Hydration Status of Geriatric Patients Is Associated with Changes in Plasma Proteome, Especially in Proteins Involved in Coagulation
Abstract
:1. Introduction
- I.
- To find out whether and, if so, to what extent moderate dehydration or hyperhydration affects the blood proteome;
- II.
- To find which proteins are differentially abundant and could serve as potential biomarkers;
- III.
- To investigate which functions these dysregulations are related to.
2. Material and Methods
2.1. Study Design
2.2. Bioelectrical Impedance Vector Analyses (BIVAs)
2.3. Selection and Characteristics of Analysed Samples
2.4. Preparation of Blood Samples for Proteomic Analyses
2.5. Data Analysis
3. Results
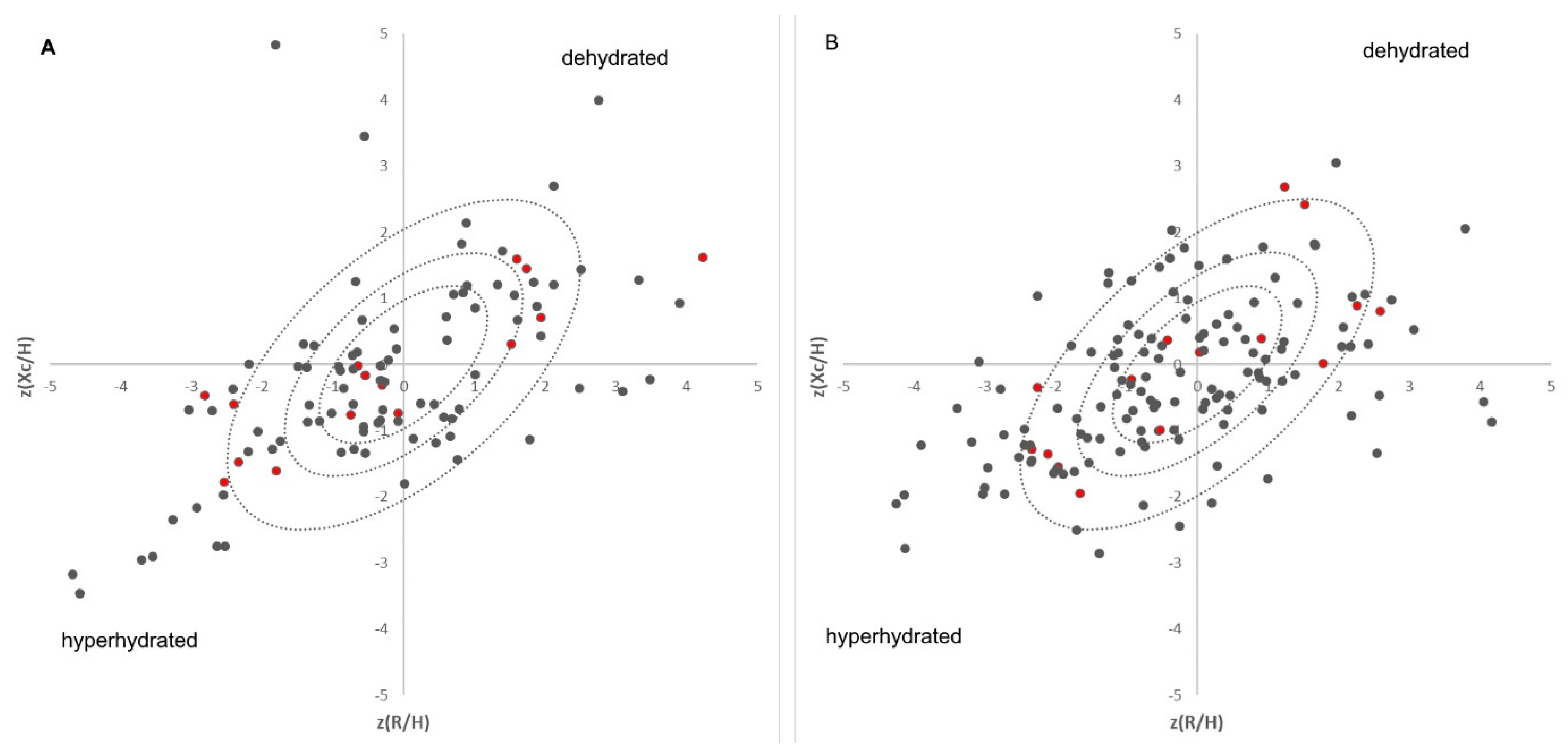
3.1. Protein Abundance Is Linked with Hydration Status
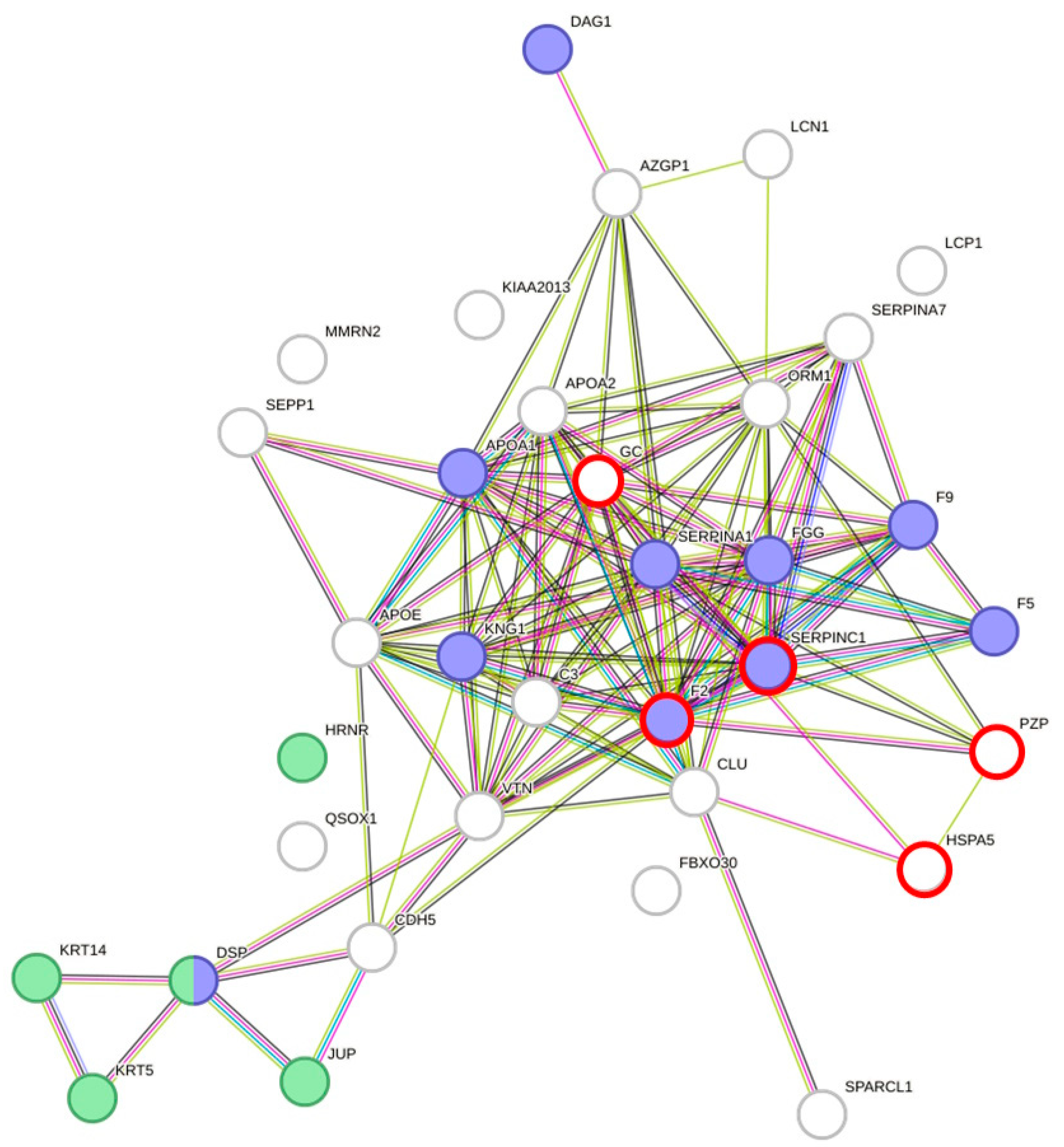
3.2. DAPs Are Involved in Wound Repair and Blood Coagulation Are Associated with Hydration
3.3. Alzheimer Prediction Marker
4. Discussion
4.1. Plasma Proteome Is Affected by Moderate Dehydration and Hyperhydration
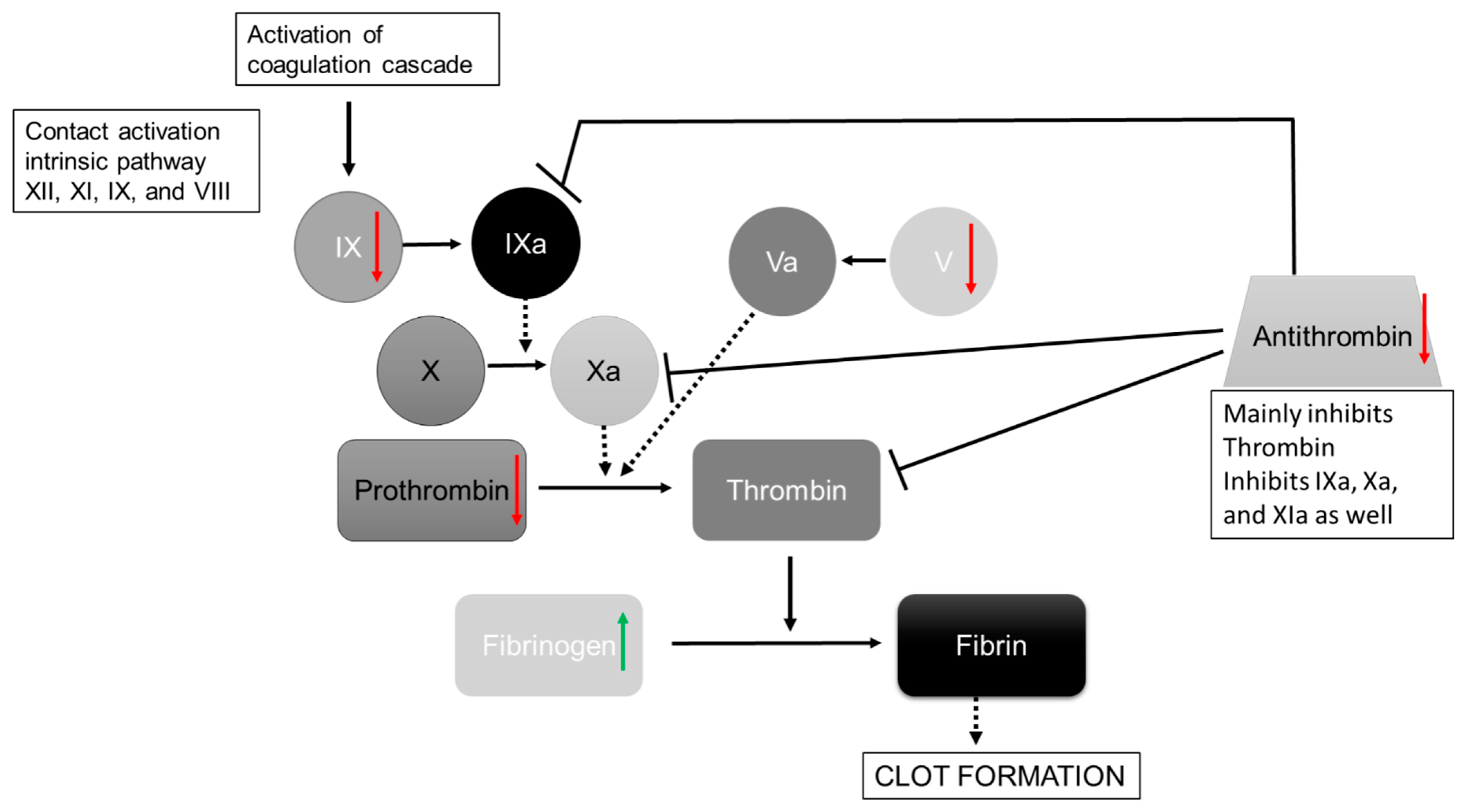
4.2. Wound Repair/Coagulation Is Affected by Hydration Status
4.3. Dehydration and Alzheimer
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, D.R.; Cote, T.R.; Lawhorne, L.; Levenson, S.A.; Rubenstein, L.Z.; Smith, D.A.; Stefanacci, R.G.; Tangalos, E.G.; Morley, J.E. Understanding clinical dehydration and its treatment. J. Am. Med. Dir. Assoc. 2008, 9, 292–301. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Attreed, N.J.; Campbell, W.W.; Channell, A.M.; Chassagne, P.; Culp, K.R.; Fletcher, S.J.; Fortes, M.B.; Fuller, N.; et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst. Rev. 2015, 2015, CD009647. [Google Scholar] [CrossRef]
- Hooper, L.; Bunn, D.; Jimoh, F.O.; Fairweather-Tait, S.J. Water-loss dehydration and aging. Mech. Ageing Dev. 2014, 136–137, 50–58. [Google Scholar] [CrossRef]
- Schmidt, S.; Hendricks, V.; Griebenow, R.; Riedel, R. Demographic change and its impact on the health-care budget for heart failure inpatients in Germany during 1995–2025. Herz 2013, 38, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.J. Dehydration in the Older Adult. J. Gerontol. Nurs. 2015, 41, 8–13. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Maughan, R.J.; Senay, L.C.; Shirreffs, S.M. Limitations to the use of plasma osmolality as a hydration biomarker. Am. J. Clin. Nutr. 2013, 98, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Kenefick, R.W.; Charkoudian, N.; Sawka, M.N. Physiologic basis for understanding quantitative dehydration assessment. Am. J. Clin. Nutr. 2013, 97, 455–462. [Google Scholar] [CrossRef]
- Nagae, M.; Umegaki, H.; Onishi, J.; Huang, C.H.; Yamada, Y.; Watanabe, K.; Komiya, H.; Kuzuya, M. Chronic Dehydration in Nursing Home Residents. Nutrients 2020, 12, 3562. [Google Scholar] [CrossRef]
- Beck, A.M.; Seemer, J.; Knudsen, A.W.; Munk, T. Narrative Review of Low-Intake Dehydration in Older Adults. Nutrients 2021, 13, 3142. [Google Scholar] [CrossRef]
- Allen, M.D.; Springer, D.A.; Burg, M.B.; Boehm, M.; Dmitrieva, N.I. Suboptimal hydration remodels metabolism, promotes degenerative diseases, and shortens life. JCI Insight 2019, 4, e130949. [Google Scholar] [CrossRef] [PubMed]
- Hoen, L.; Pfeffer, D.; Zapf, R.; Raabe, A.; Hildebrand, J.; Kraft, J.; Kalkhof, S. Association of Drug Application and Hydration Status in Elderly Patients. Nutrients 2021, 13, 1929. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Song, S.; Rhee, H.; Lee, S.H.; Kim, D.Y.; Choe, J.C.; Ahn, J.; Park, J.S.; Shin, M.J.; Jeon, Y.K.; et al. Normal Reference Plots for the Bioelectrical Impedance Vector in Healthy Korean Adults. J. Korean Med. Sci. 2019, 34, e198. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Pastori, G. BIVA Software; Department of Medical and Surgical Sciences, University of Padova: Padova, Italy, 2002. [Google Scholar]
- Wiśniewski, J.R. Filter-Aided Sample Preparation for Proteome Analysis. Methods Mol. Biol. 2018, 1841, 3–10. [Google Scholar] [CrossRef]
- Biadglegne, F.; Schmidt, J.R.; Engel, K.M.; Lehmann, J.; Lehmann, R.T.; Reinert, A.; König, B.; Schiller, J.; Kalkhof, S.; Sack, U. Mycobacterium tuberculosis Affects Protein and Lipid Content of Circulating Exosomes in Infected Patients Depending on Tuberculosis Disease State. Biomedicines 2022, 10, 783. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Snel, B.; Lehmann, G.; Bork, P.; Huynen, M.A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef]
- Schrör, K. Aspirin and platelets: The antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin. Thromb. Hemost. 1997, 23, 349–356. [Google Scholar] [CrossRef]
- Bauer, K.A.; Nguyen-Cao, T.M.; Spears, J.B. Issues in the Diagnosis and Management of Hereditary Antithrombin Deficiency. Ann. Pharmacother. 2016, 50, 758–767. [Google Scholar] [CrossRef]
- Nijholt, D.A.T.; Ijsselstijn, L.; van der Weiden, M.M.; Zheng, P.-P.; Sillevis Smitt, P.A.E.; Koudstaal, P.J.; Luider, T.M.; Kros, J.M. Pregnancy Zone Protein is Increased in the Alzheimer’s Disease Brain and Associates with Senile Plaques. J. Alzheimers Dis. 2015, 46, 227–238. [Google Scholar] [CrossRef]
- Ijsselstijn, L.; Dekker, L.J.M.; Stingl, C.; van der Weiden, M.M.; Hofman, A.; Kros, J.M.; Koudstaal, P.J.; Sillevis Smitt, P.A.E.; Ikram, M.A.; Breteler, M.M.B.; et al. Serum levels of pregnancy zone protein are elevated in presymptomatic Alzheimer’s disease. J. Proteome Res. 2011, 10, 4902–4910. [Google Scholar] [CrossRef]
- Dellinger, J.R.; Johnson, B.A.; Benavides, M.L.; Moore, M.L.; Stratton, M.T.; Harty, P.S.; Siedler, M.R.; Tinsley, G.M. Agreement of bioelectrical resistance, reactance, and phase angle values from supine and standing bioimpedance analyzers. Physiol. Meas. 2021, 42, 035003. [Google Scholar] [CrossRef]
- Silva, A.M.; Matias, C.N.; Nunes, C.L.; Santos, D.A.; Marini, E.; Lukaski, H.C.; Sardinha, L.B. Lack of agreement of in vivo raw bioimpedance measurements obtained from two single and multi-frequency bioelectrical impedance devices. Eur. J. Clin. Nutr. 2019, 73, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Makaryus, R.; Miller, T.E.; Gan, T.J. Current concepts of fluid management in enhanced recovery pathways. Br. J. Anaesth. 2018, 120, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Saghaleini, S.H.; Dehghan, K.; Shadvar, K.; Sanaie, S.; Mahmoodpoor, A.; Ostadi, Z. Pressure Ulcer and Nutrition. Indian J. Crit. Care Med. 2018, 22, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ousey, K.; Cutting, K.F.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 122, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Pötzsch, B.; Madlener, K. (Eds.) Gerinnungskonsil: Rationelle Diagnostik und Therapie von Gerinnungsstörungen; 74 Tabellen; Thieme: Stuttgart, NY, USA, 2002; ISBN 9783131353313. [Google Scholar]
- Jin, L.; Abrahams, J.P.; Skinner, R.; Petitou, M.; Pike, R.N.; Carrell, R.W. The anticoagulant activation of antithrombin by heparin. Proc. Natl. Acad. Sci. USA 1997, 94, 14683–14688. [Google Scholar] [CrossRef]
- Müller, G. Erworbene Antithrombin III-Mangelzustände. Z. Gesamte Inn. Med. 1992, 47, 74–77. [Google Scholar]
- Büller, H.R.; Ten Cate, J.W. Acquired antithrombin III deficiency: Laboratory diagnosis, incidence, clinical implications, and treatment with antithrombin III concentrate. Am. J. Med. 1989, 87, 44S–48S. [Google Scholar] [CrossRef]
- Ruttmann, T.G.; Jamest, M.F.; Lombard, E.H. Haemodilution-induced enhancement of coagulation is attenuated in vitroby restoring antithrombin III to pre-dilution concentrations. Anaesth. Intensive Care 2001, 29, 489–493. [Google Scholar] [CrossRef]
- Kelly, J.; Hunt, B.J.; Lewis, R.R.; Swaminathan, R.; Moody, A.; Seed, P.T.; Rudd, A. Dehydration and venous thromboembolism after acute stroke. QJM Int. J. Med. 2004, 97, 293–296. [Google Scholar] [CrossRef]
- Watanabe, T.; Minakami, H.; Sakata, Y.; Matsubara, S.; Tamura, N.; Obara, H.; Wada, T.; Onagawa, T.; Sato, I. Effect of labor on maternal dehydration, starvation, coagulation, and fibrinolysis. J. Perinat. Med. 2001, 29, 528–534. [Google Scholar] [CrossRef]
- Ruttmann, T.G.; James, M.F.; Viljoen, J.F. Haemodilution induces a hypercoagulable state. Br. J. Anaesth. 1996, 76, 412–414. [Google Scholar] [CrossRef]
- Janvrin, S.B.; Davies, G.; Greenhalgh, R.M. Postoperative deep vein thrombosis caused by intravenous fluids during surgery. Br. J. Surg. 1980, 67, 690–693. [Google Scholar] [CrossRef]
- Masento, N.A.; Golightly, M.; Field, D.T.; Butler, L.T.; van Reekum, C.M. Effects of hydration status on cognitive performance and mood. Br. J. Nutr. 2014, 111, 1841–1852. [Google Scholar] [CrossRef]
- Lauriola, M.; Mangiacotti, A.; D’Onofrio, G.; Cascavilla, L.; Paris, F.; Paroni, G.; Seripa, D.; Greco, A.; Sancarlo, D. Neurocognitive Disorders and Dehydration in Older Patients: Clinical Experience Supports the Hydromolecular Hypothesis of Dementia. Nutrients 2018, 10, 562. [Google Scholar] [CrossRef]
| Group | Age ± SD | Drugs ± SD |
|---|---|---|
| Normal hydrated men (control) | 89.0 ± 3.5 | 8.4 ± 4.6 |
| Hyperhydrated men | 78.2 ± 5.8 | 8.8 ± 5.6 |
| Dehydrated men | 80.6 ± 4.7 | 13.4 ± 2.0 |
| Normal hydrated female (control) | 84.0 ± 3.6 | 10.4 ± 4.4 |
| Hyperhydrated female | 80.6 ± 5.1 | 5.8 ± 7.4 |
| Dehydrated female | 86.2 ± 5.9 | 10.2 ± 3.3 |
| Female | Male | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Name | Mean | p-Value | Gene Name | Mean | p-Value | ||||||||
| DF | HF | CF | DF vs. CF | HF vs. CF | DF vs. HF | DM | HM | CM | DM vs. CM | HM vs. CM | DM vs. HM | ||
| F2 | 20.27 | 20.24 | 20.92 | 0.010 | 0.201 | 0.947 | F2 | 20.94 | 21.97 | 21.50 | 0.171 | 0.153 | 0.026 |
| SERPINC1 | 21.04 | 21.33 | 21.89 | 0.028 | 0.111 | 0.168 | SERPINC1 | 21.58 | 22.54 | 21.74 | 0.717 | 0.069 | 0.046 |
| PZP | 18.18 | 19.59 | 18.33 | 0.818 | 0.093 | 0.039 | PZP | 20.30 | 18.55 | 19.71 | 0.231 | 0.019 | 0.006 |
| GC | 20.73 | 20.91 | 21.40 | 0.003 | 0.080 | 0.412 | GC | 21.15 | 22.18 | 21.38 | 0.605 | 0.116 | 0.014 |
| HSPA5 | 15.71 | 15.52 | 16.21 | 0.047 | 0.019 | 0.019 | HSPA5 | 16.49 | 17.04 | 16.53 | 0.869 | 0.092 | 0.042 |
| QSOX1 | 15.40 | 14.52 | 15.80 | 0.407 | 0.028 | 0.018 | SERPINA1 | 22.43 | 21.27 | 22.14 | 0.514 | 0.024 | 0.026 |
| C3 | 23.28 | 23.91 | 23.83 | 0.015 | 0.811 | 0.134 | KRT14 | 19.57 | 19.79 | 19.16 | 0.069 | 0.047 | 0.399 |
| C4b | 16.16 | 15.38 | 16.23 | 0.732 | 0.012 | 0.017 | APOA1 | 22.71 | 22.42 | 23.25 | 0.049 | 0.435 | 0.769 |
| KNG1 | 19.08 | 19.47 | 19.93 | 0.032 | 0.239 | 0.212 | APOE | 21.58 | 21.27 | 21.98 | 0.281 | 0.009 | 0.378 |
| IGHG2 | 20.35 | 20.22 | 17.69 | 0.049 | 0.146 | 0.920 | APOA2 | 20.34 | 21.08 | 20.59 | 0.428 | 0.148 | 0.048 |
| VTN | 19.60 | 19.92 | 20.14 | 0.001 | 0.411 | 0.287 | FGG | 22.31 | 21.68 | 22.17 | 0.641 | 0.139 | 0.011 |
| CLU | 20.03 | 20.11 | 20.81 | 0.005 | 0.027 | 0.727 | ORM1 | 19.89 | 18.43 | 19.23 | 0.249 | 0.053 | 0.025 |
| F5 | 19.22 | 19.34 | 20.30 | 0.038 | 0.159 | 0.825 | SERPINA7 | 18.61 | 19.49 | 18.92 | 0.416 | 0.137 | 0.029 |
| F9 | 17.32 | 16.83 | 18.38 | 0.047 | 0.245 | 0.673 | KRT5 | 19.81 | 19.92 | 19.33 | 0.045 | 0.002 | 0.561 |
| LCN1 | 16.62 | 16.28 | 16.58 | 0.855 | 0.273 | 0.040 | LCP1 | 15.15 | 16.32 | 15.90 | 0.038 | 0.701 | 0.335 |
| CDH5 | 17.10 | 16.66 | 17.55 | 0.207 | 0.036 | 0.144 | JUP | 16.85 | 16.64 | 16.23 | 0.022 | 0.061 | 0.247 |
| SELENOP | 17.93 | 17.89 | 18.60 | 0.007 | 0.015 | 0.861 | AZGP1 | 19.57 | 20.38 | 19.72 | 0.731 | 0.187 | 0.046 |
| Krt86 | 16.68 | 16.49 | 16.63 | 0.829 | 0.483 | 0.023 | DAG1 | 14.61 | 14.19 | 14.58 | 0.909 | 0.174 | 0.043 |
| MMRN2 | 15.81 | 15.42 | 16.39 | 0.104 | 0.019 | 0.056 | SPARCL1 | 14.32 | 15.27 | 14.81 | 0.152 | 0.248 | 0.035 |
| DSP | 17.30 | 17.09 | 17.23 | 0.816 | 0.670 | 0.016 | dnaK | 15.83 | 16.80 | 16.25 | 0.385 | 0.236 | 0.019 |
| HRNR | 18.01 | 18.24 | 17.61 | 0.054 | 0.047 | 0.353 | |||||||
| KIAA2013 | 15.69 | 15.81 | 15.35 | 0.139 | 0.044 | 0.419 | |||||||
| FBXO30 | 16.57 | 14.87 | 15.55 | 0.011 | 0.657 | 0.365 | |||||||
| # Genes | Description | FDR Value | p-Value |
|---|---|---|---|
| 27 | Localization | 3.10 × 10−6 | 1.21 × 10−9 |
| 27 | Multicellular organismal process | 7.80 × 10−5 | 2.04 × 10−7 |
| 27 10 7 | Response to stimulus → Response to Wounding → Blood Coagulation | 0.0011 1.12 × 10−5 2.80 × 10−4 | 6.15 × 10−6 1.74 × 10−8 8.63 × 10−7 |
| 22 | Regulation of biological quality | 1.91 × 10−5 | 3.49 × 10−8 |
| 22 | Negative regulation of biological process | 0.0012 | 6.96 × 10−6 |
| 21 5 | Anatomical structure development → Keratinization | 0.0037 0.0048 | 3.32 × 10−5 4.58 × 10−5 |
| 19 | Protein metabolic process | 0.0023 | 1.53 × 10−5 |
| 14 | Cellular component assembly | 0.0027 | 1.83 × 10−5 |
| 14 | Regulation of catalytic activity | 0.0029 | 2.08 × 10−5 |
| 14 | Immune system process | 0.0037 | 3.24 × 10−5 |
| 11 | Cell activation | 3.30 × 10−4 | 1.26 × 10−6 |
| 11 | Positive regulation of multicellular organismal process | 0.0113 | 1.30 × 10−4 |
| 10 | Regulation of body fluid levels | 8.22 × 10−6 | 1.15 × 10−8 |
| 9 | Positive regulation of cellular component organization | 0.0135 | 1.70 × 10−4 |
| 8 | Cell adhesion | 0.0126 | 1.50 × 10−4 |
| 5 | Regeneration | 0.0014 | 8.31 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoen, L.; Pfeffer, D.; Schmidt, J.R.; Kraft, J.; Hildebrand, J.; Kalkhof, S. Hydration Status of Geriatric Patients Is Associated with Changes in Plasma Proteome, Especially in Proteins Involved in Coagulation. Nutrients 2023, 15, 3789. https://doi.org/10.3390/nu15173789
Hoen L, Pfeffer D, Schmidt JR, Kraft J, Hildebrand J, Kalkhof S. Hydration Status of Geriatric Patients Is Associated with Changes in Plasma Proteome, Especially in Proteins Involved in Coagulation. Nutrients. 2023; 15(17):3789. https://doi.org/10.3390/nu15173789
Chicago/Turabian StyleHoen, Laura, Daniel Pfeffer, Johannes R. Schmidt, Johannes Kraft, Janosch Hildebrand, and Stefan Kalkhof. 2023. "Hydration Status of Geriatric Patients Is Associated with Changes in Plasma Proteome, Especially in Proteins Involved in Coagulation" Nutrients 15, no. 17: 3789. https://doi.org/10.3390/nu15173789
APA StyleHoen, L., Pfeffer, D., Schmidt, J. R., Kraft, J., Hildebrand, J., & Kalkhof, S. (2023). Hydration Status of Geriatric Patients Is Associated with Changes in Plasma Proteome, Especially in Proteins Involved in Coagulation. Nutrients, 15(17), 3789. https://doi.org/10.3390/nu15173789







