent and combined influences of physical activity, screen time, and sleep quality on adiposity indicators in Indian adolescents. BMC Public Health 2021, 21, 2093. [Google Scholar] [CrossRef]
- Mineshita, Y.; Kim, H.K.; Chijiki, H.; Nanba, T.; Shinto, T.; Furuhashi, S.; Oneda, S.; Kuwahara, M.; Suwama, A.; Shibata, S. Screen time duration and timing: Effects on obesity, physical activity, dry eyes, and learning ability in elementary school children. BMC Public Health 2021, 21, 422. [Google Scholar] [CrossRef]
| Variables | All | Males | Females | χ2 | p |
|---|---|---|---|---|---|
| Race | 0.113 | 0.737 | |||
| Han | 423 (95.3) | 146 (94.8) | 277 (95.5) | ||
| Minority | 21 (4.7) | 8 (5.2) | 13 (4.5) | ||
| Location | 0.008 | 0.931 | |||
| Rural | 290 (65.3) | 101 (65.6) | 189 (65.2) | ||
| Urban | 154 (34.7) | 53 (34.4) | 101 (34.8) | ||
| The only child | 2.331 | 0.127 | |||
| No | 355 (80.0) | 117 (76.0) | 238 (82.1) | ||
| Yes | 89 (20.0) | 37 (24.0) | 52 (17.9) | ||
| Father’s education | 5.246 | 0.263 | |||
| Below elementary school | 29 (6.5) | 14 (9.1) | 15 (5.2) | ||
| Elementary school | 45 (10.1) | 12 (7.8) | 33 (11.4) | ||
| Junior school | 166 (37.4) | 62 (40.3) | 104 (35.9) | ||
| High school or technical secondary school | 107 (24.1) | 32 (20.8) | 75 (25.9) | ||
| Junior college or above | 97 (21.8) | 34 (22.1) | 63 (21.7) | ||
| Mother’s education | 2.812 | 0.590 | |||
| Below elementary school | 58 (13.1) | 24 (15.6) | 34 (11.7) | ||
| Elementary school | 65 (14.6) | 20 (13.0) | 45 (15.5) | ||
| Junior school | 131 (29.5) | 42 (27.3) | 89 (30.7) | ||
| High school or technical secondary school | 104 (23.4) | 40 (26.0) | 64 (22.1) | ||
| Junior college or above | 86 (19.4) | 28 (18.2) | 58 (20.0) | ||
| Self-rated family income | 2.804 | 0.246 | |||
| Low | 147 (33.1) | 53 (34.4) | 94 (32.4) | ||
| Middle | 270 (60.8) | 88 (57.1) | 182 (62.8) | ||
| High | 27 (6.1) | 13 (8.4) | 14 (4.8) |
| Variables | All | Males | Females | χ2 | p |
|---|---|---|---|---|---|
| Takeaway food consumption/week | 14.944 | 0.001 | |||
| 0 | 141 (31.8) | 42 (27.3) | 99 (34.1) | ||
| 1–2 servings | 188 (42.3) | 53 (34.4) | 135 (46.6) | ||
| >2 servings | 115 (25.9) | 59 (38.3) | 56 (19.3) | ||
| Breakfast consumption/week | 5.369 | 0.020 | |||
| <7 days | 276 (62.2) | 107 (69.5) | 169 (58.3) | ||
| 7 days | 168 (37.8) | 47 (30.5) | 121 (41.7) | ||
| Vegetables consumption/d | 0.737 | 0.391 | |||
| <3 servings | 342 (77.0) | 115 (74.7) | 227 (78.3) | ||
| ≥3 servings | 102 (23.0) | 39 (25.3) | 63 (21.7) | ||
| Fruits consumption/d | 16.067 | <0.001 | |||
| <1 servings | 210 (47.3) | 88 (57.1) | 122 (42.1) | ||
| 1 serving | 126 (28.4) | 42 (27.3) | 84 (29.0) | ||
| >1 servings | 108 (24.3) | 24 (15.6) | 84 (29.0) | ||
| Dried fruits consumption/d | 1.400 | 0.497 | |||
| 0 | 238 (53.6) | 82 (53.2) | 156 (53.8) | ||
| <1 servings | 140 (31.5) | 45 (29.2) | 95 (32.8) | ||
| ≥1 servings | 66 (14.9) | 27 (17.5) | 39 (13.4) | ||
| Pure juice consumption (>250 mL)/d | 11.307 | 0.004 | |||
| 0 | 289 (65.1) | 82 (53.2) | 207 (71.4) | ||
| <1 servings | 115 (25.9) | 48 (31.2) | 67 (23.1) | ||
| ≥1 servings | 40 (9.0) | 24 (15.6) | 16 (5.5) | ||
| Soft drinks consumption (>250 mL)/week | 15.286 | <0.001 | |||
| 0 | 177 (39.9) | 44 (28.6) | 133 (45.9) | ||
| 1 serving | 138 (31.1) | 46 (29.9) | 92 (31.7) | ||
| >1 servings | 129 (29.1) | 64 (41.6) | 65 (22.4) | ||
| Sugar-sweetened beverage consumption (>250 mL)/week | 3.129 | 0.209 | |||
| 0 | 111 (25.0) | 38 (24.7) | 73 (25.2) | ||
| 1 serving | 140 (31.5) | 44 (28.6) | 96 (33.1) | ||
| >1 servings | 193 (43.5) | 72 (46.8) | 121 (41.7) | ||
| Alcohol consumption | 26.115 | <0.001 | |||
| No | 391 (88.1) | 119 (77.3) | 272 (93.8) | ||
| Yes | 53 (11.9) | 35 (22.7) | 18 (6.2) | ||
| Smoking | 7.798 | 0.005 | |||
| No | 436 (98.2) | 147 (95.5) | 289 (99.7) | ||
| Yes | 8 (1.8) | 7 (4.5) | 1 (0.3) | ||
| Duration of physical exercise each time | 13.190 | <0.001 | |||
| <60 min | 411 (92.6) | 133 (86.4) | 278 (95.9) | ||
| ≥60 min | 33 (7.4) | 21 (13.6) | 12 (4.1) | ||
| Number of physical exercise/d | 2.890 | 0.089 | |||
| <1 | 371 (83.6) | 135 (87.7) | 236 (81.4) | ||
| ≥1 | 73 (16.4) | 19 (12.3) | 54 (18.6) | ||
| Weekday screen time/d | 4.031 | 0.133 | |||
| <2 h | 121 (27.3) | 32 (20.8) | 89 (30.7) | ||
| <4 h | 192 (43.2) | 75 (48.7) | 117 (40.3) | ||
| ≥4 h | 131 (29.5) | 47 (30.5) | 84 (29.0) | ||
| Weekend screen time/d | 15.653 | 0.016 | |||
| <2 h | 73 (16.4) | 27 (17.5) | 46 (15.9) | ||
| <4 h | 159 (35.8) | 50 (32.5) | 109 (37.6) | ||
| ≥4 h | 212 (47.7) | 77 (50.0) | 135 (46.6) |
| Variables | All | Males | Females | χ2/t | p |
|---|---|---|---|---|---|
| Weekday nap duration/d | 13.852 | <0.001 | |||
| ≤30 min | 330 (74.3) | 97 (63.0) | 233 (80.3) | ||
| >30 min | 114 (25.7) | 57 (37.0) | 57 (19.7) | ||
| Weekend nap duration/d | 0.338 | 0.561 | |||
| ≤30 min | 234 (52.7) | 79 (51.3) | 155 (53.4) | ||
| >30 min | 210 (47.3) | 75 (48.7) | 135 (46.6) | ||
| Average sleep duration/d | 0.102 | 0.749 | |||
| ≤7 h | 49 (11.0) | 18 (11.7) | 31 (10.7) | ||
| >7 h | 395 (89.0) | 136 (88.3) | 259 (89.3) | ||
| Social jetlag | 5.846 | 0.016 | |||
| ≤1 h | 333 (75.0) | 105 (68.2) | 228 (78.6) | ||
| >1 h | 111 (25.0) | 49 (31.8) | 62 (21.4) | ||
| Average sleep efficiency (%)/d | 95.2 ± 4.3 | 95.2 ± 4.7 | 95.3 ± 4.0 | 0.335 | 0.738 |
| Screen time before sleep (>0.5 h) | 0.043 | 0.836 | |||
| No | 50 (11.3) | 18 (11.7) | 32 (11.0) | ||
| Yes | 394 (88.7) | 136 (88.3) | 258 (89.0) |
| Variables | All | Males | Females | t | p |
|---|---|---|---|---|---|
| Visceral fat area (cm2) | 65.89 ± 25.30 | 56.33 ± 29.40 | 70.96 ± 21.19 | −5.468 | <0.001 |
| Body mass index (kg/m2) | 22.29 ± 3.58 | 22.65 ± 3.78 | 22.10 ± 3.46 | 1.536 | 0.125 |
| Waist to height ratio | 0.47 ± 0.06 | 0.46 ± 0.06 | 0.48 ± 0.05 | −3.221 | 0.001 |
| Waist to hip ratio | 0.83 ± 0.05 | 0.83 ± 0.06 | 0.83 ± 0.04 | 0.337 | 0.737 |
| Fat mass index | 6.33 ± 2.80 | 4.73 ± 2.62 | 7.18 ± 2.51 | −9.637 | <0.001 |
| Fat free mass index | 15.94 ± 2.10 | 17.89 ± 1.75 | 14.90 ± 1.43 | 18.284 | <0.001 |
| Body fat percentage (%) | 27.65 ± 8.44 | 20.00 ± 6.83 | 31.72 ± 6.06 | −18.554 | <0.001 |
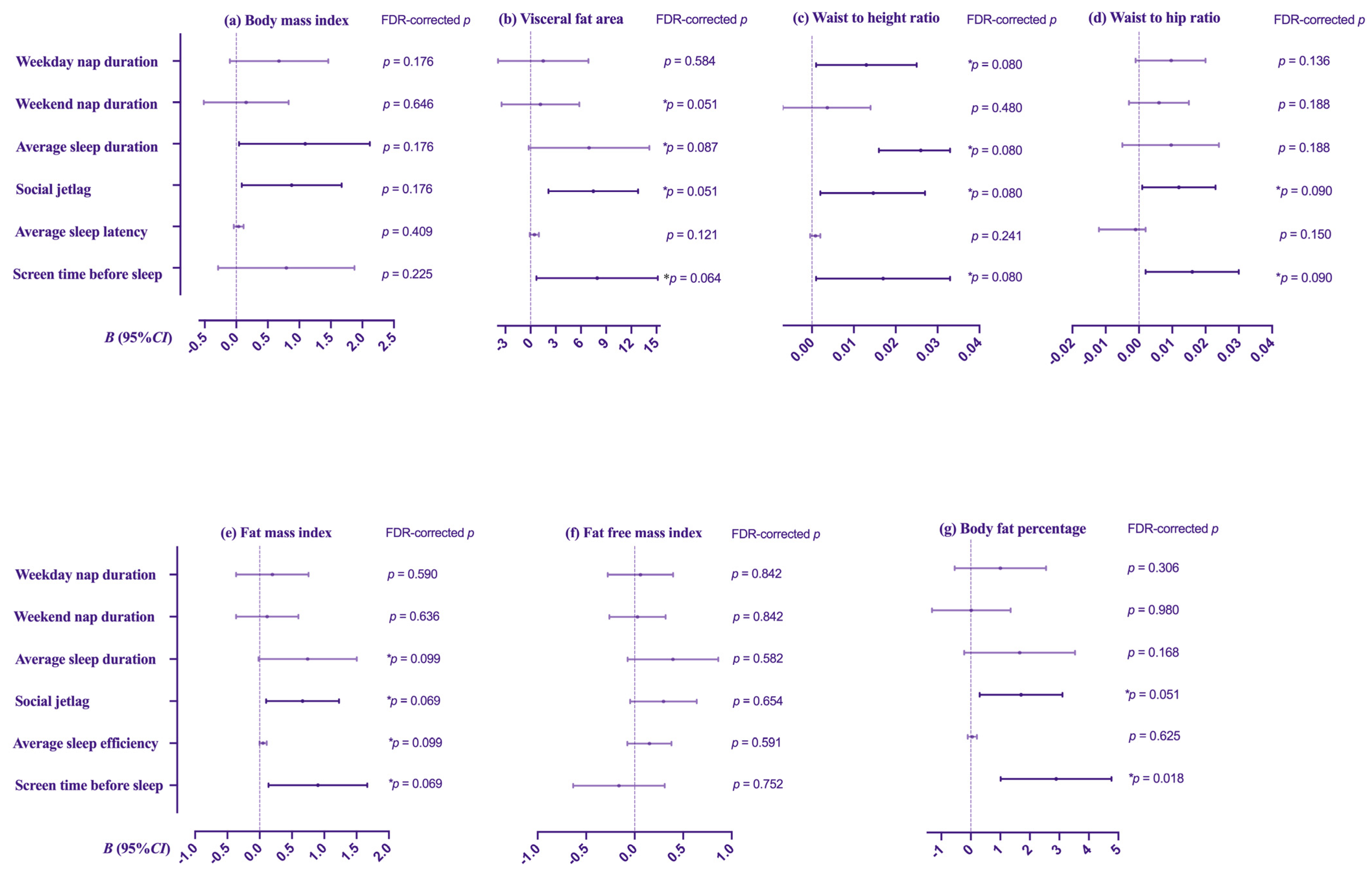
| Variables | Visceral Fat Area (cm2) | Body Mass Index (kg/m2) | Waist to Height Ratio | Waist to Hip Ratio | ||||||||||||
| Model 1 B (95%CI) | Model 2 B (95%CI) | Model 1 B (95%CI) | Model 2 B (95%CI) | Model 1 B (95%CI) | Model 2 B (95%CI) | Model 1 B (95%CI) | Model 2 B (95%CI) | |||||||||
| Weekday nap duration/d | 1.258 | (−4.148, 6.664) | 1.500 | (−3.885, 6.884) | 0.879 | (0.119, 1.640) | 0.678 | (−0.102, 1.458) | 0.011 | (<0.001, 0.023) | 0.013 | (0.001, 0.025) | 0.011 | (0.001, 0.022) | 0.010 | (−0.001, 0.020) |
| Weekend nap duration/d | 0.964 | (−3.765, 5.692) | 1.181 | (−3.451, 5.812) | 0.250 | (−0.419, 0.918) | 0.158 | (−0.515, 0.830) | 0.005 | (−0.006, 0.015) | 0.004 | (−0.007, 0.014) | 0.007 | (−0.002, 0.016) | 0.006 | (−0.003, 0.015) |
| Average sleep duration | 7.338 | (−0.170, 14.846) | 6.963 | (−0.227, 14.152) | 1.172 | (0.112, 2.232) | 1.117 | (0.045, 2.188) | 0.017 | (<0.001, 0.033) | 0.016 | (0.029, 0.033) | 0.011 | (−0.004, 0.025) | 0.010 | (−0.005, 0.024) |
| Social jetlag | 4.860 | (−0.577, 10.296) | 7.475 | (2.137, 12.813) | 0.838 | (0.071, 1.605) | 0.878 | (0.086, 1.671) | 0.011 | (−0.001, 0.023) | 0.015 | (0.002, 0.027) | 0.012 | (0.002, 0.022) | 0.012 | (0.001, 0.023) |
| Average sleep efficiency/d | 0.386 | (−0.167, 0.940) | 0.449 | (−0.088, 0.987) | 0.035 | (−0.044, 0.113) | 0.038 | (−0.040, 0.116) | 0.001 | (−0.001, 0.002) | 0.001 | (<0.001, 0.002) | 0.001 | (<0.001, 0.002) | 0.001 | (<0.001, 0.002) |
| Screen time before sleep (>0.5 h) | 6.032 | (−1.420, 13.483) | 7.934 | (0.700, 15.167) | 0.568 | (−0.487, 1.624) | 0.794 | (−0.288, 1.876) | 0.015 | (−0.001, 0.031) | 0.017 | (0.001, 0.033) | 0.015 | (0.001, 0.029) | 0.016 | (0.002, 0.030) |
| Variables | Fat mass index | Free fat mass index | Body fat percentage (%) | |||||||||||||
| model 1 B (95%CI) | model 2 B (95%CI) | model 1 B (95%CI) | model 2 B (95%CI) | model 1 B (95%CI) | model 2 B (95%CI) | |||||||||||
| Weekday nap duration/d | 0.267 | (−0.327, 0.861) | 0.196 | (−0.364, 0.757) | 0.673 | (0.127, 1.019) | 0.060 | (−0.277, 0.397) | 0.945 | (−0.858, 2.747) | 1.003 | (−0.545, 2.551) | ||||
| Weekend nap duration/d | 0.069 | (−0.451, 0.588) | 0.116 | (−0.366, 0.599) | 0.099 | (−0.293, 0.492) | 0.029 | (−0.260, 0.319) | 0.201 | (−1.378, 1.779) | 0.017 | (−1.317, 1.351) | ||||
| Average sleep duration | 0.747 | (−0.084, 1.577) | 0.743 | (−0.018, 1.503) | 0.452 | (−0.173, 1.078) | 0.395 | (−0.072, 0.862) | 1.632 | (−0.878, 4.142) | 1.655 | (−0.223, 3.534) | ||||
| Social jetlag | 0.289 | (−0.313, 0.892) | 0.663 | (0.099, 1.228) | 0.616 | (0.166, 1.066) | 0.297 | (−0.046, 0.640) | 0.094 | (−1.725, 1.914) | 1.703 | (0.301, 3.105) | ||||
| Average sleep efficiency/d | 0.045 | (−0.016, 0.105) | 0.052 | (−0.003, 0.108) | 0.011 | (−0.035, 0.057) | 0.152 | (−0.076, 0.380) | 0.025 | (−0.160, 0.210) | 0.051 | (−0.104, 0.206) | ||||
| Screen time before sleep (>0.5 h) | 0.734 | (−0.090, 1.557) | 0.902 | (0.138, 1.666) | −0.193 | (−0.814, 0.428) | −0.161 | (−0.633, 0.310) | 2.464 | (−0.018, 4.946) | 2.892 | (1.014, 4.771) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Gong, L.; Lou, X.; Zhou, H.; Hao, Y.; Chen, Q.; Zhao, Y.; Jiang, X.; Li, L.; Wang, X. Sleep-Body Composition Relationship: Roles of Sleep Behaviors in General and Abdominal Obesity in Chinese Adolescents Aged 17–22 Years. Nutrients 2023, 15, 4130. https://doi.org/10.3390/nu15194130
Song Y, Gong L, Lou X, Zhou H, Hao Y, Chen Q, Zhao Y, Jiang X, Li L, Wang X. Sleep-Body Composition Relationship: Roles of Sleep Behaviors in General and Abdominal Obesity in Chinese Adolescents Aged 17–22 Years. Nutrients. 2023; 15(19):4130. https://doi.org/10.3390/nu15194130
Chicago/Turabian StyleSong, Yalin, Lu Gong, Xiaomin Lou, Huijun Zhou, Yudan Hao, Qiuyuan Chen, Yize Zhao, Xili Jiang, Lijie Li, and Xian Wang. 2023. "Sleep-Body Composition Relationship: Roles of Sleep Behaviors in General and Abdominal Obesity in Chinese Adolescents Aged 17–22 Years" Nutrients 15, no. 19: 4130. https://doi.org/10.3390/nu15194130
APA StyleSong, Y., Gong, L., Lou, X., Zhou, H., Hao, Y., Chen, Q., Zhao, Y., Jiang, X., Li, L., & Wang, X. (2023). Sleep-Body Composition Relationship: Roles of Sleep Behaviors in General and Abdominal Obesity in Chinese Adolescents Aged 17–22 Years. Nutrients, 15(19), 4130. https://doi.org/10.3390/nu15194130






