Abstract
Background: Whether the World Cancer Research Fund and the American Institute for Cancer Research (WCRF/AICR) dietary recommendations affect the gut microbiota and inflammatory status remains unclear. We examined the association of dietary adherence scores to the WCRF/AICR with gut microbiota and inflammation in a cross-sectional setting. Methods: The WCRF/AICR diet adherence scores were calculated for 151 participants (adenoma 97, non-adenoma 54) from 7-day dietary records. The gut microbiota was analyzed by 16S rRNA gene sequencing of fecal samples. The levels of inflammatory biomarkers in both blood (i.e., IL-6, IL-8, IgA, IgM, and IgG) and fecal samples (i.e., FCP) were evaluated in 97 colorectal adenoma patients who had blood samples available. Multivariable linear regression analyses were conducted to examine the association of individual and total dietary adherence scores with gut microbiota and inflammatory biomarker levels. Results: Participants with higher adherence had lower relative abundance of Proteobacteria (β = −0.041, 95%CI: −0.073, −0.009), Enterobacteriaceae (β = −0.035, 95%CI: −0.067, −0.003), and unidentified Enterobacteriaceae at the genus level (β = −0.029, 95%CI: −0.055, −0.003) compared to those with lower adherence. Plant-based food intake was positively correlated with increased abundance of Phascolarctobacterium (β = 0.013, 95%CI: 0.001, 0.026). Restricting fast food was linked to high abundance of Bacteroidaceae (β = 0.149, 95%CI: 0.040, 0.257) and Bacteroides (β = 0.149, 95%CI: 0.040, 0.257). Limiting sugary drinks was associated with reduced abundance of Lachnospiraceae (β = −0.155, 95%CI: −0.292, −0.018). Plant-based food intake (β = −0.251, 95%CI: −0.450, −0.052) and restriction of fast food (β = −0.226, 95%CI: −0.443, −0.008) were associated with reduced IGG levels in men. Alcohol restriction was linked to lower IL-6 (β = −7.095, 95%CI: −11.286, −2.903) and IL-8 (β = −7.965, 95%CI: −14.700, −1.230) levels in women, but with higher IL-6 (β = 0.918, 95%CI: 0.161, 1.675) levels in men. Conclusions: Our findings support the association of adherence to the WCRF/AICR diet with gut microbiota and inflammation. These results need to be validated in additional prospective or interventional studies.
1. Introduction
Colorectal cancer (CRC) is a global public health concern, ranking as the third most prevalent malignancy in men and the second in women, while also being the second leading cause of cancer-related mortality worldwide [1]. The adenoma–carcinoma sequence, with adenomas as the most frequent precancerous lesions, currently serves as the classical carcinogenic pathway explaining the majority of CRC cases [2,3]. Notably, modifiable environmental factors, such as dietary habits and lifestyles, contribute to the increasing incidence of CRC due to the ongoing trend towards westernized diets and sedentary behaviors [3].
Extensive research into the etiology of colorectal cancer has shed light on the crucial role of gut microbial composition and inflammation in the intricate relationship between diet and cancer [4]. The gut microbiota, influenced by dietary factors, acts as a key mediator in the interaction between the immune system and the host’s overall health and disease outcomes [5]. Consequently, therapeutic approaches targeting the gut microbiota and immune system through nutritional interventions, specifically diet modifications, have emerged as potential strategies for combating CRC [6].
In 2018, the World Cancer Research Fund and American Institute for Cancer Research released their updated 10 cancer prevention recommendations (2018 WCRF/AICR), providing evidence-based guidelines for maintaining a healthy diet, incorporating supplements, breastfeeding, weight management, and engaging in physical activity [7]. Studies have indicated that adhering to these recommendations not only enhances the quality of life for colorectal cancer survivors but also improves patient-reported outcomes following a cancer diagnosis [8,9,10,11]. The WCRF/AICR dietary recommendations, recognized as an anti-inflammatory diet, have demonstrated promising effects on human metabolism and immune function [12,13]. Previous investigations have shown that adherence to the 2007 WCRF/AICR dietary recommendations and energy-related dietary guidelines correlates with reduced oxidative stress and a more favorable inflammatory biomarker profile [14,15]. Furthermore, Laura et al. identified that higher consumption of animal-derived foods, processed foods, sugar, and alcohol coincided with a microbiome signature indicative of inflammation and was associated with elevated levels of intestinal inflammatory biomarkers, while plant-based foods were linked to a lower abundance of pathobionts [16].
Despite these findings, no studies have specifically examined the potential impact of diets adhering to the 2018 WCRF/AICR recommendations on gut microbiota composition and inflammatory pathways. Therefore, this study aimed to examine the association between adherence to these recommendations and gut microbiota composition, as well as its effects on inflammation in high-risk individuals for CRC, considering potential gender differences. Understanding this relationship could offer insights into CRC prevention and management strategies.
2. Materials and Methods
2.1. Study Design and Population
The present study used cross-sectional data obtained from the Colorectal Cancer Screening Project at Tianjin Nankai Hospital. All participants underwent colonoscopies between January 2019 and January 2021. The sample population consisted of 151 participants, of which 97 patients with colorectal adenoma were diagnosed by colonoscopy and 54 healthy volunteers without any colorectal cancer or precancerous/adenomatous lesions identified during the same period (non-adenomatous population). All participants signed informed consent.
A structured questionnaire was used to collect information from participants on (1) socio-demographic characteristics and anthropometric measures (e.g., age, gender); (2) selected lifestyle habits (e.g., smoking, alcohol drinking, yogurt consumption, and physical activity); (3) disease and health status (e.g., presence of comorbidities (including hypertension, hyperlipidemia, diabetes, coronary heart disease, gout, fatty liver, atherosclerosis, chronic gastroenteritis, appendicitis, duodenal ulcer), medication history, and self-reported height and weight); and (4) the utilization of dietary and health supplements. Body mass index (BMI) was calculated as weight divided by height2 (kg/m2). Participants’ adherence scores to the WCRF/AICR dietary recommendations were calculated from a detailed, consecutive 7-day dietary record, completed by each participant at the time of enrollment. Seven-day dietary records have been widely used to assess the nutrient intake of participants in epidemiological studies [17,18,19]. To minimize the possibility of information bias, participants were not provided with the specific WCRF/AICR dietary recommendations while filling out the dietary record. In addition, participants were required to provide blood and stool samples for subsequent measurement of inflammatory biomarker levels (adenoma participants) and gut microbiota, respectively. This study was approved by the Ethics Committee of Tianjin Nankai Hospital (No. NKYY_YXKT_IRB_2021_048_01).
2.2. World Cancer Research Fund/American Institute for Cancer Research Diet Adherence Score
We assessed adherence to the 2018 WCRF/AICR dietary recommendations using the scoring criteria proposed by Shams-White and Esposito et al. [20,21]. Specifically, we focused on 5 dietary recommendations: (R1) eating a diet rich in vegetables, fruits, and whole grains, (R2) limiting consumption of fast foods and other processed foods high in fat, sugar, or starch content, (R3) limiting consumption of red and processed meat, (R4) limiting consumption of sugary drinks, and (R5) limiting alcohol consumption. R1 was split into two sub-recommendations (as suggested by the standard scoring system [20,21]): 1a for vegetables and fruits and 1b for whole grains. For each dietary recommendation, participants were assigned 1, 0.5, and 0 points for complete adherence, partial adherence, and non-adherence, respectively. For 1a and 1b, participants were given 0.5 points for complete adherence, 0.25 for partial adherence, and 0 for non-adherence. These scores were then summed to calculate the total WCRF/AICR diet adherence score (ranging from 0 to 5), with higher scores indicating greater adherence to the dietary recommendations.
2.3. Collection of Fecal and Blood Samples
Fresh fecal samples were collected using a stool collector, rapidly frozen in liquid nitrogen, and transferred to the laboratory within 48 h. Venous blood samples from adenoma patients were collected using vacuum anticoagulated (EDTA) blood collection tubes, centrifuged within 4 h of the collection according to standard procedures, and plasma components were isolated and stored at −80 °C for subsequent testing.
2.4. DNA Extraction
Using the PowerMax (stool/soil) DNA isolation kit (MoBio Laboratories, Carlsbad, CA, USA), total bacterial genomic DNAs were extracted from fecal samples. They were then refrigerated at 20 °C for future analysis. The quantity and quality of isolated DNAs were measured using an agarose gel electrophoresis and a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
2.5. 16S rDNA Amplicon Pyrosequencing
The gut microbial species and abundance of participants were determined by 16S rRNA Illumina NovaSeq high-throughput sequencing technology. The V3-V4 region of the bacterial 16 S rRNA gene was amplified by PCR using forward primer 341F CCTACGGGNGGCWGCAG and reverse primer 805R GACTACHVGGGTATCTAATCC. For multiplex sequencing, sample-specific paired-end 6 bp barcodes were added to the TrueSeq adaptors. The PCR components consisted of 10 μL DNA Template, 25 μL of Phusion High-Fidelity PCR Master Mix, 3 μL DMSO, 3 μL (10 μM) of Forward and Reverse primer, and 6 μL ddH2O. The thermal cycling comprised initial denaturation, followed by 25 cycles of denaturation, annealing, and extension, and final extension. Purification of PCR products was performed using Agencourt AMPure XP Beads (Beckman Coulter, Indianapolis, IN, USA), and quantification was performed by the Qubit dsDNA HS Assay. Subsequently, the PCR products were sequenced using the Illumina NovaSeq6000 platform at D I J I Info Technology Co., Ltd. (Nanjing, China).
2.6. Sequence Analysis
The Quantitative Insights Into Microbial Ecology (QIIME, v1.9.0) pipeline was used to process the sequencing data, as described previously [22]. In brief, raw sequencing reads with exact barcode matches were assigned to corresponding samples and identified as valid sequences. The screening criteria for low-quality sequences were as follows [23,24]: sequences <150 bp in length, sequences containing ambiguous bases, sequences with an average Phred score of <20, and sequences with mononucleotide repeats of >8 bp. Vsearch V2.4.4 was used to assemble pairs of end reads (--fastq_mergepairs --fastq_minovlen 5) and to perform operational taxonomic unit (OTU) picking, including dereplication, clustering, and detecting chimeras [25]. Using the default parameters to select a representative sequence from each OTU, a Vsearch of representative sequences against the Greengeen database was used to classify OTUs.
After generating the OTU table, the abundance of each OTU and its classification were recorded for each sample. In all samples, OTUs containing <0.001% of the total sequence were discarded. As an averaged, rounded, rarefied OTU table, we minimized differences in sequencing depth between samples by averaging over a subset of 100 uniformly resampled OTUs below 90% of the minimum sequencing depth.
2.7. Bioinformatics Analysis
The OTU table in QIIME (Quantitative Insights Into Microbial Ecology, v1.8.0, http://qiime.org, last accessed on 1 January 2018) was used to calculate OTU-level alpha diversity indexes, including the Shannon diversity index, Simpson index, Chao1 index, and ACE index. The results of clustering and annotation of OTUs, based on taxonomic information, allowed statistical analysis of community composition at different taxonomic levels (e.g., phylum, family, and genus levels). We used an online website (https://www.bioinformatics.com.cn, last accessed on 10 July 2023) to generate bubble plots of the relative abundance of gut microbiota according to dietary adherence subgroups.
2.8. Assessment of Inflammatory Biomarkers
We evaluated both systemic inflammation and local intestinal inflammation by measuring the levels of five inflammatory biomarkers (i.e., interleukin-6 (IL-6), interleukin-8 (IL-8), immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG)) in the blood, as well as fecal calprotectin (FCP). The concentrations of IgA, IgM, IgG, IL-6, and IL-8 were quantified in the serum sample using the Merck MILLIPLEX®® Protein Multifactor Liquid Chromatography Panel (Human Cytokine Autoantibody Panel). The assay was performed by Beijing Huatai Yikang Biotechnology Co. The quantification of FCP was performed using the Calprotectin ELISA Assay Kit (Eagle BioSciences, NH, USA) through an enzyme-linked immunosorbent assay (ELISA) method.
3. Statistical Analysis
The highest total score for adherence to dietary recommendations was 5 (complete adherence). A cut-off of 3 was used to divide participants into two groups: a low adherence group (0 to <3 points) and a high adherence group (3 to <5 points). Each separate score and the total score of the dietary recommendations were also considered for analysis as continuous variables.
The distributions of biomarker concentrations were skewed, and thus, we performed a natural log transformation of all biomarkers before analysis. The main characteristics of participants were described using means and standard deviations or frequencies and percentages. The t-test (continuous variables) and the χ2 test (categorical variables) were used to test the differences in the distribution of each variable between the two groups. Multivariable linear regression models were used to assess overall adherence scores (categorical and continuous variables) and adherence scores for each of the individual dietary recommendations (continuous variables) with gut microbiota and inflammation. Potential confounding variables included in the analysis of the association between adherence scores and inflammation were gender, age, BMI category (underweight, normal, overweight, and obese), number of comorbidities (0, 1, ≥2), long-term use of anti-inflammatory drugs (yes/no), smoking status (current, former, never). In the analysis of adherence scores and gut microbiota, additional adjusted for adenoma (yes/no), yogurt consumption (yes/no), and physical activity (</≥150 min/week). Furthermore, drinking status was also modeled as a categorical variable (never drinking, current drinking, former drinking) in the adherence scores of the first four dietary recommendations.
We explored whether associations differed between men and women by stratifying the analyses by sex. All analyses were performed with SAS 9.3 (SAS Institute, Cary, NC, USA). p < 0.05 (two-sided) was considered statistically significant.
4. Results
A total of 151 participants with a mean age of 61 years were included. The mean adherence score to the WCRF/AICR diet recommendations was 3.5 (maximum score of 5 and minimum score of 2). Participants with higher levels of adherence were older, more likely to be female, never smoked, never drank alcohol, and had ≥2 comorbidities compared to those with lower levels of adherence (Table 1). No statistical differences emerged for the other factors considered.

Table 1.
Characteristics of participants within categories of adherence to the WCRF/AICR diet score (n = 151). [n (%)/(Mean values ± standard deviations)].
The degree of adherence to the WCRF/AICR dietary recommendations ranged from 2.6% to 94.0% (Table 2). None of the separate dietary recommendations were entirely adhered to by all participants. A total of 49.7% met the recommended daily intake of 400 g of fruit and vegetables. Only four persons (2.6%) met the recommendation to consume 30 g/day of dietary fiber. The ranking of adherence to dietary recommendations from highest to lowest was as follows: limit consumption of sugar-sweetened drinks (94.0%), limit consumption of ultra-processed foods (75.5%), limit alcoholic drink consumption (60.9%), eat adequate vegetables and fruits (49.0%), limit intakes of red and processed meat (45.0%), and eat diets rich in dietary fiber (2.6%).

Table 2.
Dietary WCRF/AICR cancer prevention recommendations and adherence in participants (n = 151) *.
4.1. WCRF/AICR Dietary Adherence and Gut Microbiota
We characterized the effect of adherence scores on the relative abundance of different bacterial taxa at the phylum, family, and genus levels. We restricted our analysis to gut microbiota with a relative abundance of 1% and higher. Examining the relative abundance of gut microbiota in relation to low and high adherence groups revealed that, at the phylum level, both groups were predominantly composed of Proteobacteria (48%) and Bacteroidetes (41%). At the family level, the high adherence group was marked by the dominance of Ruminococcaceae (22%) and an unidentified Clostridiales (20%), whereas the low adherence group was characterized by Ruminococcaceae (25%) and Enterobacteriaceae (18%). No discernible differences in microbiota composition between the two groups were observed at the genus level (Figure 1).
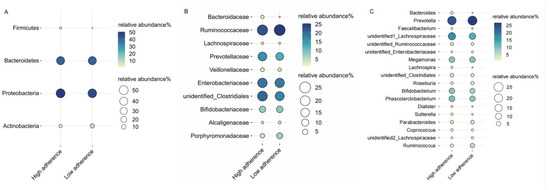
Figure 1.
The distribution of gut bacteria’s relative abundance is depicted across phylum (A), family (B), and genus (C) levels, categorized by the WCRF/AICR dietary adherence scores. The size and color intensity of the circles represents the relative abundance (%) of the respective gut bacteria. Each gut bacteria’s name is presented on the left side, while the high/low adherence group label for each gut bacteria is displayed at the bottom.
Using the low adherence group as a reference, the high adherence group exhibited a significant decrease in the relative abundance of Proteobacteria (β = −0.041, 95%CI: −0.073, −0.009) and Enterobacteriaceae (β = −0.035, 95%CI: −0.067, −0.003) (Table 3). Meanwhile, an unidentified Enterobacteriaceae had a lower relative abundance at the genus level (β = −0.029, 95%CI: −0.055, −0.003).

Table 3.
Differences in the relative abundance of gut microbiota among participants grouped according to the WCRF/AICR dietary adherence scores at the phylum, family, and genus levels (n = 151). (β-Coefficients and 95% confidence intervals).
The results of the analysis of adherence to each dietary recommendation and gut microbiota are shown in Table 4. Higher adherence to vegetable, fruit, and whole grain recommendations was associated with a higher relative abundance of Phascolarctobacterium (β = 0.013, 95%CI: 0.001,0.026). Higher adherence to the fast food recommendations was positively correlated with the abundance of Bacteroides at both the family and genus levels (β = 0.149, 95%CI: 0.040, 0.257), while adherence to the sugary drinks recommendations was significantly associated with a decrease in the abundance of Lachnospiraceae (β = −0.155, 95%CI: −0.292, −0.018).

Table 4.
Relationship between the WCRF/AICR dietary adherence scores and the relative abundance of gut microbiota at the phylum, family, and genus levels (n = 151). (β-Coefficients and 95% confidence intervals).
We further explored the potential relationship between the WCRF/AICR dietary adherence scores and the gut microbiota α-diversity (Shannon, Simpson, chao1, and Ace) indexes. However, our findings did not yield any significant results (Supplementary Tables S1 and S2).
4.2. WCRF/AICR Dietary Adherence and Inflammation
The overall WCRF/AICR diet adherence scores were not associated with the level of inflammation, with the low adherence group as the reference group, and this finding was consistent across gender stratifications. Furthermore, our analysis of adherence to the WCRF/AICR dietary recommendations as a continuous score yielded consistent findings compared to the results obtained from analyzing the exposures as categorical variables (Table 5).

Table 5.
Association between adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) diet score and biomarkers of inflammation (n = 97). (β-Coefficients and 95% confidence intervals).
In our analysis, we employed a gender-stratified approach and observed significant gender differences between adherence scores to each of the WCRF/AICR dietary recommendations and inflammatory biomarkers (Table 6). Specifically, the negative associations of IgG levels with adherence scores for recommendations on vegetables, fruits, and whole grains, as well as fast food consumption, were evident in men only (R1: β = −0.251, 95%CI: −0.450, −0.052; R2, β = −0.226; 95%CI: −0.443, −0.008). Moreover, limited alcohol consumption was positively correlated with IL-6 levels in men (β = 0.918, 95%CI: 0.161, 1.675) but conversely displayed a negative association in women (β = −7.095, 95%CI: −11.286, −2.093). Additionally, limited alcohol consumption was associated with lower IL-8 levels in women (β = −7.965, 95%CI: −14.700, −1.230).

Table 6.
Association between adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) diet score and biomarkers of inflammation (n = 97). (β-Coefficients and 95% confidence intervals).
5. Discussion
Understanding the role of the WCRF/AICR diet in modulating gut microbiota and inflammation can help identify new intervention targets, as well as optimize available methods to prevent or delay CRC. Our study stands as the only investigation exploring the relationship between the WCRF/AICR diet and gut microbiota. The key finding of our study was that adherence to the WCRF/AICR dietary recommendations had a significant effect on the relative abundance of gut microbiota; specifically, individuals with higher adherence to overall dietary recommendations showed a lower abundance of Proteobacteria, Enterobacteriaceae, and an unidentified Enterobacteriaceae at the genus level compared to those with lower adherence. Moreover, the intake of vegetables, fruits, and grains was positively associated with Phascolarctobacterium abundance. Limiting fast food consumption was associated with increased abundance of Bacteroidaceae and Bacteroides. In comparison, limiting sugary drinks was associated with decreased abundance of Lachnospiraceae.
Recent studies have found that the Proteobacteria and Enterobacteriaceae were significantly increased in CRC samples compared to normal samples [26,27]. Numerous studies have demonstrated that the proliferation of potentially harmful Proteobacteria, especially Enterobacteriaceae, can lead to an increased inflammatory response in the host [28,29,30]. The Enterobacteriaceae family has been reported to interfere with intestinal metabolic processes, leading to alterations in the host’s bile acid (BA) metabolism [31], whereas reductions in BA levels seem to favor the growth of pathogenic and pro-inflammatory Enterobacteriaceae [32]. Thus, following the WCRF/AICR dietary recommendations can regulate the composition of the gut microbiota, probably by reducing the amounts of harmful or cancer-causing bacteria, such as Proteobacteria and Enterobacteriaceae.
We observed that a higher intake of whole grains, fruits, and vegetables was associated with a higher abundance of Phascolarctobacterium. As a potentially beneficial bacterium, reduced numbers of Phascolarctobacterium were associated with the presence of colonic inflammation and disruption of gut homeostasis [33]. Higher contents of dietary fibers from fruits and vegetables have been reported to have beneficial effects on the gut microbiome in adults. Intervention studies conducted in both murine and human subjects have consistently reported a significant increase in the abundance of Phascolarctobacterium following soluble corn fiber supplementations [34,35]. In addition, higher dietary vitamin supplementation can also significantly increase the abundance of Phascolarctobacterium in the gut microbiota [36].
Our results indicated that adherence to the dietary recommendations for limiting the intakes of ‘fast food’ and other processed foods characterized by high fat, starch, or sugar content increased the abundance of both Bacteroidaceae and Bacteroides taxa in the gut microbiota. High-fat/carbohydrate diets have been reported to be associated with unfavorable changes in the gut microbiota, leaving it deficient in beneficial genera such as Bacteroides [37]. A systematic literature review on the effects of consuming ultra-processed, very-low-energy foods on the gut microbiota of obese patients found contradictory results that such diets may result in increased or decreased abundance from Bacteroides taxa [38]. Moreover, studies on Spanish populations observed that higher consumption of ultra-processed foods increased the abundance of Bacteroidetaceae in men but impacted differently in women [39]. Given the considerable individual variation in the response of the gut microbiota to dietary habits, the effects of different food groups, compositions, and contents on the human gut are complex. We also observed that limiting sugary drinks was associated with a reduced abundance of Lachnospiraceae, which is consistent with previous reports [16,40]. The progression of CRC may be affected by a higher abundance of the Lachnospiraceae family in the gut microbiome [41]. As previously reported, some Lachnospiraceae strains produce metabolites that are toxic or trigger ecological dysregulation in the host and may exert harmful effects on host health [42]. Their effects on host physiology remain variable and inconclusive across various investigations.
The improved biomarker profile primarily resulted from following the guidelines for consuming vegetables, fruits, and whole grains, and limiting intake of processed foods and alcohol. In contrast, limiting red and processed meat intake and sugary drinks consumption did not appear to affect inflammatory status. Thus, different dietary habits may have important effects in determining the concentrations of biomarkers such as IL-6, IL-8, and IgG. Adherence to limit alcohol consumption and IL-6 levels showed inconsistent relationships across genders, with possible explanations due to the different types of alcohol consumed by men and women. As described in previous studies, distinct characteristics of drinking patterns (binge or regular) or different types of alcoholic drinks (wine, beer, hard liquor, or mixed) may lead to significant changes in biomarkers status and metabolic differences that predict adverse health outcomes [43]. Based on prior literature, the stimulation of oxidative stress and inflammatory consequences of excessive alcohol consumption may be influenced by gender-specific factors [44,45]. These results emphasize the sex-related differences in alcohol-induced inflammation or immune responses. The pro-inflammatory factor IL-6 plays a major role in the chronic inflammatory condition of the body, and its increased levels are thought to stimulate CRC progression [46], while previous studies have reported no significant association between adherence to the 2007 WCRF/AICR recommendations for limiting alcohol consumption and IL-6 [15]. This contradictory result may be attributed to the utilization of distinct operationalization criteria for alcohol consumption (ethanol intake vs. frequency of alcohol intake). IL-8 has been validated to have the potential to screen for CRC and its precancerous lesions [47]. We found that adherence to limiting alcohol consumption had a notable inhibitory impact on IL-8. These results are in line with a prior controlled human study that demonstrated a significant increase in IL-8 levels after alcohol consumption [48].
Our results showed that higher whole grains, vegetables, and fruit intakes were associated with lower IGG levels. Increased IgG in serum is usually accompanied by various inflammations in the body [49]. The presence of multiple bioactive compounds in fruits and vegetables was reported to have a protective effect in reducing the risk of developing non-communicable diseases due to chronic inflammation. Among them, dietary polyphenols have been identified as significant contributors [50], which affect immune function by affecting the synthesis of pro-inflammatory cytokines and gene expression [51]. In addition, we found that limiting the intake of high-energy processed foods was associated with a significant decrease in serum IgG levels. Consumption of ultra-processed foods may have an adverse effect on the inflammatory state [39,52]. An intervention study showed that controlling dietary energy intake led to a marked decrease in IgG, thereby enhancing anti-inflammatory responses and promoting overall immune system health [53]. Additionally, animal experiments have shown that a high-sugar and high-fat diet can significantly increase serum IGG levels and induce immune system dysfunction, resulting in chronic intestinal inflammation [49].
As we are aware, this is the first study examining the association of adherence to WCRF/AICR dietary recommendations with gut microbiota and inflammation, taking into account differences between gender in a Chinese population. A notable strength of this study is the use of a continuous 7-day dietary record approach that enables quantitative assessment of the precise intake of individual foods and nutrients. To elaborate, meticulous instructions on completing the food record were provided by the dietitians, including information about the detailed composition of each food item, as well as accurate assessments of portion sizes and units of food. Supplementary data were also requested from participants when required, thus enhancing the credibility and validity of the reported consumption of food and drink.
The current study is limited by the cross-sectional design, which hampers our analysis of the dynamic diet-microbiome relationship over time and the causal inferences regarding the effects of the WCRF/AICR diet on gut microbiota and inflammation. Addressing these constraints necessitates a long-term follow-up in longitudinal studies. The potential for reporting bias should be considered when interpreting self-reported dietary intake data, as participants tended to report food consumption close to social expectations [54]. For instance, there is a tendency to overestimate the intake of fruits, vegetables, and other health-conscious choices, while underestimating the consumption of energy-dense foods and alcohol. Hence, it is possible that adherence to dietary recommendations could be slightly overestimated. Moreover, a comprehensive investigation involving a broader range of inflammatory biomarkers (e.g., TNF-alpha and IL-17) has the potential to provide a deeper and more nuanced comprehension of the intricate relationship between the WCRF/AICR diet and inflammation. In addition, the lack of references in the literature on WCRF/AICR adherence scores with gut microbiota and inflammation may also make the selection of an appropriate cut-off point difficult, given that different grouping criteria may cause variations in the study results.
6. Conclusions
The present study suggested that adherence to the WCRF/AICR dietary recommendations may contribute to a favorable gut microbial environment and improve the inflammatory status of adenoma patients. Intervention studies are urgently needed in the future to verify whether adherence to these dietary recommendations can improve and fine-tune the quality of life and outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15173705/s1. Table S1: Association between adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) diet score and gut microbiota diversity (n = 151). (β-Coefficients and 95 % confidence intervals). Table S2: Association between adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) diet score and gut microbiota α-diversity(n = 151). (β-Coefficients and 95 % confidence intervals).
Author Contributions
The work reported in the paper has been performed by the authors, unless clearly specified in the text. Conceptualization, D.W., S.M. and Y.Z.; Data curation, D.W., S.M., J.L., Y.W. (Yu Wang) and M.D.; Formal analysis, D.W. and Y.Z.; Investigation, D.W., S.M., J.L., J.Z., Y.W. (Yu Wang) and M.D.; Methodology, Y.Z.; Software, Y.W. (Yuan Wang), W.L. and Y.Z.; Writing—Original draft, D.W.; Writing—Review and editing, D.W., S.M., J.L., J.Z., Y.W. (Yu Wang), M.D., Y.W. (Yuan Wang), W.L. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 82003533) and the CNS-ZD Tizhi and Health Fund (No. CNS-ZD2020-82).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Tianjin Nankai Hospital (No. NKYY_YXKT_IRB_2021_048_01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Conteduca, V.; Sansonno, D.; Russi, S.; Dammacco, F. Precancerous colorectal lesions (Review). Int. J. Oncol. 2013, 43, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.V.; de Camargo, M.R.; Russo, E.; Amedei, A. Role of diet and gut microbiota on colorectal cancer immunomodulation. World J. Gastroenterol. 2019, 25, 151–162. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Schröder, L.; Kaiser, S.; Flemer, B.; Hamm, J.; Hinrichsen, F.; Bordoni, D.; Rosenstiel, P.; Sommer, F. Nutritional Targeting of the Microbiome as Potential Therapy for Malnutrition and Chronic Inflammation. Nutrients 2020, 12, 3032. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Available online: http://dietandcancerreport.org (accessed on 11 April 2023).
- Kenkhuis, M.F.; Mols, F.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; van Duijnhoven, F.J.B.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers 2022, 14, 417. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; van der Linden, B.W.A.; Breedveld-Peters, J.J.L.; Koole, J.L.; van Roekel, E.H.; Breukink, S.O.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Associations of the dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations with patient-reported outcomes in colorectal cancer survivors 2–10 years post-diagnosis: A cross-sectional analysis. Br. J. Nutr. 2021, 125, 1188–1200. [Google Scholar] [CrossRef]
- Schroeder, J.; Reitz, L.K.; Vieira, F.G.K.; da Silva, E.L.; Di Pietro, P.F. Low to moderate adherence to 2018 diet and physical exercise recommendations of the World Cancer Research Fund/American Institute for Cancer Research is associated with prooxidant biochemical profile in women undergoing adjuvant breast cancer treatment. Nutr. Res. 2023, 109, 1–11. [Google Scholar] [CrossRef]
- Song, R.; Petimar, J.; Wang, M.; Tabung, F.K.; Song, M.; Liu, L.; Lee, D.H.; Giovannucci, E.L.; Zhang, X.; Smith-Warner, S.A. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Colorectal Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1816–1825. [Google Scholar] [CrossRef]
- Holthuijsen, D.D.B.; Bours, M.J.L.; Roekel, E.H.V.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Ueland, P.M.; Midttun, Ø.; Brezina, S.; Gigic, B.; et al. Longitudinal Associations of Adherence to the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and Dutch Healthy Diet (DHD) Recommendations with Plasma Kynurenines in Colorectal Cancer Survivors after Treatment. Nutrients 2022, 14, 5151. [Google Scholar] [CrossRef]
- Quagliariello, V.; D’Aiuto, G.; Iaffaioli, R.V.; Berretta, M.; Buccolo, S.; Iovine, M.; Paccone, A.; Cerrone, F.; Bonanno, S.; Nunnari, G.; et al. Reasons why COVID-19 survivors should follow dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations: From hyper-inflammation to cardiac dysfunctions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3898–3907. [Google Scholar] [CrossRef]
- Morimoto, Y.; Beckford, F.; Cooney, R.V.; Franke, A.A.; Maskarinec, G. Adherence to cancer prevention recommendations and antioxidant and inflammatory status in premenopausal women. Br. J. Nutr. 2015, 114, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Fung, T.T.; Chavarro, J.E.; Smith-Warner, S.A.; Willett, W.C.; Giovannucci, E.L. Associations between adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and biomarkers of inflammation, hormonal, and insulin response. Int. J. Cancer 2017, 140, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Laura, A.B.; Vich, V.A.; Floris, I.; Valerie, C.; Ranko, G.; Vera, P.; Cisca, W.; Alexander, K.; Marjo, J.E.C.-K.; Jingyuan, F.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287. [Google Scholar] [CrossRef]
- Magklis, E.; Howe, L.D.; Johnson, L. Eating Style and the Frequency, Size and Timing of Eating Occasions: A cross-sectional analysis using 7-day weighed dietary records. Sci. Rep. 2019, 9, 15133. [Google Scholar] [CrossRef]
- Ostan, R.; Guidarelli, G.; Giampieri, E.; Lanzarini, C.; Berendsen, A.A.M.; Januszko, O.; Jennings, A.; Lyon, N.; Caumon, E.; Gillings, R.; et al. Cross-Sectional Analysis of the Correlation between Daily Nutrient Intake Assessed by 7-Day Food Records and Biomarkers of Dietary Intake among Participants of the NU-AGE Study. Front. Physiol. 2018, 9, 1359. [Google Scholar] [CrossRef]
- Vitale, M.; Bruno, V.; D’Abbronzo, G.; Rivellese, A.A.; Bozzetto, L.; Scidà, G.; Annuzzi, G. Evaluation of eating habits by 7-day food record: Web-PC vs. traditional paper format. Int. J. Food Sci. Nutr. 2023, 74, 580–587. [Google Scholar] [CrossRef]
- Esposito, G.; Turati, F.; Serraino, D.; Crispo, A.; Negri, E.; Parazzini, F.; La Vecchia, C. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and endometrial cancer risk: A multicentric case-control study. Br. J. Nutr. 2023, 129, 2133–2141. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P.; Romaguera, D.; Brown, S.; Bender, A.; Kahle, L.L.; Reedy, J. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Y.; Shao, L.; Ling, Z. Alterations of the Predominant Fecal Microbiota and Disruption of the Gut Mucosal Barrier in Patients with Early-Stage Colorectal Cancer. BioMed Res. Int. 2020, 2020, 2948282. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, H.; Wu, D.; Cao, D.; Ma, W. A comprehensive analysis of the microbiota composition and gene expression in colorectal cancer. BMC Microbiol. 2020, 20, 308. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Aldeguer, X.; Gonzalez-Huix, F.; Acero, D.; Garcia-Gil, L.J. Abnormal microbiota composition in the ileocolonic mucosa of Crohn’s disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm. Bowel Dis. 2006, 12, 1136–1145. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Alves, J.M.; Hylemon, P.B.; Bajaj, J.S. Cirrhosis, bile acids and gut microbiota. Gut Microbes 2013, 4, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Vega, L.; Bohórquez, L.; Ramírez, J.D.; Muñoz, M. Do we need to change our perspective about gut biomarkers? A public data mining approach to identify differentially abundant bacteria in intestinal inflammatory diseases. Front. Cell. Infect. Microbiol. 2022, 12, 918237. [Google Scholar] [CrossRef] [PubMed]
- Hooda, S.; Boler, B.M.V.; Serao, M.C.R.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C.; Swanson, K.S. 454 Pyrosequencing Reveals a Shift in Fecal Microbiota of Healthy Adult Men Consuming Polydextrose or Soluble Corn Fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Cousin, F.J.; Lynch, D.B.; Menon, R.; Brulc, J.; Brown, J.R.M.; O’Herlihy, E.; Butto, L.F.; Power, K.; Jeffery, I.B.; et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 2019, 7, 39. [Google Scholar] [CrossRef]
- Gan, L.; Zhao, Y.; Mahmood, T.; Guo, Y. Effects of dietary vitamins supplementation level on the production performance and intestinal microbiota of aged laying hens. Poult. Sci. 2020, 99, 3594–3605. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Lane, M.; Howland, G.; West, M.; Hockey, M.; Marx, W.; Loughman, A.; O’Hely, M.; Jacka, F.; Rocks, T. The effect of ultra-processed very low-energy diets on gut microbiota and metabolic outcomes in individuals with obesity: A systematic literature review. Obes. Res. Clin. Pract. 2020, 14, 197–204. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Milagro, F.I.; Aranaz, P.; Martinez, J.A.; Riezu-Boj, J.I. Gut Microbiota Differences According to Ultra-Processed Food Consumption in a Spanish Population. Nutrients 2021, 13, 2710. [Google Scholar] [CrossRef]
- Beilharz, J.E.; Kaakoush, N.O.; Maniam, J.; Morris, M.J. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain Behav. Immun. 2016, 57, 304–313. [Google Scholar] [CrossRef]
- Hexun, Z.; Miyake, T.; Maekawa, T.; Mori, H.; Yasukawa, D.; Ohno, M.; Nishida, A.; Andoh, A.; Tani, M. High abundance of Lachnospiraceae in the human gut microbiome is related to high immunoscores in advanced colorectal cancer. Cancer Immunol. Immunother. 2023, 72, 315–326. [Google Scholar] [CrossRef]
- Abdugheni, R.; Wang, W.-Z.; Wang, Y.-J.; Du, M.-X.; Liu, F.-L.; Zhou, N.; Jiang, C.-Y.; Wang, C.-Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta 2022, 1, e58. [Google Scholar] [CrossRef]
- Niemela, O.; Aalto, M.; Bloigu, A.; Bloigu, R.; Halkola, A.S.; Laatikainen, T. Alcohol Drinking Patterns and Laboratory Indices of Health: Does Type of Alcohol Preferred Make a Difference? Nutrients 2022, 14, 4529. [Google Scholar] [CrossRef]
- Orio, L.A.-O.; Antón, M.; Rodríguez-Rojo, I.C.; Correas, Á.; García-Bueno, B.; Corral, M.; de Fonseca, F.R.; García-Moreno, L.M.; Maestú, F.; Cadaveira, F. Young alcohol binge drinkers have elevated blood endotoxin, peripheral inflammation and low cortisol levels: Neuropsychological correlations in women. Addict. Biol. 2018, 23, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Montesinos, J.; Marcos, M.; Torres, J.-L.; Costa-Alba, P.; García-García, F.; Laso, F.-J.; Guerri, C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict. Biol. 2017, 22, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Ruiz-Limon, P.; Crujeiras, A.B.; de Luque, V.; Tinahones, F.J.; Macias-Gonzalez, M. 25-Hydroxyvitamin D status is associated with interleukin-6 methylation in adipose tissue from patients with colorectal cancer. Food Funct. 2021, 12, 9620–9631. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Nielsen, H.J.; Christensen, I.J. Evaluation of a 92 multiplex protein panel in detection of colorectal cancer and high-risk adenoma in 784 symptomatic individuals. Cancer Biomark. 2021, 32, 73–84. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Nadim, H.; Devine, L.; Jatlow, P.; O’Malley, S.S. Acute alcohol consumption alters the peripheral cytokines IL-8 and TNF-α. Alcohol 2020, 85, 95–99. [Google Scholar] [CrossRef]
- Tan, R.; Dong, H.; Chen, Z.; Jin, M.; Yin, J.; Li, H.; Shi, D.; Shao, Y.; Wang, H.; Chen, T.; et al. Intestinal Microbiota Mediates High-Fructose and High-Fat Diets to Induce Chronic Intestinal Inflammation. Front. Cell. Infect. Microbiol. 2021, 11, 654074. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Nestares, T.; Martin-Masot, R.; Flor-Alemany, M.; Bonavita, A.; Maldonado, J.; Aparicio, V.A. Influence of Ultra-Processed Foods Consumption on Redox Status and Inflammatory Signaling in Young Celiac Patients. Nutrients 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Valentina, L.G.; Ana, C.; Tamara, Š.; Niall, J.D.; Domagoj, K.; Helena, D.; Ana, C.; Frano, V.; Mario, F.; Richard, S.G.; et al. Extensive weight loss can reduce immune age by altering IgG N-glycosylation. medRxiv 2020. [Google Scholar] [CrossRef]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31, 38–45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).