Feasibility of Home Parenteral Nutrition in Patients with Intestinal Failure Due to Neuroendocrine Tumours: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Quality Assessment
3. Results
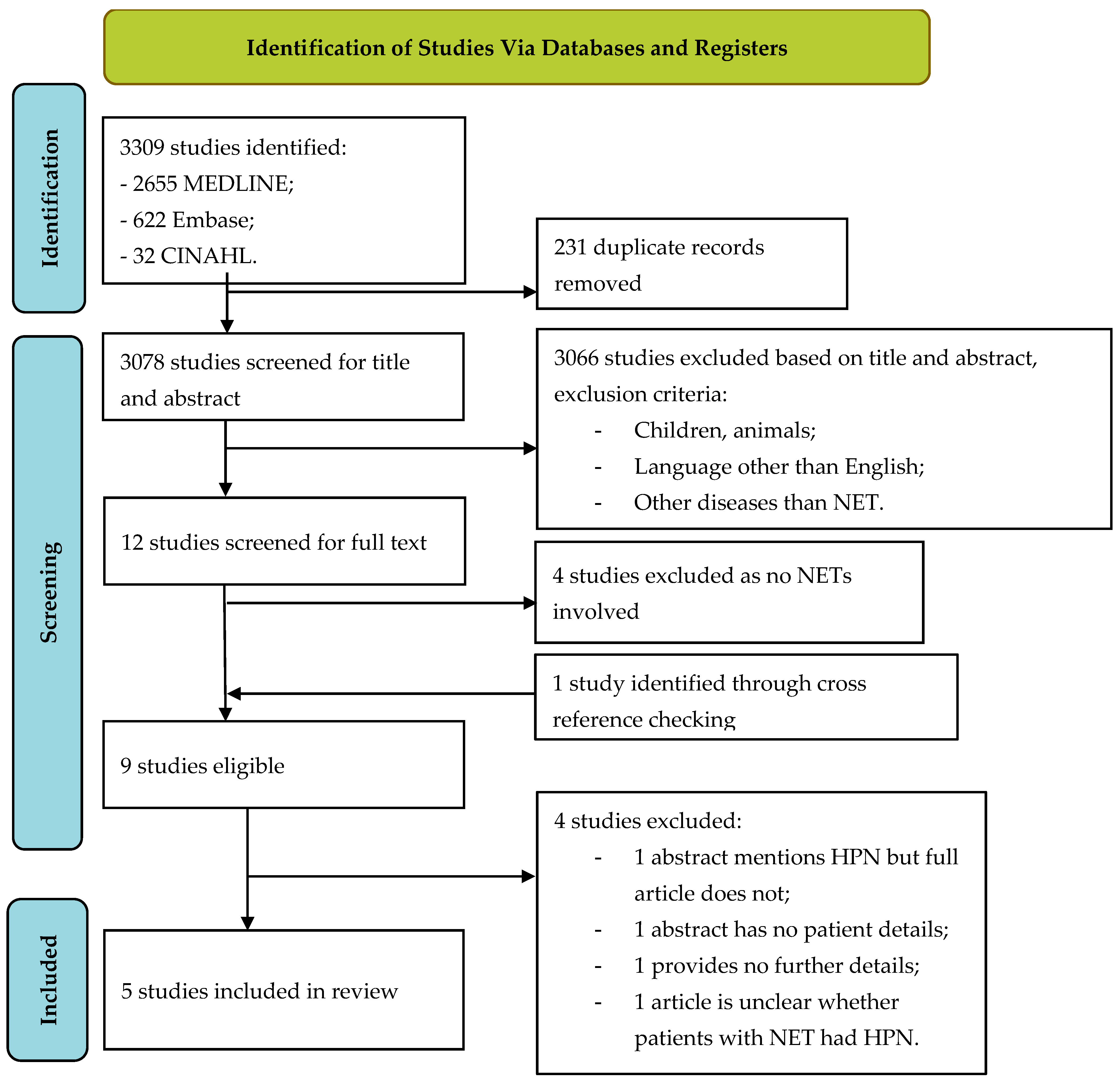
3.1. Literature Search
3.2. Study Selection
3.3. Feasibility of HPN
3.4. Complications of HPN
3.5. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search String
Search Strategy
References
- Genus, T.S.E.; Bouvier, C.; Wong, K.F.; Srirajaskanthan, R.; Rous, B.A.; Talbot, D.C.; Valle, J.W.; Khan, M.; Pearce, N.; Elshafie, M.; et al. Impact of Neuroendocrine Morphology on Cancer Outcomes and Stage at Diagnosis: A UK Nationwide Cohort Study 2013–2015. Br. J. Cancer 2019, 121, 966–972. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Pironi, L.; Boeykens, K.; Bozzetti, F.; Joly, F.; Klek, S.; Lal, S.; Lichota, M.; Mühlebach, S.; Van Gossum, A.; Wanten, G.; et al. ESPEN Guideline on Home Parenteral Nutrition. Clin. Nutr. 2020, 39, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Sandor, A. An Analysis of 8305 Cases of Carcinoid Tumors. Cancer 1997, 79, 813–829. [Google Scholar] [CrossRef]
- Lardière-Deguelte, S.; De Mestier, L.; Appéré, F.; Vullierme, M.P.; Zappa, M.; Hoeffel, C.; Noaves, M.; Brixi, H.; Hentic, O.; Ruszniewski, P.; et al. Toward a Preoperative Classification of Lymph Node Metastases in Patients with Small Intestinal Neuroendocrine Tumors in the Era of Intestinal-Sparing Surgery. Neuroendocrinology 2016, 103, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Koumarianou, A.; Alexandraki, K.I.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Pathogenesis and Clinical Management of Mesenteric Fibrosis in Small Intestinal Neuroendocine Neoplasms: A Systematic Review. J. Clin. Med. 2020, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Blažević, A.; Hofland, J.; Hofland, L.J.; Feelders, R.A.; De Herder, W.W. Small Intestinal Neuroendocrine Tumours and Fibrosis: An Entangled Conundrum. Endocr.-Relat. Cancer 2018, 25, R115–R130. [Google Scholar] [CrossRef]
- Daskalakis, K.; Karakatsanis, A.; Stålberg, P.; Norlén, O.; Hellman, P. Clinical Signs of Fibrosis in Small Intestinal Neuroendocrine Tumours. Br. J. Surg. 2017, 104, 69–75. [Google Scholar] [CrossRef]
- Öberg, K.; Knigge, U.; Kwekkeboom, D.; Perren, A. Neuroendocrine Gastro-Entero-Pancreatic Tumors: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2012, 23, vii124–vii130. [Google Scholar] [CrossRef]
- Deguelte, S.; Perrier, M.; Hammoutene, C.; Cadiot, G.; Kianmanesh, R. Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 2319. [Google Scholar] [CrossRef]
- Simion, N.I.; Muntean, V.; Fabian, O. Current State of Knowledge on Neuroendocrine Small Bowel Tumours: Non-Systematic Review of the Literature Based on One Case. BMJ Case Rep. 2013, 2013, bcr2012007217. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN Guidelines on Chronic Intestinal Failure in Adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed]
- Marín Caro, M.M.; Laviano, A.; Pichard, C. Nutritional Intervention and Quality of Life in Adult Oncology Patients. Clin. Nutr. 2007, 26, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Niederle, B.; Pape, U.F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, K.; Karakatsanis, A.; Hessman, O.; Stuart, H.C.; Welin, S.; Tiensuu Janson, E.; Öberg, K.; Hellman, P.; Norlén, O.; Stålberg, P. Association of a Prophylactic Surgical Approach to Stage IV Small Intestinal Neuroendocrine Tumors With Survival. JAMA Oncol. 2018, 4, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jann, H.; Roll, S.; Couvelard, A.; Hentic, O.; Pavel, M.; Müller-Nordhorn, J.; Koch, M.; Röcken, C.; Rindi, G.; Ruszniewski, P.; et al. Neuroendocrine Tumors of Midgut and Hindgut Origin: Tumor-Node-Metastasis Classification Determines Clinical Outcome. Cancer 2011, 117, 3332–3341. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Clement, D.S.V.M.; Srirajaskanthan, R.; Ramage, J.K.; Tesselaar, M.E.T.; Khan, M.S.; Verbeek, W.H.M.; Wanten, G.J.A.; Naghibi, M. Outcomes and Survival in Patients with Advanced Intestinal Neuroendocrine Tumours on Home Parenteral Nutrition, an International Multicentre Retrospective Cohort Study. Clin. Nutr. ESPEN 2023, 54, 106–112. [Google Scholar] [CrossRef]
- Sagar, V.M.; Shah, T.; Malhi, H.; Parkinson, S.; Shetty, S.; Cooper, S.C. Home Parenteral Nutrition in Neuroendocrine Tumour Intestinal Failure: Improved Quality of Life and Longevity. BMJ Support. Palliat. Care 2020, 1–4. [Google Scholar] [CrossRef]
- Liu, M.; Laskaratos, F.-M.; Bennell, J.; Chen, J.; Toumpanakis, C.; Mandair, D.; Caplin, M. Home Total Parenteral Nutrition for Intestinal Failure in Patients with Advanced Small Intestinal Neuroendocrine Neoplasms. Nutr. Cancer 2020, 73, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Hoda, D.; Jatoi, A.; Burnes, J.; Loprinzi, C.; Kelly, D. Should Patients with Advanced, Incurable Cancers Ever Be Sent Home with Total Parenteral Nutrition? Cancer 2005, 103, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Nehra, V.; Camilleri, M.; Burton, D.; Oenning, L.; Kelly, D.G. An Open Trial of Octreotide Long-Acting Release in the Management of Short Bowel Syndrome. Am. J. Gastroenterol. 2001, 96, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Ladefoged, K. Quality of Life in Patients on Permanent Home Parenteral Nutrition. JPEN J. Parenter. Enter. Nutr. 1981, 5, 132–137. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Abrams, H.B.; L’Abbe, K.A.; Whitwell, J.; Bombardier, C.; Jeejeebhoy, K.N. Quality of Life of Patients on Long-Term Total Parenteral Nutrition at Home. J. Gen. Intern. Med. 1986, 1, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.F.; Hagan, E.; Wetle, T.; Smith, C.; O’Sullivan Maillet, J.; Touger-Decker, R. An Exploration of Quality of Life and the Experience of Living with Home Parenteral Nutrition. J. Parenter. Enter. Nutr. 2010, 34, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Langholz, E.; Mortensen, P.B. Quality of Life in Patients Receiving Home Parenteral Nutrition. Gut 1999, 44, 844–852. [Google Scholar] [CrossRef]
- Oz, V.; Theilla, M.; Singer, P. Eating Habits and Quality of Life of Patients Receiving Home Parenteral Nutrition in Israel. Clin. Nutr. 2008, 27, 95–99. [Google Scholar] [CrossRef]
- Baxter, J.P.; Fayers, P.M.; McKinlay, A.W. The Clinical and Psychometric Validation of a Questionnaire to Assess the Quality of Life of Adult Patients Treated with Long-Term Parenteral Nutrition. J. Parenter. Enter. Nutr. 2010, 34, 131–142. [Google Scholar] [CrossRef]
- Culine, S.; Chambrier, C.; Tadmouri, A.; Senesse, P.; Seys, P.; Radji, A.; Rotarski, M.; Balian, A.; Dufour, P. Home Parenteral Nutrition Improves Quality of Life and Nutritional Status in Patients with Cancer: A French Observational Multicentre Study. Support. Care Cancer 2014, 22, 1867–1874. [Google Scholar] [CrossRef]
- Girke, J.; Seipt, C.; Markowski, A.; Luettig, B.; Schettler, A.; Momma, M.; Schneider, A.S. Quality of Life and Nutrition Condition of Patients Improve under Home Parenteral Nutrition: An Exploratory Study. Nutr. Clin. Pract. 2016, 31, 659–665. [Google Scholar] [CrossRef]
- Obling, S.R.; Wilson, B.V.; Pfeiffer, P.; Kjeldsen, J. Home Parenteral Nutrition Increases Fat Free Mass in Patients with Incurable Gastrointestinal Cancer. Results of a Randomized Controlled Trial. Clin. Nutr. 2017, 38, 182–190. [Google Scholar] [CrossRef]
- Senesse, P.; Tadmouri, A.; Culine, S.; Dufour, P.R.; Seys, P.; Radji, A.; Rotarski, M.; Balian, A.; Chambrier, C. A Prospective Observational Study Assessing Home Parenteral Nutrition in Patients with Gastrointestinal Cancer: Benefits for Quality of Life. J. Pain Symptom Manag. 2015, 49, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Cozzaglio, L.; Biganzoli, E.; Chiavenna, G.; De Cicco, M.; Donati, D.; Gilli, G.; Percolla, S.; Pironi, L. Quality of Life and Length of Survival in Advanced Cancer Patients on Home Parenteral Nutrition. Clin. Nutr. 2002, 21, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Dahlk, S.; Popiel, B.; Lammersfeld, C.A.; Ireton-Jones, C.; Gupta, D. A Longitudinal Study Investigating Quality of Life and Nutritional Outcomes in Advanced Cancer Patients Receiving Home Parenteral Nutrition. BMC Cancer 2014, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- Dibb, M.; Soop, M.; Teubner, A.; Shaffer, J.; Abraham, A.; Carlson, G.; Lal, S. Survival and Nutritional Dependence on Home Parenteral Nutrition: Three Decades of Experience from a Single Referral Centre. Clin. Nutr. 2017, 36, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Baxter, J.; Staun, M.; Kelly, D.G.; Hwa, Y.L.; Corcos, O.; De Francesco, A.; Agostini, F.; Klek, S.; Santarpia, L.; et al. Five-Year Survival and Causes of Death in Patients on Home Parenteral Nutrition for Severe Chronic and Benign Intestinal Failure. Clin. Nutr. 2018, 37, 1415–1422. [Google Scholar] [CrossRef]
- Lloyd, D.A.J.; Vega, R.; Bassett, P.; Forbes, A.; Gabe, S.M. Survival and Dependence on Home Parenteral Nutrition: Experience over a 25-Year Period in a UK Referral Centre. Aliment. Pharmacol. Ther. 2006, 24, 1231–1240. [Google Scholar] [CrossRef]
- White, B.E.; Rous, B.; Chandrakumaran, K.; Wong, K.; Bouvier, C.; Van Hemelrijck, M.; George, G.; Russell, B.; Srirajaskanthan, R.; Ramage, J.K. Incidence and Survival of Neuroendocrine Neoplasia in England 1995–2018: A Retrospective, Population-Based Study. Lancet Reg. Health Eur. 2022, 23, 100510. [Google Scholar] [CrossRef]
- O’Hanlon, F.J.; Fragkos, K.C.; Fini, L.; Patel, P.S.; Mehta, S.J.; Rahman, F.; Di Caro, S. Home Parenteral Nutrition in Patients with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2020, 73, 943–955. [Google Scholar] [CrossRef]
- Noelting, J.; Gramlich, L.; Whittaker, S.; Armstrong, D.; Marliss, E.; Jurewitsch, B.; Raman, M.; Duerksen, D.R.; Stevenson, D.; Lou, W.; et al. Survival of Patients With Short-Bowel Syndrome on Home Parenteral Nutrition: A Prospective Cohort Study. J. Parenter. Enter. Nutr. 2020, 45, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, M.; Smith, T.R.; Elia, M. A Systematic Review with Meta-Analysis of Survival, Quality of Life and Cost-Effectiveness of Home Parenteral Nutrition in Patients with Inoperable Malignant Bowel Obstruction. Clin. Nutr. 2015, 34, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Santarpia, L.; Pironi, L.; Thul, P.; Klek, S.; Gavazzi, C.; Tinivella, M.; Joly, F.; Jonkers, C.; Baxter, J.; et al. The Prognosis of Incurable Cachectic Cancer Patients on Home Parenteral Nutrition: A Multi-Centre Observational Study with Prospective Follow-up of 414 Patients. Ann. Oncol. 2014, 25, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wouters, Y.; Theilla, M.; Singer, P.; Tribler, S.; Jeppesen, P.B.; Pironi, L.; Vinter-Jensen, L.; Rasmussen, H.H.; Rahman, F.; Wanten, G.J.A. Randomised Clinical Trial: 2% Taurolidine versus 0.9% Saline Locking in Patients on Home Parenteral Nutrition. Aliment. Pharmacol. Ther. 2018, 48, 410–422. [Google Scholar] [CrossRef]
- Cotogni, P.; Mussa, B.; Degiorgis, C.; De Francesco, A.; Pittiruti, M. Comparative Complication Rates of 854 Central Venous Access Devices for Home Parenteral Nutrition in Cancer Patients: A Prospective Study of Over 169,000 Catheter-Days. J. Parenter. Enter. Nutr. 2021, 45, 768–776. [Google Scholar] [CrossRef]
- Guerra, E.M.; Cortés-Salgado, A.; Mateo-Lobo, R.; Nattero, L.; Riveiro, J.; Vega-Piñero, B.; Valbuena, B.; Carabaña, F.; Carrero, C.; Grande, E.; et al. Role of Parenteral Nutrition in Oncologic Patients with Intestinal Occlusion and Peritoneal Carcinomatosis. Nutr. Hosp. 2015, 32, 1222–1227. [Google Scholar]

| Author, Year | Country | Study Period | Study Design | Sample Size | Gender | Median Age (Year) | Intestinal Failure Subtypes | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Clement D 2023 [21] | The Netherlands and the United Kingdom | 2000–2019 | Retrospective case series | n = 41 | Male n = 18 (44%) Female n = 23 (56%) | 65 | SBS n = 27 (66%) IMBO n = 14 (34%) | Survival, catheter-related bloodstream infection rate |
| Sagar V 2020 [22] | United Kingdom | 2000–2017 | Retrospective case series | n = 8 | Male n = 5 (63%) Female n = 3 (37%) | NR | SBS n = 4 (50%) IMBO n = 2 (25%) Fistula n = 2 (25%) | Survival, catheter-related bloodstream infection rate, quality of life |
| Liu M 2020 [23] | United Kingdom | 2010–2019 | Retrospective case series | n = 5 | Male n = 2 (40%) Female n = 3 (60%) | 63 | SBS n = 2 (40%) IMBO n = 3 (60%) | Period on HPN, survival, HPN-related complications |
| Hoda D 2005 [24] | United States | 1979–1999 | Retrospective case series | n = 5 ** | Male n = 1 (20%) Female n = 4 (80%) | 64 | SBS n = 3 (60%) IMBO n = 2 (40%) | Survival and HPN-related complications |
| Nehra V 2001 [25] | United States | NR | Prospective case series (one arm) | n = 1 *** | Female n = 1 (100%) | 72 | SBS n = 1 | Body weight, stool fat, sodium and potassium, gastric- and small bowel transit times |
| Number of Patients with NETs | Median Survival (Months on HPN) | Median Survival (Months) Short Bowel Syndrome | Median Survival (Months) Inoperable Malignant Bowel Obstruction | Number of Patients on HPN Longer than 1 Year | Number of Patients on HPN Longer than 2 Years | Number of Patients on HPN Longer than 3 Years | |
|---|---|---|---|---|---|---|---|
| Clement D 2023 [21] | n = 41 | 19 months (IQR 7–50) | 24 months (IQR 12–52) | 7 months (IQR 3–19) | n = 20 | n = 11 | n = 6 |
| Sagar V 2020 [22] | n = 8 | 27 months (IQR 0–54) | NR | NR | n = 5 | n = 3 | n = 2 |
| Liu M 2020 [23] | n = 5 | 12 months (IQR 9–54) | 35.5 months (no IQR) | 12 months (no IQR) | n = 4 | n = 1 | n = 1 |
| Hoda D 2005 [24] | n = 5 | 74 months (IQR 16–115) | 74 months (no IQR) | 44.5 months (no IQR) | n = 5 | n = 3 | n = 3 |
| Nehra V 2001 [25] | n = 1 | 120 months | 120 months | 0 | n = 1 | n = 1 | n = 1 |
| Total | n = 60 | n = 35 (58%) | n = 19 (32%) | n = 13 (22%) |
| Number of Patients with NETs | Number of Catheter-Related Bloodstream Infections Reported | Catheter-Related Bloodstream Infection Rate/1000 Catheter Days | Central Venous Thrombosis | HPN-Related Liver Disease | |
|---|---|---|---|---|---|
| Clement D 2023 [21] | n = 41 | n = 23 | 1/1000 catheter days | NR | NR |
| Sagar V 2020 [22] | n = 8 | n = 4 | 2/1000 catheter days | NR | NR |
| Liu M 2020 [23] | n = 5 | n = 2 | 0.64/1000 catheter days | n = 1 (20%) | n = 1 (20%) |
| Hoda D 2005 ** [24] | n = 5 | n = 18 | 0.97/1000 catheter days | n = 4 (8%) | n = 2 (4%) |
| Nehra V 2001 [25] | n = 1 | NR | NR | NR | NR |
| Randomisation Process (D1) | Deviations from the Intended Interventions (D2) | Missing Outcome Data (D3) | Measurement of the Outcome (D4) | Selection of the Reported Result (D5) | Overall | |
|---|---|---|---|---|---|---|
| Clement D 2023 [21] |  |  |  |  |  |  |
| Sagar V 2020 [22] |  |  |  |  |  |  |
| Liu M 2020 [23] |  |  |  |  |  |  |
| Hoda D 2005 [24] |  |  |  |  |  |  |
| Nehra V 2001 [25] |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clement, D.S.V.M.; Brown, S.E.; Naghibi, M.; Cooper, S.C.; Tesselaar, M.E.T.; van Leerdam, M.E.; Ramage, J.K.; Srirajaskanthan, R. Feasibility of Home Parenteral Nutrition in Patients with Intestinal Failure Due to Neuroendocrine Tumours: A Systematic Review. Nutrients 2023, 15, 3787. https://doi.org/10.3390/nu15173787
Clement DSVM, Brown SE, Naghibi M, Cooper SC, Tesselaar MET, van Leerdam ME, Ramage JK, Srirajaskanthan R. Feasibility of Home Parenteral Nutrition in Patients with Intestinal Failure Due to Neuroendocrine Tumours: A Systematic Review. Nutrients. 2023; 15(17):3787. https://doi.org/10.3390/nu15173787
Chicago/Turabian StyleClement, Dominique S. V. M., Sarah E. Brown, Mani Naghibi, Sheldon C. Cooper, Margot E. T. Tesselaar, Monique E. van Leerdam, John K. Ramage, and Rajaventhan Srirajaskanthan. 2023. "Feasibility of Home Parenteral Nutrition in Patients with Intestinal Failure Due to Neuroendocrine Tumours: A Systematic Review" Nutrients 15, no. 17: 3787. https://doi.org/10.3390/nu15173787
APA StyleClement, D. S. V. M., Brown, S. E., Naghibi, M., Cooper, S. C., Tesselaar, M. E. T., van Leerdam, M. E., Ramage, J. K., & Srirajaskanthan, R. (2023). Feasibility of Home Parenteral Nutrition in Patients with Intestinal Failure Due to Neuroendocrine Tumours: A Systematic Review. Nutrients, 15(17), 3787. https://doi.org/10.3390/nu15173787






