Association between Dietary Vitamin E Intake and Human Papillomavirus Infection among US Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey
Abstract
:1. Introduction
2. Materials and Methods
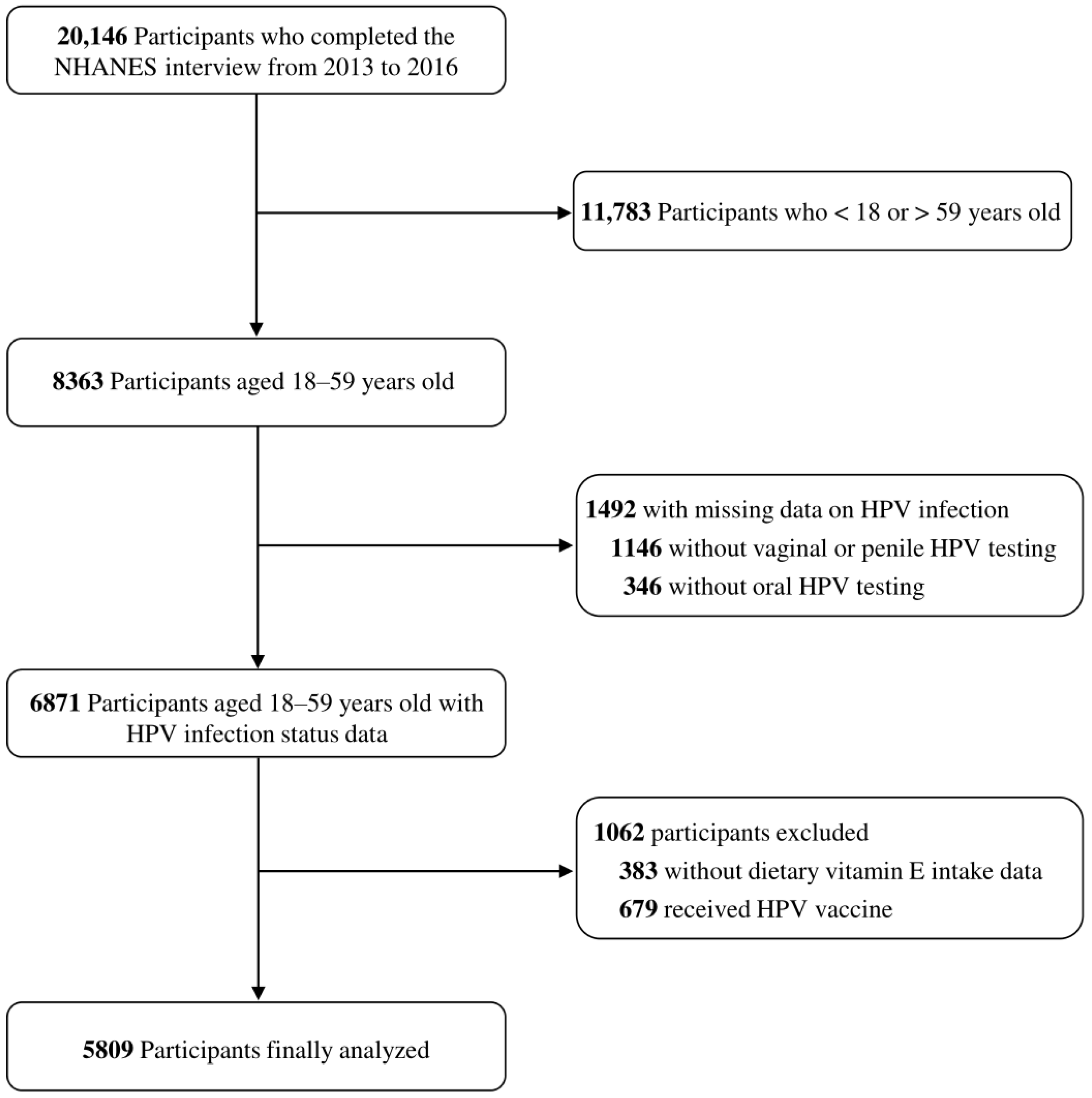
2.1. Data Source and Study Population
2.2. Detection and Classification of HPV Infections
2.3. Assessment of Dietary Vitamin E Intake and Covariates
2.4. Statistical Analyses
3. Results
3.1. Population Characteristics
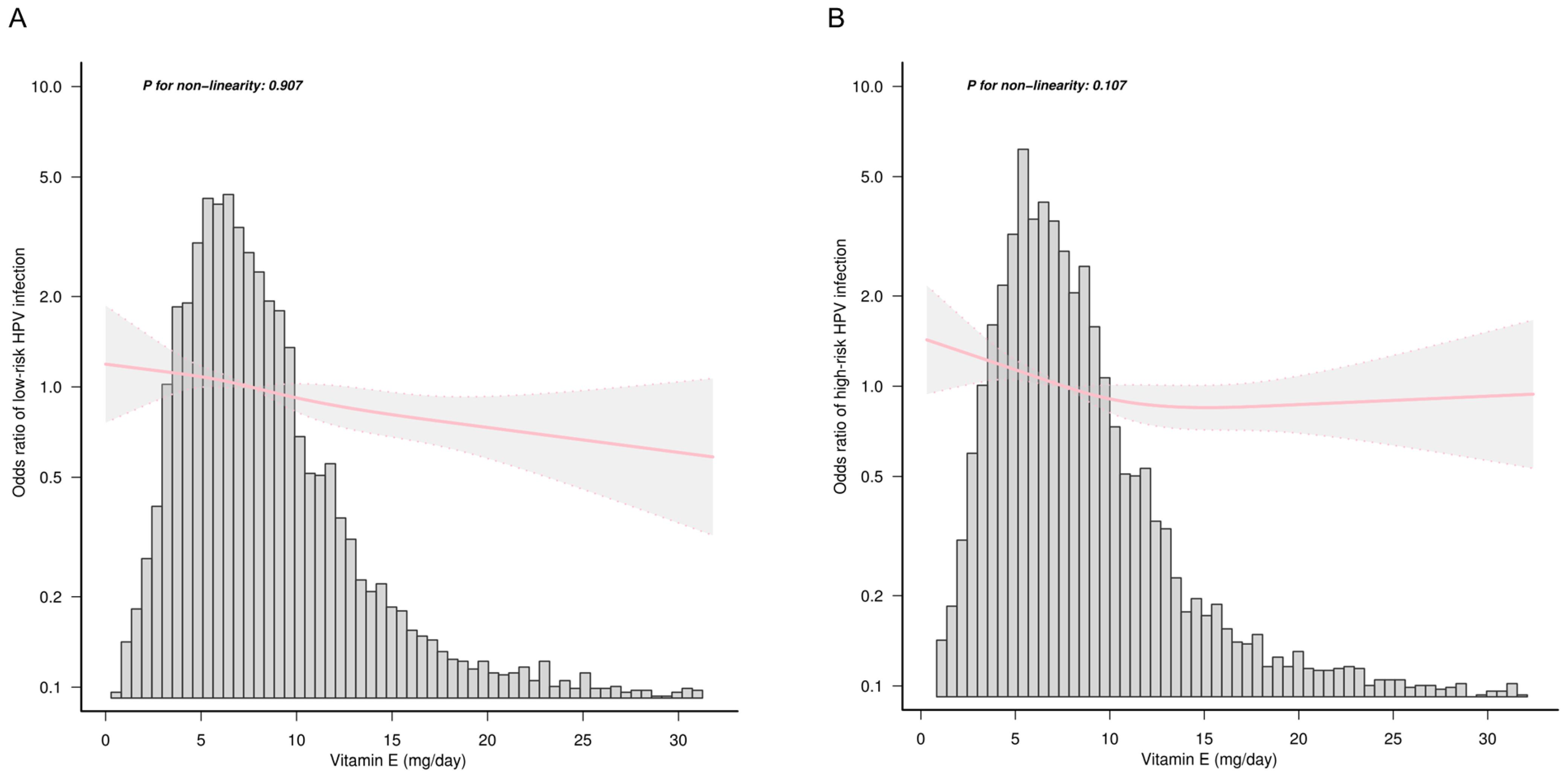
3.2. Association between Dietary Vitamin E Intake and HPV Infection
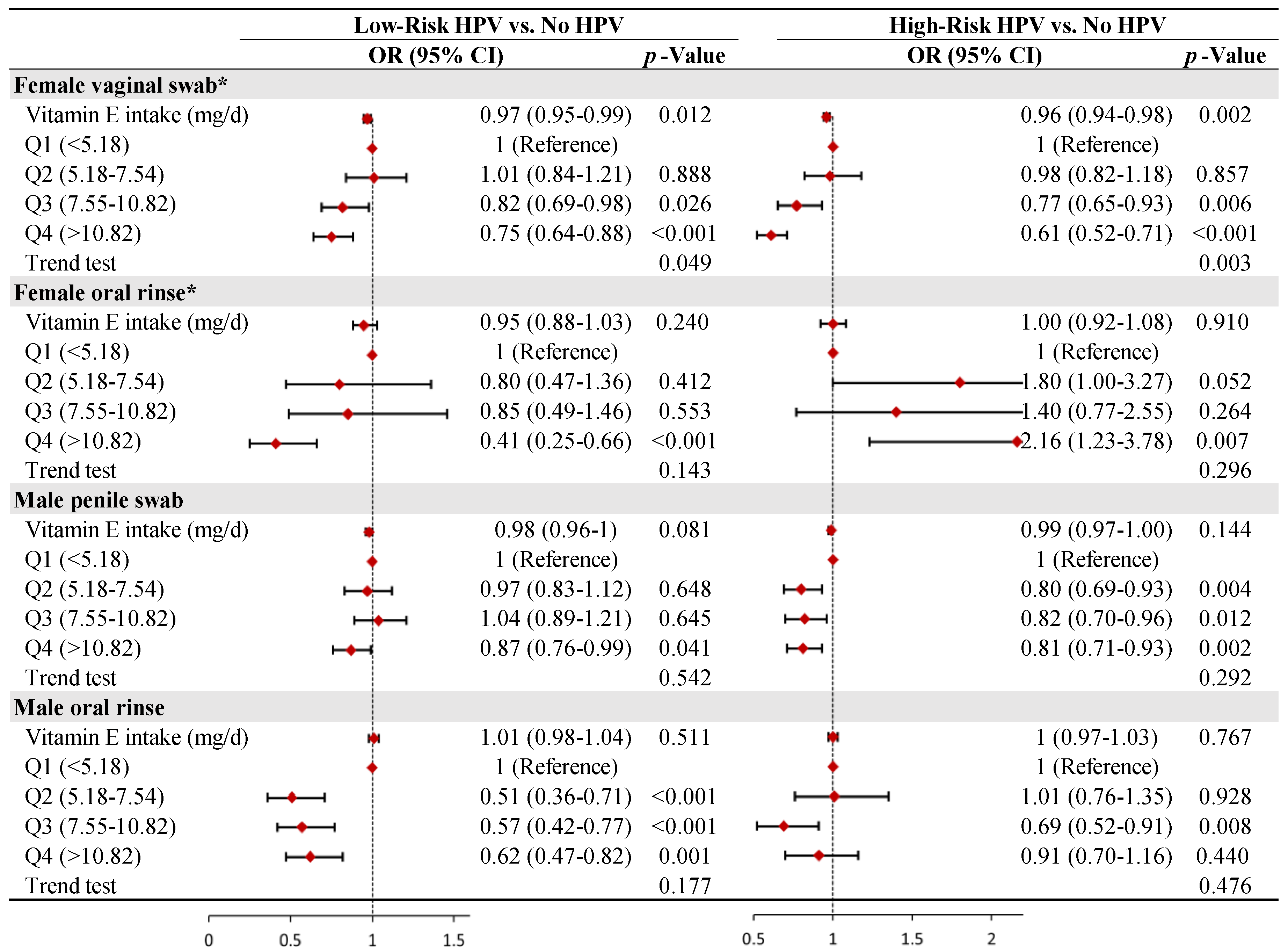
3.3. Stratified Analysis by Gender and Site
3.4. Sensitivity Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Schiffman, M.; Herrero, R.; Wacholder, S.; Hildesheim, A.; Castle, P.E.; Solomon, D.; Burk, R.; On behalf of the Proyecto Epidemiológico Guanacaste Group. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J. Natl. Cancer Inst. 2008, 100, 513–517. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjose, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- Alemany, L.; Saunier, M.; Alvarado-Cabrero, I.; Quiros, B.; Salmeron, J.; Shin, H.R.; Pirog, E.C.; Guimera, N.; Hernandez-Suarez, G.; Felix, A.; et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer 2015, 136, 98–107. [Google Scholar] [CrossRef]
- De Vuyst, H.; Clifford, G.M.; Nascimento, M.C.; Madeleine, M.M.; Franceschi, S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. Int. J. Cancer 2009, 124, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Alemany, L.; Cubilla, A.; Halec, G.; Kasamatsu, E.; Quiros, B.; Masferrer, E.; Tous, S.; Lloveras, B.; Hernandez-Suarez, G.; Lonsdale, R.; et al. Role of Human Papillomavirus in Penile Carcinomas Worldwide. Eur. Urol. 2016, 69, 953–961. [Google Scholar] [CrossRef]
- Castellsague, X.; Alemany, L.; Quer, M.; Halec, G.; Quiros, B.; Tous, S.; Clavero, O.; Alos, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef]
- Lopes, R.; Teixeira, J.A.; Marchioni, D.; Villa, L.L.; Giuliano, A.R.; Luiza Baggio, M.; Fisberg, R.M. Dietary intake of selected nutrients and persistence of HPV infection in men. Int. J. Cancer 2017, 141, 757–765. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Arshad, A.; Lassi, Z.S.; Bhutta, Z.A. Systematic Review and Meta-Analysis of Interventions to Improve Access and Coverage of Adolescent Immunizations. J. Adolesc. Health 2016, 59, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Markowitz, L.E. Human papillomavirus vaccination coverage among females and males, National Health and Nutrition Examination Survey, United States, 2007–2016. Vaccine 2018, 36, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Scalisi, A.; Agodi, A. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients 2018, 10, 469. [Google Scholar] [CrossRef]
- Ono, A.; Koshiyama, M.; Nakagawa, M.; Watanabe, Y.; Ikuta, E.; Seki, K.; Oowaki, M. The Preventive Effect of Dietary Antioxidants on Cervical Cancer Development. Medicina 2020, 56, 604. [Google Scholar] [CrossRef]
- Koshiyama, M. The Effects of the Dietary and Nutrient Intake on Gynecologic Cancers. Healthcare 2019, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Chih, H.J.; Lee, A.H.; Colville, L.; Binns, C.W.; Xu, D. A review of dietary prevention of human papillomavirus-related infection of the cervix and cervical intraepithelial neoplasia. Nutr. Cancer 2013, 65, 317–328. [Google Scholar] [CrossRef]
- Lin, H.Y.; Fu, Q.; Kao, Y.H.; Tseng, T.S.; Reiss, K.; Cameron, J.E.; Ronis, M.J.; Su, J.; Nair, N.; Chang, H.M.; et al. Antioxidants Associated with Oncogenic Human Papillomavirus Infection in Women. J. Infect. Dis. 2021, 224, 1520–1528. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; La Mastra, C.; Rosa, M.C.; Favara, G.; Lio, R.M.S.; Agodi, A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients 2020, 12, 1384. [Google Scholar] [CrossRef]
- Xiao, D.; Li, W.; Zhang, W.H.; Wen, Z.; Dai, B.; Mo, W.; Qiu, S.; Yang, L. Dietary Zinc, Copper, and Selenium Intake and High-Risk Human Papillomavirus Infection among American Women: Data from NHANES 2011–2016. Nutr. Cancer 2022, 74, 1958–1967. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Siegel, E.M.; Roe, D.J.; Ferreira, S.; Baggio, M.L.; Galan, L.; Duarte-Franco, E.; Villa, L.L.; Rohan, T.E.; Marshall, J.R.; et al. Dietary intake and risk of persistent human papillomavirus (HPV) infection: The Ludwig-McGill HPV Natural History Study. J. Infect. Dis. 2003, 188, 1508–1516. [Google Scholar] [CrossRef]
- Peterhans, E. Oxidants and antioxidants in viral diseases: Disease mechanisms and metabolic regulation. J. Nutr. 1997, 127 (Suppl. 5), 962S–965S. [Google Scholar] [CrossRef] [PubMed]
- Siegel, E.M.; Craft, N.E.; Duarte-Franco, E.; Villa, L.L.; Franco, E.L.; Giuliano, A.R. Associations between serum carotenoids and tocopherols and type-specific HPV persistence: The Ludwig-McGill cohort study. Int. J. Cancer 2007, 120, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; Shvetsov, Y.B.; McDuffie, K.; Wilkens, L.R.; Zhu, X.; Franke, A.A.; Bertram, C.C.; Kessel, B.; Bernice, M.; Sunoo, C.; et al. Hawaii cohort study of serum micronutrient concentrations and clearance of incident oncogenic human papillomavirus infection of the cervix. Cancer Res. 2007, 67, 5987–5996. [Google Scholar] [CrossRef]
- Peterson, C.E.; Sedjo, R.L.; Davis, F.G.; Beam, C.A.; Giuliano, A.R. Combined antioxidant carotenoids and the risk of persistent human papillomavirus infection. Nutr. Cancer 2010, 62, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Closas, R.; Castellsague, X.; Bosch, X.; Gonzalez, C.A. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. Int. J. Cancer 2005, 117, 629–637. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Zhou, L.; Zhao, M.; Zhu, X. Effect of vitamin E supplementation on uterine cervical neoplasm: A meta-analysis of case-control studies. PLoS ONE 2017, 12, e0183395. [Google Scholar] [CrossRef] [PubMed]
- Tomita, L.Y.; Roteli-Martins, C.M.; Villa, L.L.; Franco, E.L.; Cardoso, M.A.; Team, B.S. Associations of dietary dark-green and deep-yellow vegetables and fruits with cervical intraepithelial neoplasia: Modification by smoking. Br. J. Nutr. 2011, 105, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Ouh, Y.T.; Hong, J.H.; Min, K.J.; So, K.A.; Kim, T.J.; Paik, E.S.; Lee, J.W.; Moon, J.H.; Lee, J.K. Comparison of urine, self-collected vaginal swab, and cervical swab samples for detecting human papillomavirus (HPV) with Roche Cobas, HPV, Anyplex, II HPV, and RealTime HR-S HPV assay. J. Virol. Methods 2019, 269, 77–82. [Google Scholar] [CrossRef]
- Saville, M.; Hawkes, D.; Keung, M.; Ip, E.; Silvers, J.; Sultana, F.; Malloy, M.J.; Velentzis, L.S.; Canfel, L.K.; Wrede, C.D.; et al. Analytical performance of HPV assays on vaginal self-collected vs practitioner-collected cervical samples: The SCoPE study. J. Clin. Virol. 2020, 127, 104375. [Google Scholar] [CrossRef]
- National Center for Health Statistics. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 1 March 2023).
- National Health and Nutrition Examination Survey. Measuring Guides for the Dietary Recall Interview. Available online: https://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm (accessed on 1 March 2023).
- Liu, H.; Wang, L.; Chen, C.; Dong, Z.; Yu, S. Association between Dietary Niacin Intake and Migraine among American Adults: National Health and Nutrition Examination Survey. Nutrients 2022, 14, 3052. [Google Scholar] [CrossRef]
- Dempsey, A.F. Human papillomavirus: The usefulness of risk factors in determining who should get vaccinated. Rev. Obstet. Gynecol. 2008, 1, 122–128. [Google Scholar]
- Itarat, Y.; Kietpeerakool, C.; Jampathong, N.; Chumworathayi, B.; Kleebkaow, P.; Aue-Aungkul, A.; Nhokaew, W. Sexual behavior and infection with cervical human papillomavirus types 16 and 18. Int. J. Womens Health 2019, 11, 489–494. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Hatam, L.J.; Kayden, H.J. A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J. Lipid Res. 1979, 20, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Coquette, A.; Vray, B.; Vanderpas, J. Role of vitamin E in the protection of the resident macrophage membrane against oxidative damage. Arch. Int. Physiol. Biochim. 1986, 94, S29–S34. [Google Scholar]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Harris, S.G.; Padilla, J.; Koumas, L.; Ray, D.; Phipps, R.P. Prostaglandins as modulators of immunity. Trends Immunol. 2002, 23, 144–150. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rocca, B.; FitzGerald, G.A. Cyclooxygenases and prostaglandins: Shaping up the immune response. Int. Immunopharmacol. 2002, 2, 603–630. [Google Scholar] [CrossRef] [PubMed]
- Hayek, M.G.; Mura, C.; Wu, D.; Beharka, A.A.; Han, S.N.; Paulson, K.E.; Hwang, D.; Meydani, S.N. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J. Immunol. 1997, 159, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Mossavar-Rahmani, Y.; Huang, Y.; Van Horn, L.; Beresford, S.A.; Caan, B.; Tinker, L.; Schoeller, D.; Bingham, S.; Eaton, C.B.; et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am. J. Epidemiol. 2011, 174, 591–603. [Google Scholar] [CrossRef]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The OPEN study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef]
| Characteristic | Dietary Vitamin E Intake, mg/d | |||||
|---|---|---|---|---|---|---|
| Total | Q1 (<5.18) | Q2 (5.18–7.54) | Q3 (7.55–10.82) | Q4 (>10.82) | p-Value | |
| No. | 5809 | 1452 | 1452 | 1450 | 1455 | |
| Age (years), mean (SD) | 39.1 (11.9) | 38.8 (12.3) | 38.9 (11.9) | 39.4 (11.8) | 39.2 (11.6) | 0.448 |
| Sex, n (%) | <0.001 | |||||
| Male | 2907 (50.0) | 616 (42.4) | 683 (47) | 773 (53.3) | 835 (57.4) | |
| Female | 2902 (50.0) | 836 (57.6) | 769 (53) | 677 (46.7) | 620 (42.6) | |
| Race, n (%) | <0.001 | |||||
| Non-Hispanic white | 2081 (35.8) | 471 (32.4) | 498 (34.3) | 534 (36.8) | 578 (39.7) | |
| Non-Hispanic black | 1207 (20.8) | 304 (20.9) | 320 (22) | 281 (19.4) | 302 (20.8) | |
| Mexican American | 978 (16.8) | 241 (16.6) | 260 (17.9) | 254 (17.5) | 223 (15.3) | |
| Others | 1543 (26.6) | 436 (30) | 374 (25.8) | 381 (26.3) | 352 (24.2) | |
| Education level (years), n (%) | <0.001 | |||||
| <9 | 365 (6.3) | 137 (9.4) | 97 (6.7) | 84 (5.8) | 47 (3.2) | |
| 9–12 | 1939 (33.4) | 591 (40.7) | 487 (33.5) | 462 (31.9) | 399 (27.4) | |
| >12 | 3189 (54.9) | 635 (43.7) | 773 (53.2) | 828 (57.1) | 953 (65.5) | |
| NA | 316 (5.4) | 89 (6.1) | 95 (6.5) | 76 (5.2) | 56 (3.8) | |
| Marital status, n (%) | <0.001 | |||||
| Living in a couple | 3439 (59.2) | 794 (54.7) | 866 (59.6) | 879 (60.6) | 900 (61.9) | |
| Living alone | 2054 (35.4) | 569 (39.2) | 491 (33.8) | 494 (34.1) | 500 (34.4) | |
| NA | 316 (5.4) | 89 (6.1) | 95 (6.5) | 77 (5.3) | 55 (3.8) | |
| No. of persons in household, n (%) | 0.003 | |||||
| 1 | 490 (8.4) | 110 (7.6) | 115 (7.9) | 131 (9) | 134 (9.2) | |
| 2–3 | 2369 (40.8) | 577 (39.7) | 589 (40.6) | 599 (41.3) | 604 (41.5) | |
| 4–6 | 2508 (43.2) | 616 (42.4) | 646 (44.5) | 614 (42.3) | 632 (43.4) | |
| >6 | 442 (7.6) | 149 (10.3) | 102 (7.0) | 106 (7.3) | 85 (5.8) | |
| Family income, n (%) | <0.001 | |||||
| Low | 1821 (31.3) | 593 (40.8) | 486 (33.5) | 403 (27.8) | 339 (23.3) | |
| Medium | 1924 (33.1) | 469 (32.3) | 474 (32.6) | 482 (33.2) | 499 (34.3) | |
| High | 1616 (27.8) | 270 (18.6) | 391 (26.9) | 444 (30.6) | 511 (35.1) | |
| NA | 448 (7.7) | 120 (8.3) | 101 (7.0) | 121 (8.3) | 106 (7.3) | |
| Smoking status, n (%) | <0.001 | |||||
| Never | 3532 (60.8) | 830 (57.2) | 917 (63.2) | 900 (62.1) | 885 (60.8) | |
| Current | 1309 (22.5) | 429 (29.5) | 322 (22.2) | 277 (19.1) | 281 (19.3) | |
| Former | 967 (16.6) | 193 (13.3) | 213 (14.7) | 273 (18.8) | 288 (19.8) | |
| NA | 1 (0.0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) | |
| Sleep hours, n (%) | 0.051 | |||||
| <8 | 3476 (59.8) | 852 (58.7) | 877 (60.4) | 868 (59.9) | 879 (60.4) | |
| 8–9 | 1944 (33.5) | 476 (32.8) | 470 (32.4) | 502 (34.6) | 496 (34.1) | |
| >9 | 372 (6.4) | 117 (8.1) | 101 (7) | 76 (5.2) | 78 (5.4) | |
| NA | 17 (0.3) | 7 (0.5) | 4 (0.3) | 4 (0.3) | 2 (0.1) | |
| Age at first sexual intercourse (years), n (%) | 0.016 | |||||
| Never | 279 (4.8) | 83 (5.7) | 75 (5.2) | 71 (4.9) | 50 (3.4) | |
| <16 | 1545 (26.6) | 397 (27.3) | 368 (25.3) | 399 (27.5) | 381 (26.2) | |
| 16–17 | 1551 (26.7) | 383 (26.4) | 384 (26.4) | 381 (26.3) | 403 (27.7) | |
| 18–19 | 1034 (17.8) | 246 (16.9) | 287 (19.8) | 247 (17.0) | 254 (17.5) | |
| >19 | 1070 (18.4) | 238 (16.4) | 266 (18.3) | 276 (19.0) | 290 (19.9) | |
| NA | 330 (5.7) | 105 (7.2) | 72 (5.0) | 76 (5.2) | 77 (5.3) | |
| No. of sexual intercourse past year, n (%) | <0.001 | |||||
| 0 | 157 (2.7) | 37 (2.5) | 40 (2.8) | 44 (3.0) | 36 (2.5) | |
| 1–11 | 1256 (21.6) | 332 (22.9) | 322 (22.2) | 306 (21.1) | 296 (20.3) | |
| 12–51 | 1552 (26.7) | 337 (23.2) | 368 (25.3) | 427 (29.4) | 420 (28.9) | |
| 52–103 | 923 (15.9) | 200 (13.8) | 223 (15.4) | 235 (16.2) | 265 (18.2) | |
| 104–364 | 585 (10.1) | 134 (9.2) | 151 (10.4) | 136 (9.4) | 164 (11.3) | |
| 365 or more | 53 (0.9) | 20 (1.4) | 16 (1.1) | 7 (0.5) | 10 (0.7) | |
| NA | 1283 (22.1) | 392 (27) | 332 (22.9) | 295 (20.3) | 264 (18.1) | |
| No. of sex partners during lifetime, n (%) | <0.001 | |||||
| 0 | 327 (5.6) | 90 (6.2) | 86 (5.9) | 81 (5.6) | 70 (4.8) | |
| ≤5 | 2593 (44.6) | 681 (46.9) | 664 (45.7) | 643 (44.3) | 605 (41.6) | |
| >5 | 2568 (44.2) | 579 (39.9) | 632 (43.5) | 654 (45.1) | 703 (48.3) | |
| NA | 321 (5.5) | 102 (7.0) | 70 (4.8) | 72 (5.0) | 77 (5.3) | |
| Illegal substance use, n (%) | <0.001 | |||||
| Yes | 2906 (50.0) | 696 (47.9) | 700 (48.2) | 710 (49.0) | 800 (55.0) | |
| No | 2573 (44.3) | 653 (45) | 678 (46.7) | 667 (46) | 575 (39.5) | |
| NA | 330 (5.7) | 103 (7.1) | 74 (5.1) | 73 (5.0) | 80 (5.5) | |
| No. of alcohol consumption past year, Median (IQR) | 1.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 1.5 (0.0, 3.0) | 2.0 (0.0, 3.0) | <0.001 |
| Body mass index (kg/m2), mean (SD) | 29.2 (7.5) | 29.3 (7.7) | 29.6 (7.5) | 29.1 (7.5) | 28.8 (7.4) | 0.035 |
| Calorie consumption (kcal/day), Mean (SD) | 2141.2 (901.0) | 1451.4 (532.5) | 1958.6 (562.1) | 2313.1 (658.4) | 2840.3 (1088.8) | <0.001 |
| HPV infection status, n (%) | 0.012 | |||||
| Negative | 3200 (55.1) | 748 (51.5) | 785 (54.1) | 822 (56.7) | 845 (58.1) | |
| Low-risk HPV | 1234 (21.2) | 326 (22.5) | 319 (22.0) | 308 (21.2) | 281 (19.3) | |
| High-risk HPV | 1375 (23.7) | 378 (26.0) | 348 (24.0) | 320 (22.1) | 329 (22.6) | |
| Variables | Low-Risk HPV vs. No HPV | High-Risk HPV vs. No HPV | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 1.02 (1.01–1.02) | <0.001 | 1.00 (1.00–1.01) | 0.454 |
| Sex | ||||
| Male | 1 (Reference) | 1 (Reference) | ||
| Female | 0.92 (0.81–1.05) | 0.238 | 0.68 (0.60–0.78) | <0.001 |
| Race | ||||
| Non-Hispanic white | 1 (Reference) | 1 (Reference) | ||
| Non-Hispanic black | 2.50 (2.09–2.99) | <0.001 | 2.10 (1.76–2.49) | <0.001 |
| Mexican American | 0.92 (0.75–1.12) | 0.398 | 0.75 (0.62–0.91) | 0.004 |
| Others | 0.87 (0.73–1.04) | 0.127 | 0.75 (0.64–0.89) | 0.001 |
| Education level (years) | ||||
| <9 | 1 (Reference) | 1 (Reference) | ||
| 9–12 | 1.33 (1.01–1.77) | 0.046 | 1.95 (1.46–2.60) | <0.001 |
| >12 | 1.00 (0.76–1.31) | 0.978 | 1.09 (0.82–1.45) | 0.542 |
| Marital status | ||||
| Living in a couple | 1 (Reference) | 1 (Reference) | ||
| Living alone | 1.65 (1.44–1.89) | <0.001 | 2.19 (1.91–2.50) | <0.001 |
| No. of persons in household | ||||
| 1 | 1 (Reference) | 1 (Reference) | ||
| 2–3 | 0.84 (0.65–1.07) | 0.163 | 0.63 (0.50–0.79) | <0.001 |
| 4–6 | 0.63 (0.49–0.80) | <0.001 | 0.47 (0.38–0.59) | <0.001 |
| >6 | 0.64 (0.46–0.89) | 0.008 | 0.49 (0.36–0.67) | <0.001 |
| Family income | ||||
| Low | 1 (Reference) | 1 (Reference) | ||
| Medium | 0.92 (0.78–1.08) | 0.310 | 0.83 (0.71–0.96) | 0.015 |
| High | 0.68 (0.58–0.81) | <0.001 | 0.60 (0.51–0.71) | <0.001 |
| Smoking status | ||||
| Never | 1 (Reference) | 1 (Reference) | ||
| Current | 2.24 (1.91–2.63) | <0.001 | 2.72 (2.34–3.18) | <0.001 |
| Former | 1.40 (1.17–1.68) | <0.001 | 1.51 (1.26–1.79) | <0.001 |
| Sleep hours | ||||
| <8 | 1 (Reference) | 1 (Reference) | ||
| 8–9 | 0.85 (0.73–0.98) | 0.023 | 0.84 (0.73–0.96) | 0.013 |
| >9 | 0.79 (0.60–1.05) | 0.110 | 0.96 (0.74–1.24) | 0.728 |
| Age at first sexual intercourse (years) | ||||
| Never | 1 (Reference) | 1 (Reference) | ||
| <16 | 5.83 (3.79–8.96) | <0.001 | 8.99 (5.49–14.73) | <0.001 |
| 16–17 | 4.49 (2.92–6.91) | <0.001 | 7.16 (4.37–11.74) | <0.001 |
| 18–19 | 3.56 (2.29–5.53) | <0.001 | 5.28 (3.19–8.73) | <0.001 |
| >19 | 1.86 (1.19–2.91) | 0.007 | 2.84 (1.71–4.72) | <0.001 |
| No. of sexual intercourse past year | ||||
| 0 | 1 (Reference) | 1 (Reference) | ||
| 1–11 | 1.22 (0.80–1.84) | 0.356 | 1.28 (0.85–1.94) | 0.234 |
| 12–51 | 0.90 (0.59–1.36) | 0.607 | 1.00 (0.66–1.50) | 0.983 |
| 52–103 | 0.88 (0.57–1.35) | 0.564 | 1.08 (0.71–1.64) | 0.727 |
| 104–364 | 1.21 (0.77–1.88) | 0.408 | 1.52 (0.99–2.34) | 0.058 |
| 365 or more | 1.90 (0.90–4.00) | 0.092 | 1.52 (0.70–3.30) | 0.289 |
| No. of sex partners during lifetime | ||||
| 0 | 1 (Reference) | 1 (Reference) | ||
| ≤5 | 2.04 (1.41–2.96) | <0.001 | 2.02 (1.37–2.98) | <0.001 |
| >5 | 5.10 (3.52–7.38) | <0.001 | 7.01 (4.78–10.28) | <0.001 |
| Illegal substance use | ||||
| Yes | 1 (Reference) | 1 (Reference) | ||
| No | 0.55 (0.48–0.63) | <0.001 | 0.43 (0.37–0.49) | <0.001 |
| No. of alcohol consumption past year | 1.01 (1.00–1.01) | 0.058 | 1.01 (1.00–1.01) | 0.070 |
| Body mass index (kg/m2) | 1.02 (1.01–1.02) | <0.001 | 1.00 (0.99–1.01) | 0.955 |
| Calorie consumption (kcal/day) | 1.00 (1.00–1.00) | 0.469 | 1.00 (1.00–1.00) | <0.001 |
| Crude a | Model 1 b | Model 2 c | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Low-Risk HPV vs. No HPV | ||||||
| Vitamin E intake (mg/d) | 0.99 (0.97–1.00) | 0.010 | 0.99 (0.97–1.00) | 0.016 | 0.98 (0.97–1.00) | 0.021 |
| Q1 (<5.18) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Q2 (5.18–7.54) | 0.93 (0.78–1.12) | 0.456 | 0.96 (0.79–1.16) | 0.665 | 0.96 (0.84–1.08) | 0.475 |
| Q3 (7.55–10.82) | 0.86 (0.71–1.03) | 0.109 | 0.89 (0.74–1.08) | 0.238 | 0.89 (0.79–1.00) | 0.042 |
| Q4 (>10.82) | 0.76 (0.63–0.92) | 0.005 | 0.78 (0.64–0.95) | 0.015 | 0.75 (0.68–0.84) | <0.001 |
| Trend test | 0.003 | 0.012 | 0.018 | |||
| High-Risk HPV vs. No HPV | ||||||
| Vitamin E intake (mg/d) | 0.99 (0.98–1.00) | 0.015 | 0.99 (0.97–1.00) | 0.010 | 0.98 (0.97–0.99) | 0.004 |
| Q1 (<5.18) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Q2 (5.18–7.54) | 0.88 (0.74–1.05) | 0.146 | 0.92 (0.77–1.11) | 0.379 | 0.91 (0.81–1.03) | 0.134 |
| Q3 (7.55–10.82) | 0.77 (0.64–0.92) | 0.004 | 0.80 (0.66–0.97) | 0.021 | 0.77 (0.69–0.87) | <0.001 |
| Q4 (>10.82) | 0.77 (0.65–0.92) | 0.004 | 0.79 (0.65–0.95) | 0.013 | 0.72 (0.65–0.80) | <0.001 |
| Trend test | 0.001 | 0.005 | 0.002 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Fan, M.; Wang, Y.; Ma, Y.; Si, H.; Dai, G. Association between Dietary Vitamin E Intake and Human Papillomavirus Infection among US Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey. Nutrients 2023, 15, 3825. https://doi.org/10.3390/nu15173825
Zhou Q, Fan M, Wang Y, Ma Y, Si H, Dai G. Association between Dietary Vitamin E Intake and Human Papillomavirus Infection among US Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey. Nutrients. 2023; 15(17):3825. https://doi.org/10.3390/nu15173825
Chicago/Turabian StyleZhou, Qian, Mengjiao Fan, Yanrong Wang, Yue Ma, Haiyan Si, and Guanghai Dai. 2023. "Association between Dietary Vitamin E Intake and Human Papillomavirus Infection among US Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey" Nutrients 15, no. 17: 3825. https://doi.org/10.3390/nu15173825
APA StyleZhou, Q., Fan, M., Wang, Y., Ma, Y., Si, H., & Dai, G. (2023). Association between Dietary Vitamin E Intake and Human Papillomavirus Infection among US Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey. Nutrients, 15(17), 3825. https://doi.org/10.3390/nu15173825







