A Scoping Review of Tools to Assess Diet in Children and Adolescents with Autism Spectrum Disorder
Highlights
- A wide variety of dietary tools are used in published studies to assess diet and related factors in children and adolescents with Autism Spectrum Disorders (ASDs).
- The Brief Assessment scale for Mealtime Behavior in Children (BAMBI) and 24-hour dietary recalls are the most frequently used dietary tools in children and adolescents with ASDs.
- BAMBI is used to evaluate mealtime behavior and food selectivity, while 24-hour dietary recalls assess the dietary intake in children and adolescents with ASDs.
- This study provides us with an improved understanding of the various dietary tools used to assist professionals in selecting the most appropriate dietary assessment for their intervention goals.
- This study promotes the use of standardized dietary assessment approaches within ASD interventions, enhancing the comparability of results across studies.
- This study emphasizes the need to develop and validate additional dietary assessment tools for children and adolescents with ASDs, particularly in Spain and other European countries.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Review Criteria and Study Selection
2.3. Data Extraction
2.4. Quality Assessment
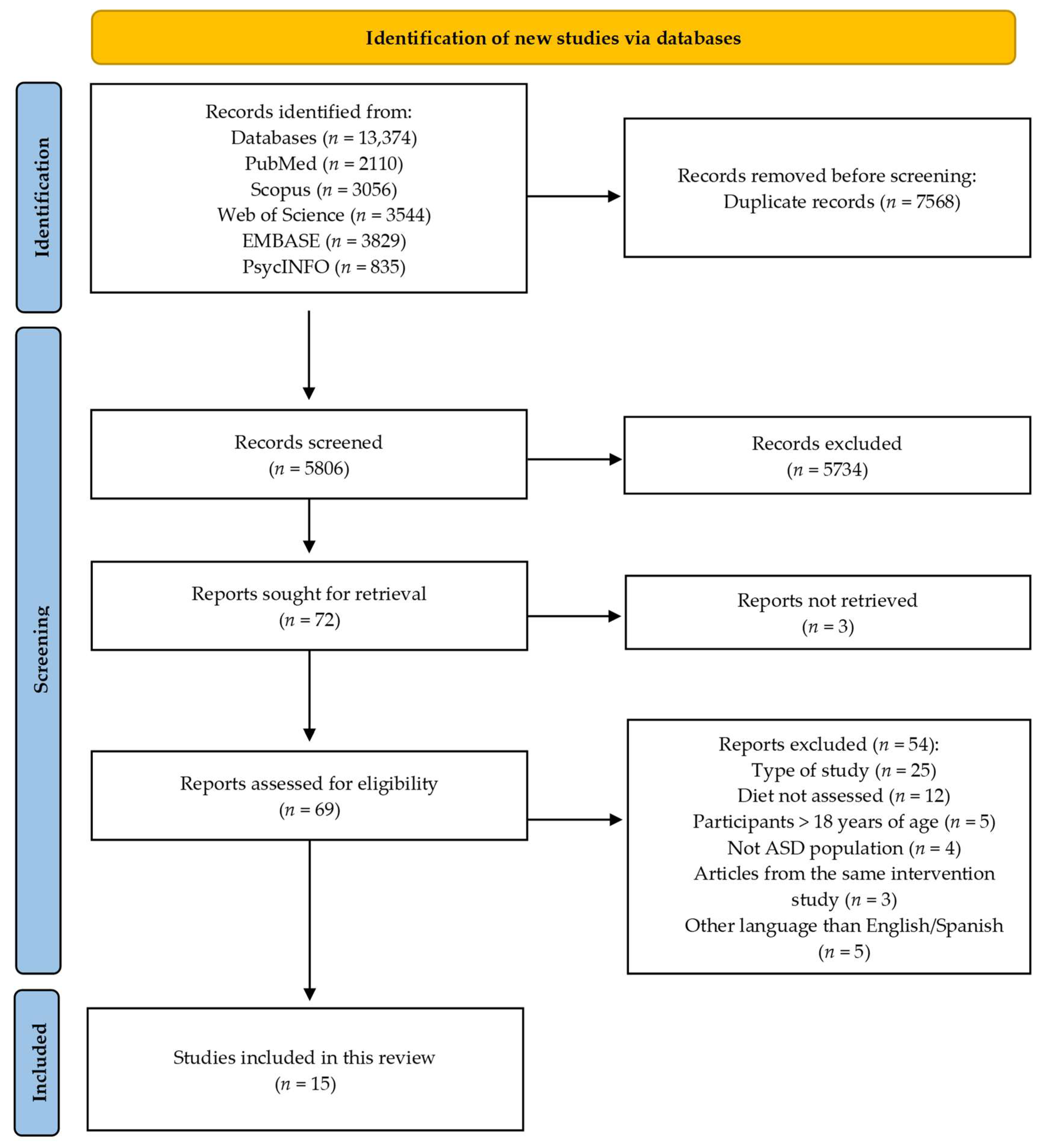
3. Results
3.1. Main Characteristics of the Included Studies
3.2. Main Variables in the Included Studies
3.3. Dietary Assessment Tools and Questionnaires
3.4. The BAMBI and the BAMBI-R
3.5. Twenty-four-h Dietary Recall
3.6. Main Limitations Reported in the Included Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Sauer, A.K.; Stanton, J.E.; Hans, S.; Grabrucker, A.M. Autism Spectrum Disorders: Etiology and Pathology. In Autism Spectrum Disorders; Grabrucker, A.M., Ed.; Exon Publications: Brisbane City, Australia, 2021; pp. 1–16. ISBN 978-0-645-00178-5. [Google Scholar]
- Taylor, M.J.; Rosenqvist, M.A.; Larsson, H.; Gillberg, C.; D’Onofrio, B.M.; Lichtenstein, P.; Lundström, S. Etiology of Autism Spectrum Disorders and Autistic Traits over Time. JAMA Psychiatry 2020, 77, 936. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, R.; Garrison, W.T.; Tokatli, V. Early Identification of Autism Spectrum Disorder (ASD): Strategies for Use in Local Communities. Indian J. Pediatr. 2023, 90, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Bilder, D.A.; McArthur, D.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Early Identification of Autism Spectrum Disorder Among Children Aged 4 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Donovan, S.M.; Lee, S.-Y. Considering Nature and Nurture in the Etiology and Prevention of Picky Eating: A Narrative Review. Nutrients 2020, 12, 3409. [Google Scholar] [CrossRef]
- Van Dijk, M.W.G.; Buruma, M.E.; Blijd-Hoogewys, E.M.A. Detecting Feeding Problems in Young Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2021, 51, 4115–4127. [Google Scholar] [CrossRef]
- Sathe, N.; Andrews, J.C.; McPheeters, M.L.; Warren, Z.E. Nutritional and Dietary Interventions for Autism Spectrum Disorder: A Systematic Review. Pediatrics 2017, 139, e20170346. [Google Scholar] [CrossRef]
- Saini, V.; Kadey, H.J.; Paszek, K.J.; Roane, H.S. A Systematic Review of Functional Analysis in Pediatric Feeding Disorders. J. Appl. Behav Anal. 2019, 52, 1161–1175. [Google Scholar] [CrossRef]
- Emond, A.; Emmett, P.; Steer, C.; Golding, J. Feeding Symptoms, Dietary Patterns, and Growth in Young Children with Autism Spectrum Disorders. Pediatrics 2010, 126, e337–e342. [Google Scholar] [CrossRef]
- Johnson, C.R.; Turner, K.; Stewart, P.A.; Schmidt, B.; Shui, A.; Macklin, E.; Reynolds, A.; James, J.; Johnson, S.L.; Manning Courtney, P.; et al. Relationships between Feeding Problems, Behavioral Characteristics and Nutritional Quality in Children with ASD. J. Autism Dev. Disord. 2014, 44, 2175–2184. [Google Scholar] [CrossRef]
- Suarez, M.A.; Nelson, N.W.; Curtis, A.B. Longitudinal Follow-up of Factors Associated with Food Selectivity in Children with Autism Spectrum Disorders. Autism 2014, 18, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.L.; Llewellyn, C.; Fildes, A.; Ronald, A. Autism Spectrum Disorder and Food Neophobia: Clinical and Subclinical Links. Am. J. Clin. Nutr. 2018, 108, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Curtin, C.; Phillips, S.; Anderson, S.E.; Maslin, M.; Must, A. Changes in Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 439–446. [Google Scholar] [CrossRef]
- Esteban-Figuerola, P.; Canals, J.; Fernández-Cao, J.C.; Arija Val, V. Differences in Food Consumption and Nutritional Intake between Children with Autism Spectrum Disorders and Typically Developing Children: A Meta-Analysis. Autism 2019, 23, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.A.; Mou, Y.; Dieleman, G.C.; Voortman, T.; Jansen, P.W. Child Autistic Traits, Food Selectivity, and Diet Quality: A Population-Based Study. J. Nutr. 2022, 152, 856–862. [Google Scholar] [CrossRef]
- Charman, T.; Gotham, K. Measurement Issues: Screening and Diagnostic Instruments for Autism Spectrum Disorders—Lessons from Research and Practise. Child Adolesc. Ment. Health 2013, 18, 52–63. [Google Scholar] [CrossRef]
- Craigie, A.M.; Lake, A.A.; Kelly, S.A.; Adamson, A.J.; Mathers, J.C. Tracking of Obesity-Related Behaviours from Childhood to Adulthood: A Systematic Review. Maturitas 2011, 70, 266–284. [Google Scholar] [CrossRef]
- De Souza Silva, E.; Castro, K.; Valle, S.C.; Dos Santos Vaz, J. Dietary Assessment Methods Applied in Clinical and Epidemiological Studies in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2023. [Google Scholar] [CrossRef]
- Holloway, J.M.; Gray, H.L.; Buro, A.W.; Thomas, J.; Sauls, R.; Howard, A.M. Measurement Tools to Assess Usual Dietary Intake and Physical Activity in Individuals with Autism Spectrum Disorder: A Scoping Review. Rev. J. Autism Dev. Disord. 2022. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021); Cochrane: London, UK, 2021; Available online: https://training.cochrane.org/handbook/archive/v6.2 (accessed on 20 July 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Gough, D.; Thomas, J.; Oliver, S. Clarifying Differences between Review Designs and Methods. Syst. Rev. 2012, 1, 28. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Santini, A. The Importance of Referencing. J. Crit. Care Med. 2018, 4, 3–4. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Olafsdottir, A.S. Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial. Nutrients 2022, 14, 4000. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Bjarnason, R.; Eliasdottir, H.G.; Olafsdottir, A.S. Body Composition in Fussy-Eating Children, with and without Neurodevelopmental Disorders, and Their Parents, Following a Taste Education Intervention. Nutrients 2023, 15, 2788. [Google Scholar] [CrossRef] [PubMed]
- Burrell, T.L.; Scahill, L.; Nuhu, N.; Gillespie, S.; Sharp, W. Exploration of Treatment Response in Parent Training for Children with Autism Spectrum Disorder and Moderate Food Selectivity. J. Autism Dev. Disord. 2023, 53, 229–235. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Haraldsson, H.; Olafsdottir, A.S. Taste Education—A Food-Based Intervention in a School Setting, Focusing on Children with and without Neurodevelopmental Disorders and Their Families. A Randomized Controlled Trial. Appetite 2021, 167, 105623. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Burrell, T.L.; Berry, R.C.; Stubbs, K.H.; McCracken, C.E.; Gillespie, S.E.; Scahill, L. The Autism Managing Eating Aversions and Limited Variety Plan vs. Parent Education: A Randomized Clinical Trial. J. Pediatr. 2019, 211, 185–192.e1. [Google Scholar] [CrossRef]
- Taylor, T.; Kozlowski, A.M.; Girolami, P.A. Comparing Behavioral Treatment of Feeding Difficulties and Tube Dependence in Children with Cerebral Palsy and Autism Spectrum Disorder. NeuroRehabilitation 2017, 41, 395–402. [Google Scholar] [CrossRef]
- Patton, S.R.; Odar Stough, C.; Pan, T.Y.; Holcomb, L.O.; Dreyer Gillette, M.L. Associations between Autism Symptom Severity and Mealtime Behaviors in Young Children Presented with an Unfamiliar Food. Res. Dev. Disabil. 2020, 103, 103676. [Google Scholar] [CrossRef] [PubMed]
- Kral, T.V.E.; O’Malley, L.; Johnson, K.; Benvenuti, T.; Chittams, J.; Quinn, R.J.; Thomas, J.G.; Pinto-Martin, J.A.; Levy, S.E.; Kuschner, E.S. Effects of a Mobile Health Nutrition Intervention on Dietary Intake in Children Who Have Autism Spectrum Disorder. Front. Pediatr. 2023, 11, 1100436. [Google Scholar] [CrossRef] [PubMed]
- Javadfar, Z.; Abdollahzad, H.; Moludi, J.; Rezaeian, S.; Amirian, H.; Foroughi, A.A.; Nachvak, S.M.; Goharmehr, N.; Mostafai, R. Effects of Vitamin D Supplementation on Core Symptoms, Serum Serotonin, and Interleukin-6 in Children with Autism Spectrum Disorders: A Randomized Clinical Trial. Nutrition 2020, 79–80, 110986. [Google Scholar] [CrossRef]
- Doaei, S.; Bourbour, F.; Teymoori, Z.; Jafari, F.; Kalantari, N.; Abbas Torki, S.; Ashoori, N.; Nemat Gorgani, S.; Gholamalizadeh, M. The Effect of Omega-3 Fatty Acids Supplementation on Social and Behavioral Disorders of Children with Autism: A Randomized Clinical Trial. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 12–18. [Google Scholar] [CrossRef]
- Miyajima, A.; Tateyama, K.; Fuji, S.; Nakaoka, K.; Hirao, K.; Higaki, K. Development of an Intervention Programme for Selective Eating in Children with Autism Spectrum Disorder. Hong Kong J. Occup. Ther. 2017, 30, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Fujii, Y.; Hijikata, N. Support and Development of Autistic Children with Selective Eating Habits. Brain Dev. 2020, 42, 121–128. [Google Scholar] [CrossRef]
- González-Domenech, P.J.; Díaz Atienza, F.; García Pablos, C.; Fernández Soto, M.L.; Martínez-Ortega, J.M.; Gutiérrez-Rojas, L. Influence of a Combined Gluten-Free and Casein-Free Diet on Behavior Disorders in Children and Adolescents Diagnosed with Autism Spectrum Disorder: A 12-Month Follow-Up Clinical Trial. J. Autism Dev. Disord. 2020, 50, 935–948. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-Aguilar, M.J.; Gomez-Fernandez, A.; Flores-Rojas, K.; Martin-Borreguero, P.; Mesa, M.D.; Perez-Navero, J.L.; Olivares, M.; Gil, A.; Gil-Campos, M. Docosahexaenoic and Eicosapentaenoic Intervention Modifies Plasma and Erythrocyte Omega-3 Fatty Acid Profiles But Not the Clinical Course of Children With Autism Spectrum Disorder: A Randomized Control Trial. Front. Nutr. 2022, 9, 790250. [Google Scholar] [CrossRef]
- Galpin, J.; Osman, L.; Paramore, C. Sensory Snack Time: A School-Based Intervention Addressing Food Selectivity in Autistic Children. Front. Educ. 2018, 3, 77. [Google Scholar] [CrossRef]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-Free Diet in Children with Autism Spectrum Disorders: A Randomized, Controlled, Single-Blinded Trial. J. Autism Dev. Disord. 2020, 50, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.M.Y.; Law, Q.P.S.; Fong, S.S.M. Using Physical Food Transformation to Enhance the Sensory Approval of Children with Autism Spectrum Disorders for Consuming Fruits and Vegetables. J. Altern. Complement. Med. 2020, 26, 1074–1079. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chung, K.-M.; Jung, S. Effects of Repeated Food Exposure on Increasing Vegetable Consumption in Preschool Children with Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2018, 47, 26–35. [Google Scholar] [CrossRef]
- Sammels, O.; Karjalainen, L.; Dahlgren, J.; Wentz, E. Autism Spectrum Disorder and Obesity in Children: A Systematic Review and Meta-Analysis. Obes. Facts 2022, 15, 305–320. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.L.; Levy, S.E.; Myers, S.M.; Council On Children with Disabilities. Section on Developmental and Behavioral Pediatrics Identification, Evaluation, and Management of Children with Autism Spectrum Disorder. Pediatrics 2020, 145, e20193447. [Google Scholar] [CrossRef]
- Malhi, P.; Saini, S.; Bharti, B.; Attri, S.; Sankhyan, N. Sensory Processing Dysfunction and Mealtime Behavior Problems in Children With Autism. Indian Pediatr. 2021, 58, 842–845. [Google Scholar] [CrossRef]
- Margari, L.; Marzulli, L.; Gabellone, A.; de Giambattista, C. Eating and Mealtime Behaviors in Patients with Autism Spectrum Disorder: Current Perspectives. Neuropsychiatr. Dis. Treat. 2020, 16, 2083–2102. [Google Scholar] [CrossRef]
- DeMand, A.; Johnson, C.; Foldes, E. Psychometric Properties of the Brief Autism Mealtime Behaviors Inventory. J. Autism Dev. Disord. 2015, 45, 2667–2673. [Google Scholar] [CrossRef]
- Lukens, C.T.; Linscheid, T.R. Development and Validation of an Inventory to Assess Mealtime Behavior Problems in Children with Autism. J. Autism Dev. Disord. 2008, 38, 342–352. [Google Scholar] [CrossRef]
- Salvador Castell, G. ¿Qué y Cuánto Comemos? Método de Recuerdo 24 Horas. Nutr. Hosp. 2015, 21, 46–48. [Google Scholar] [CrossRef]
- Foster, E.; Adamson, A. Challenges Involved in Measuring Intake in Early Life: Focus on Methods. Proc. Nutr. Soc. 2014, 73, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.; Bradley, J. Methodological Considerations and Future Insights for 24-Hour Dietary Recall Assessment in Children. Nutr. Res. 2018, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Databases | Search Strategy 10-August-2023 | Results |
|---|---|---|
| PubMed | ||
| #1 | “arthropod struct dev” [Journal] OR “agron sustain dev” [Journal] OR “asd” [All Fields] OR (“autism s” [All Fields] OR “autisms” [All Fields] OR “autistic disorder” [MeSH Terms] OR (“autistic” [All Fields] AND “disorder” [All Fields]) OR “autistic disorder” [All Fields] OR “autism” [All Fields]) OR (“autistic disorder” [MeSH Terms] OR (“autistic” [All Fields] AND “disorder” [All Fields]) OR “autistic disorder” [All Fields] OR “autistic” [All Fields] OR “autistics” [All Fields] OR “autists” [All Fields]) OR (“asperger” [All Fields] OR “asperger s” [All Fields] OR “aspergers” [All Fields]) OR “Rett” [All Fields] OR (“pervasive” [All Fields] OR “pervasively” [All Fields] OR “pervasiveness” [All Fields]) OR “disintegrative” [All Fields] | 109,644 |
| #2 | (“food” [MeSH Terms] OR “food” [All Fields] OR “diet” [MeSH Terms] OR “diet” [All Fields]) | 1,876,154 |
| #1 AND #2 | 4396 | |
| #1 AND #2 in the last 5 years | 2110 | |
| Scopus | ||
| #1 | TITLE-ABS-KEY ((asd OR autism OR autistic OR asperger OR rett OR pervasive OR disintegrative)) | 213,111 |
| #2 | TITLE-ABS-KEY ((food OR diet)) | 2,463,041 |
| #1 AND #2 | 6555 | |
| #1 AND #2 in the last 5 years | 3056 | |
| EMBASE | ||
| #1 | ‘asd’/exp OR asd OR ‘autism’/exp OR autism OR autistic OR asperger OR rett OR pervasive OR disintegrative | 178,684 |
| #2 | ‘food’/exp OR food OR ‘diet’/exp OR diet | 2,099,081 |
| #1 AND #2 | 7083 | |
| #1 AND #2 in the last 5 years | 3829 | |
| Web of Science | ||
| #1 | asd OR autism OR autistic OR asperger OR rett OR pervasive OR disintegrative (Topic) | 209,781 |
| #2 | food OR diet (Topic) | 2,679,024 |
| #1 AND #2 | 5994 | |
| #1 AND #2 in the last 5 years | 3544 | |
| PsycINFO | ||
| #1 | (ASD OR autism OR autistic OR asperger OR Rett OR pervasive OR disintegrative) | 113,239 |
| #2 | (food OR diet) | 136,365 |
| #1 AND #2 | 2076 | |
| #1 AND #2 in the last 5 years | 835 |
| Author, Year | Design | Sample (n), Country | Participants | Intervention/Comparator | Evaluation | Dietary Study Outcomes |
|---|---|---|---|---|---|---|
| Miyajima et al. [39], 2017 | nRCT | 49, Japan Loss to follow up (n = 26) | 23 parents of children with ASD. Age between 3 and 6 years (mean age 4.40 years) | PEP on selective feeding/NA | 2 months prior to the intervention, pre- and post- evaluation | Variety and number of foods consumed, and food selectivity |
| Taylor et al. [34], 2017 | nRCT | 58, United States Loss to follow up (n = 0) | 58 children (25 with ASD, 33 with CP). Age between 1 and 12 years (mean age 5.8 years) | Intensive feeding program/NA | Pre-, and post-evaluation | Grams of food consumed, eating difficulties and mealtime behaviours |
| Galpin et al. [43], 2018 | nRCT | 23, United Kingdom Loss to follow up (n = 4) | 19 children with ASD. Age between 4.5 and 10.5 years (mean age 6 years) | Sensory based selective eating intervention/NA | Pre- and post- evaluation | Food variety and mealtime behaviours |
| Kim et al. [46], 2018 | RCT | 42, Korea Loss to follow up (n = 7) | 35 children with ASD. Age between 2 and 5.5 years (mean age 4.2 years) | Exposure program to vegetables/Usual treatment, applied behaviour analysis | Pre- and post- evaluation | Dietary intake |
| Sharp et al. [33], 2019 | RCT | 111, United States Loss to follow up (n = 73) | 38 parent-child with ASD dyads. Age between 3 and 7 years (mean age 4.9 years) | MEAL/PEP | Pre-, at week 12 and post- evaluation. And 4 weeks after for intervention group. | Children’s eating problems improvement and mealtime behaviours. |
| Piwowarczyk et al. [44], 2019 | RCT | 79, Poland Loss to follow up (n = 13) | 66 children with ASD. Age between 3 and 6 years (mean age 4 years). | Gluten-free diet/Gluten-containing diet | Pre- and post-evaluation | Adherence to the gluten-containing and to the gluten-free diet |
| González-Domenech et al. [41], 2019 | RCT | 40, Spain Loss to follow up (n = 11) | 29 children with ASD. Age between 2 and 18 years (mean age 8.9 years). | Began with normal diet and ended with gluten-free and casein-free diet/Began with gluten-free and casein-free diet and ended with normal diet | Pre-, after first diet and after second diet evaluation | Adherence to the Diet Protocol |
| Yamane et al. [40], 2019 | nRCT | 40, Japan Loss to follow up (n = 0) | 40 children with ASD. Age between 3 and 6 years (mean age NS). | Diet based on sensory factors/Diet based on visual appearance of foods/Diet based on familiar foods | Pre- and post-evaluation | Eating habits |
| Javadfar et al. [37], 2020 | RCT | 52, Iran Loss to follow up (n = 9) | 43 children with ASD. Age between 3 and 13 years (mean age 8.9 years). | 300–6000 IU/kg of Vitamin D/Placebo | Pre-, at week 8 and post- evaluation | Dietary intake |
| Chung et al. [45], 2020 | nRCT | 56, China Loss to follow up (n = 0) | 56 children with ASD. Age between 8 and 15 years (mean age 10.7 years). | Exposure program to fruits and vegetables/NA | Pre- and post- evaluation | Fruits and vegetables acceptance, habitual fruits and vegetables consumption and mealtime behaviours |
| Patton et al. [35], 2020 | nRCT | 73, United States Loss to follow up (n = 0) | 73 children with ASD. Age between 2 and 8 years (mean age 5.4 years). | Unfamiliar food presentation /NA | Pre- and post-evaluation | Mealtime behaviour |
| Doaei et al. [38], 2021 | RCT | 64, Iran Loss to follow up (n = 10) | 54 children with ASD. Age between 5 and 15 years (mean age 8.2 years). | 1000 mg omega-3/Placebo | Pre- and post-evaluation | Dietary intake |
| Thorsteinsdottir et al. [32], 2021 | RCT | 190, Iceland Loss to follow up (n = 109) | 81 parent–child with ASD, ADHD or another ND dyads. Age between 8 and 12 years (mean age 10.4 years). | “Taste Education” program, immediate intervention/“Taste Education” program, delayed intervention | Pre-, post- and 6 months follow up evaluation | Fussy eating Food acceptance and variety |
| De la Torre-Aguilar et al. [42], 2022 | RCT | 117, Spain Loss to follow up (n = 9) | 54 children with ASD. Age between 2 and 6 years (mean age 3.6 years). | 800 mg omega-3 + 25 mg EPA/Placebo | Pre- and post-evaluation | Dietary intake and adequacy of food consumption |
| Kral et al. [36], 2023 | RCT | 38, United States Loss to follow up (n = 0) | 38 parent–child with ASD dyads. Age between 6 and 10 years (mean age 8.6 years). | mHealth intervention/Education about healthy eating | Pre- and post-evaluation | Dietary intake |
| Dietary Assessment Tool Used | Author, Year | Participants and Diagnosis | Dietary Assessment Tool Description | Scores | Assessment Manager |
|---|---|---|---|---|---|
| BAMBI | Galpin et al. [43], 2018 | 19 children with ASD | 18 items. A parent-reported standardized measure of mealtime behaviours. Three subscale scores were included: limited diversity, food refusal and features of autism. | Likert scale was used as scoring system ranging from 1 (Never/Rarely) to 5 (At almost every meal) for each question. Higher scores reflected more problematic mealtime behaviours. | Teachers, therapists and parents |
| Chung et al. [45], 2020 | 56 children with ASD | 18 items. A parent-reported standardized measure of mealtime behaviours with three subscale scores: limited variety, features of autism and food refusal. | Likert scale ranging from 1 (Never/Rarely) to 5 (always) was used for each question. Problematic mealtime behaviours were reflected by higher scores. | Not clearly stated | |
| Patton et al. [35], 2020 | 73 children with ASD | 18 items. A parent-reported standardised measure of mealtime behaviours with three subscale scores: limited variety, features of autism and food refusal. | Scoring system based on a Likert scale ranging from 1 (Never/Rarely) to 5 (At Almost Every Meal) for each question. Higher scores reflected more problematic mealtime behaviours. | Study personnel | |
| BAMBI-R | Sharp et al. [33], 2019 | 38 parent-child with ASD dyads | 15 items. Questionnaire on mealtime behaviours common to children with ASD that contains four aspects (food selectivity, food refusal, mealtime rigidity disruptive and mealtime behaviours). | Five-point Likert scale measure each item, from 1 (never) to 5 (always). A total score ≥ 34 is considered clinically meaningful. Greater eating behaviour problems are reflected by higher scores. | Treatment assignment was performed by an independent evaluator. |
| 24-h dietary recall | Kim et al. [46], 2018 | 25 children with ASD | Parent-reported food diary for three self-selected days (2 weekdays and 1 weekend day). | Dietary intakes were analysed via CAN-PRO 4.0, which provides the amount of intake across 60 nutrients. | Graduate students |
| González-Domenech et al. [41], 2019 | 37 children with ASD | Unspecific number of items. Parents completed two 24-h recall per week which consisted of listing each food and beverage intake during the preceding 24 h. | Good compliant (adherence of 80–100% of the diet), intermediate compliant (adherence of 50–79%) and poor compliant (<50%). | Psychiatrist/ Psychologist | |
| De la Torre Aguilar et al. [42], 2022 | 54 children with ASD | Three non-consecutive 24-h dietary registrations. Parent-reported children’s following the Guidance on the Menu Methodology of the European Food Safety Agency | Not clearly stated. | Not clearly stated. | |
| Kral et al. [36], 2023 | 81 parent-child with ASD dyads | Three non-consecutive 24-h dietary registrations. Parent-reported questionnaires. | Calories consumption from salty and sugary snacks, sugar-sweetened beverages, water and fruits and vegetables were calculated using the University of Minnesota Nutrition Coordinating Center’s Food and Nutrient Database. | Dietitians | |
| FFQ | Chung et al. [45], 2020 | 56 children with ASD | Number of items not stated. Pre- and post- caregiver-reported questionnaires were used to assess habitual fruits and vegetables consumption. | The frequencies of fruit and vegetables consumption were assessed using a five-point Likert scale ranging from 1 (never) to 5 (always). | Not clearly stated |
| Doaei et al. [38], 2021 | 54 children with ASD | 168 items. Parent-reported semi-quantitative validated tool to assess habitual food consumption. | Scores of questionnaires were transformed to grams/day using Iranian standard portions. | Nutritionist | |
| De la Torre-Aguilar et al. [42], 2022 | 54 children with ASD | FFQ: number of items not stated. Parent-reported measure to assess children’s dietary intake. | Not clearly stated. | Not clearly stated. | |
| 3-day food records | Piwowarczyk et al. [44], 2019 | 66 children with ASD | These records were obtained in two different moments of follow-up (week 2–4 and at week 12). | Adherence to the gluten-containing diet was described as the consumption in more than one meal every day of some gluten-containing foods. Adherence to the gluten-free diet was described as suitable when no intake of gluten was stated in the food record. | Study coordinators and psychologist |
| Javadfar et al. [37], 2020 | 43 children with ASD | These records were collected at baseline, 8 and 15 weeks of the intervention. | Nutritionist IV software evaluated dietary intake. Energy and micro/macronutrients were calculated. Higher scores reflect higher intakes. | Dietitian | |
| Food questionnaire | Miyajima et al. [39], 2017 | 23 parents of children with ASD | 47 items. A non-standardized list of food which included carbohydrate-rich foods, liquids, meat, fish, beans, potatoes, seaweed, vegetables, mushrooms and eggs | Scoring system from 0 to 47 points. Higher scores represented higher food items consumed by children. | OT |
| Galpin et al. [43], 2018 | 19 children with ASD | 60 items. A non-standardized list which included 52 types of food, 3 liquids and 5 sauces. | Scoring system from 0 to 60 points. Higher scores represented higher variety of food consumed. | Teachers, therapists and parents | |
| SAPS | Miyajima et al. [39], 2017 | 23 parents of children with ASD | 12 items. It measures the degree of parental self-efficacy in three areas: rudimentary attitudes to eating, factors related to likes and dislikes and agreement to recommendations for selective eating. | Scoring system from 12 to 60 points. A Likert type scale was used; higher scores represented higher parental self-efficacy. | OT |
| CEBI | Taylor et al. [34], 2017 | 58 children (25 with ASD, 33 with CP). | 40 items. Caregiver-report measure intended to assess eating and mealtime problems. | Two scores are obtained from the questionnaire: (i) the Total Eating Problems score which include a rate of 19 types of eating behaviours) and (ii) the Total Perceived Problems score (Behaviours that may or may not present a problem for the family). Higher scores represented higher eating and mealtime problems. | Feeding therapists |
| CGI-I | Sharp et al. [33], 2019 | 38 parent-child with ASD dyads | Unspecific number of items. Independent evaluator-rated, seven-point scale developed to measure the two most important feeding difficulties improvement. | Scores range from 1 (Very Much Improved) to 4 (Unchanged) to 7 (Very Much Worse). Very much improved or fairly improved (i.e., 2 or 1) were used to define the positive response; negative response was indicated by all other scores. | Treatment assignment was performed by an independent evaluator. |
| Household eating records | Yamane et al. [40], 2019 | 38 children with ASD | Records which included food preferences, environment and sensory tendencies. | Scores range from 1-5 (only milk; only carbohydrates; protein and carbohydrates; some vegetables; everything). | Nutritionist |
| DINE | Patton et al. [35], 2020 | 73 children with ASD | Unspecific number of items. This questionnaire included information about child’s eating and behaviour, and parent behaviour. | Family mealtime behaviours using the DINE were recorded and then videos were coded and analysed. This instrument does not have a final score. | Graduate students |
| CEBQ | Thorsteinsdottir et al. [32], 2021 | 81 parent-child with ASD, ADHD or another ND dyads | 35 items. Parent-reported measure to assess children´s fussy eating. | Five-point Likert scale with 35 items, from “never” to “always”. Higher levels of fussy eating were indicated by a higher score for food fussiness. High levels of enjoyment were indicated by a high score for enjoyment of food. | Psychologist/ Nutritionist |
| Food indices | Thorsteinsdottir et al. [32], 2021 | 81 parent-child with ASD, ADHD or another ND dyads | 57 items. Parent-reported intake of designated food items, clustered into three food indices (Fruit; nuts, seeds, and dried fruits). | Percentages of change in food acceptance and variety calculated with a dichotomous variable (accept or reject) | Psychologist/ Nutritionist |
| Author, Year | Main Limitations | Funding/Support | Conflicts of Interest |
|---|---|---|---|
| Miyajima et al. [39], 2017 | - A lack of an operational definition of selective eating. - No significant change in the degree of food items acceptable by children. - Loss of follow-up due to the difficulty of the parents to follow the recommendations. - Difficulties in addressing the subject in children who had solid selective eating. | No financial support of any kind. | None declared. |
| Taylor et al. [34], 2017 | - Retrospective study. - The severity of CP and ASD diagnoses is unknown. - A lack of categorization based on the severity of motor impairment. | Not stated. | None declared. |
| Galpin et al. [43], 2018 | - Small sample size. - Low generalizability of the results due to the heterogeneous group. - A lack of control group. - A lack of baseline control period. - A lack of meaningful standardized assessment measures. - Experimenter bias. | Not stated. | None declared. |
| Kim et al. [46], 2018 | - Small sample size. - Convenient sampling method was used to select participants. - Large variability among participants because the different characteristics included in the feeding problems. - The observed variables did not meet normal distribution. - Non-significant changes in nutritional intake. | Not stated. | None declared. |
| Sharp et al. [33], 2019 | - Small sample size. - Parent-completed questionnaires. - Interaction between food selectivity and disruptive behaviour. - A lack of standardized measures to assess dietary variety. - Changes in food selectivity severity were not assessed. - Treatment assignment was not blinded from parents. | Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study by grants to Emory University (MH081148). | None declared. |
| Piwowarczyk et al. [44], 2019 | - Possible randomization bias due to some children possibly following a gluten free diet before the study. - Single blinding. - A lack of adherence to the allocated diet. | The Nutricia Foundation research Grant [RG8/2013] funded the study. | Some authors collaborate with Nutricia Foundation. |
| González-Domenech et al. [41], 2019 | - Small sample size. - Difficulty for caregivers to follow recommendations. - Dietary errors outside the scope of the main caregiver. - Interindividual variability in relation to the age variable. - No conclusive results were found. - A lack of washing period between two interventions. | Not stated. | None declared. |
| Yamane et al. [40], 2019 | - The support group classification was only based on observations. | Not stated. | None declared. |
| Javadfar et al. [37], 2020 | - Small sample size. - The supplementation period was short. | Vice chancellor of research and technology of Kermanshah University of Medical Sciences funded the study as a thesis proposal for the MSc degree. | None declared. |
| Chung et al. [45], 2020 | - Small sample size. - Low generalizability of the snack preparation to all type of foods. - A lack of control group. - A lack of statistical significance. - Only three fruits and three vegetables were studied. | No financial support of any kind. | None declared. |
| Patton et al. [35], 2020 | - Families knew they were being observed during meals, fact that can reduce mealtime interactions. - Evaluators were not blinded. - Low internal consistency on some of the BAMBI subscales. - Specific questionnaires to assess sensory sensitivity were not used. | The Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R21HD076116); the Doctoral Student Research Award from the University of Kansas; and the Brown-Kirschmanv Award for Research Excellence from the University of Kansas supported the study. | None declared. |
| Doaei et al. [38], 2021 | - Small sample size. | Not stated. | None declared. |
| Thorsteinsdottir et al. [32], 2021 | - Low generalizability of the results due to not including children with ASD and lower-functioning. - No control comparison group with parental education sessions only. - No measurement of the weight of the food consumed by the children in the sessions was carried out. - Changes in children’s medications doses were not registered. - A lack of direct observation by researchers. - The sample had a high proportion of parents with higher education and full-time jobs, while there was a low proportion of single-parent homes. | The University of Iceland’s Research fund and the Public Health Fund of the Directorate of Health supported the study. | None declared. |
| De la Torre-Aguilar et al. [42], 2022 | - Small sample size. - Loss of follow-up. - Low generalizability of the results due to the inclusion of only one centre. - Methods are different from other trials making it difficult to compare all their results. | Maternal-Infant and Developmental Health Network, Carlos III Health Institute | One author collaborated with Biosearch Life, a company that promoted the placebo and the nutritional supplement |
| Kral et al. [36], 2023 | - Small sample size. - Some final evaluations could not be carried out due to COVID-19 pandemic restrictions. - Difficulties in enrolment due to COVID-19 pandemic restrictions. - Short duration of the intervention. | The Eunice Kennedy Shriver National Institute of Child Health and Human Development | One author had a financial conflict of interest related to the intellectual property of the mHealth nutrition intervention that was used in the study. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compañ-Gabucio, L.M.; Ojeda-Belokon, C.; Torres-Collado, L.; García-de-la-Hera, M. A Scoping Review of Tools to Assess Diet in Children and Adolescents with Autism Spectrum Disorder. Nutrients 2023, 15, 3748. https://doi.org/10.3390/nu15173748
Compañ-Gabucio LM, Ojeda-Belokon C, Torres-Collado L, García-de-la-Hera M. A Scoping Review of Tools to Assess Diet in Children and Adolescents with Autism Spectrum Disorder. Nutrients. 2023; 15(17):3748. https://doi.org/10.3390/nu15173748
Chicago/Turabian StyleCompañ-Gabucio, Laura María, Carolina Ojeda-Belokon, Laura Torres-Collado, and Manuela García-de-la-Hera. 2023. "A Scoping Review of Tools to Assess Diet in Children and Adolescents with Autism Spectrum Disorder" Nutrients 15, no. 17: 3748. https://doi.org/10.3390/nu15173748
APA StyleCompañ-Gabucio, L. M., Ojeda-Belokon, C., Torres-Collado, L., & García-de-la-Hera, M. (2023). A Scoping Review of Tools to Assess Diet in Children and Adolescents with Autism Spectrum Disorder. Nutrients, 15(17), 3748. https://doi.org/10.3390/nu15173748






