Low Levels of Serum and Intracellular Vitamin C in Hospitalized COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Collection of Clinical Data
2.3. Collection and Analysis of Blood Samples
2.4. Data Analysis
3. Results
3.1. Inclusion
3.2. Baseline Characteristics
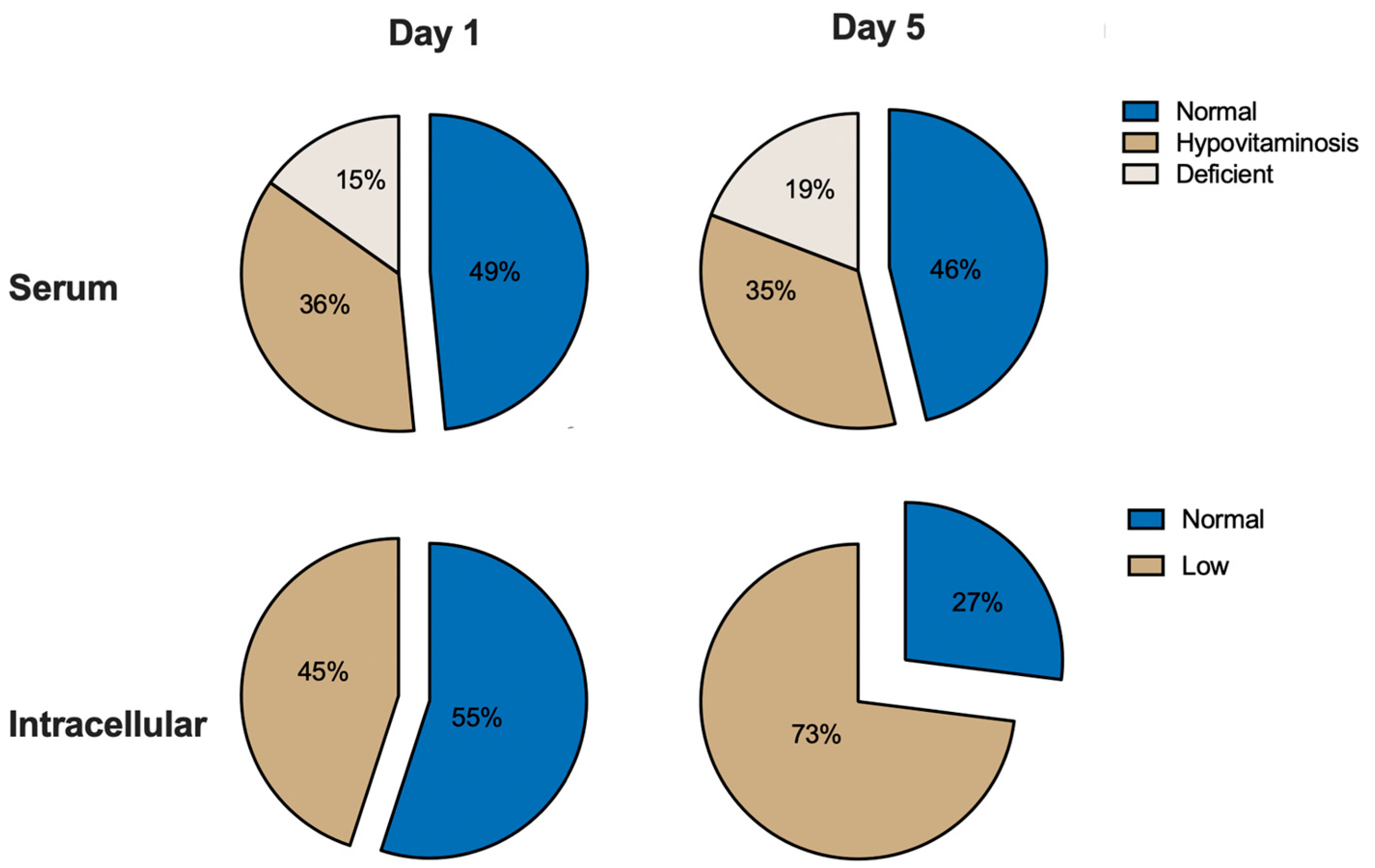
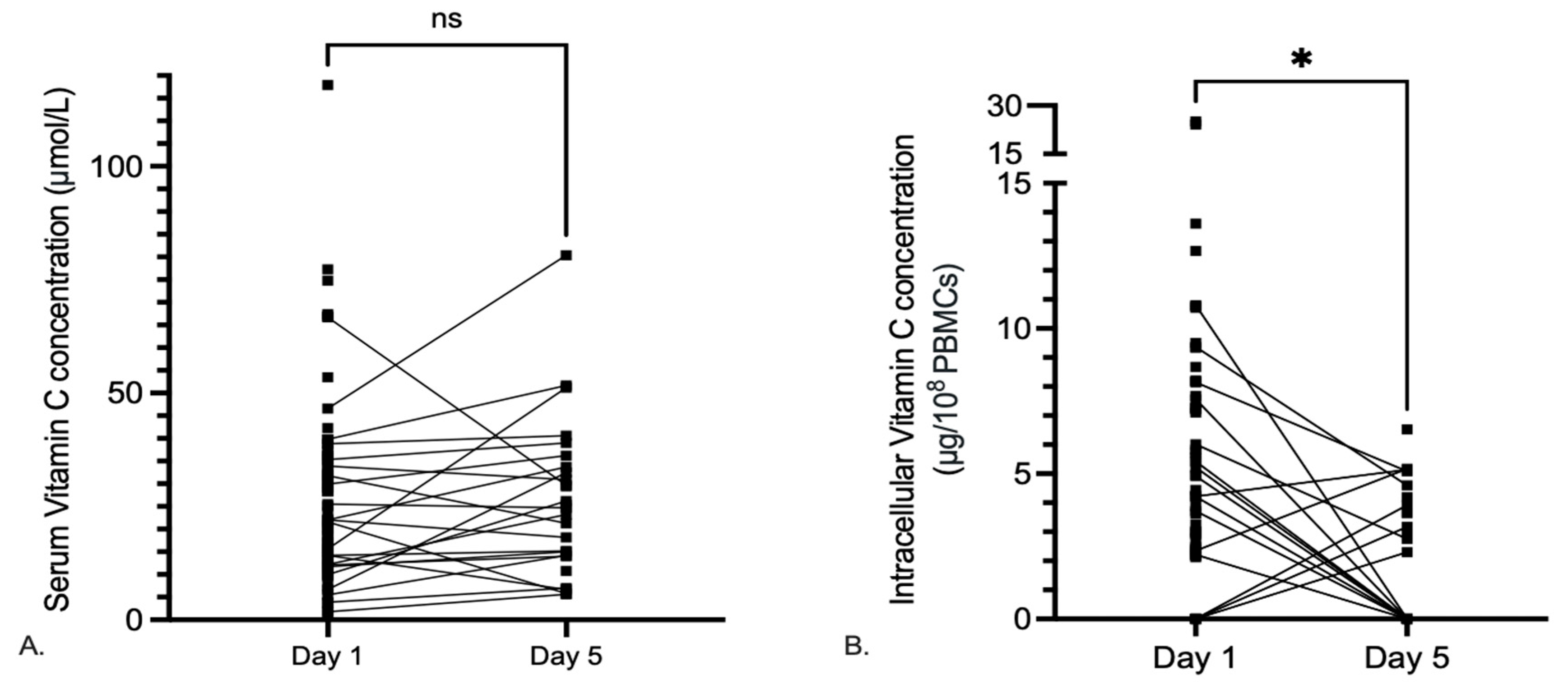
3.3. Serum Vitamin C Levels
3.4. Dynamics of Serum and Intracellular Vitamin C
3.5. Correlation of Serum and Intracellular Vitamin C with COVID-19 Severity
3.6. Correlation of Serum and Intracellular Vitamin C with COVID Severity Markers
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bendich, A.; Machlin, L.; Scandurra, O.; Burton, G.; Wayner, D. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Nabzdyk, C.S.; Bittner, E. Vitamin C in the critically ill—Indications and controversies. World J. Crit. Care Med. 2018, 7, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, A.K.; Johnston, C.S. Vitamin C: Overview and update. J. Evid. Based Complement. Altern. Med. 2011, 16, 49–57. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020, 12, 100190. [Google Scholar] [CrossRef] [PubMed]
- Bozonet, S.M.; Carr, A.C. The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant genera-tion following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Huijskens, M.J.A.J.; Walczak, M.; Koller, N.; Briedé, J.J.; Senden-Gijsbers, B.L.M.G.; Schnijderberg, M.C.; Bos, G.M.J.; Germeraad, W.T.V. Technical Advance: Ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 2014, 96, 1165–1175. [Google Scholar] [CrossRef]
- Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Nganga, V.; Swanson, P.C.; May, J.M.; Tantin, D.; et al. Vitamin C Promotes Maturation of T-Cells. Antioxid. Redox Signal 2013, 19, 2054–2067. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Huijskens, M.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.; Tilanus, M.G.; Bos, G.M.; Wieten, L.; Germeraad, W.T. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef]
- Omaye, S.T.; Schaus, E.E.; Kutnink, M.A.; Hawkes, W.C. Measurement of vitamin C in blood components by high-performance liq-uid chromatography. Implication in assessing vitamin C status. Ann. N. Y. Acad Sci. 1987, 498, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Van Gorkom, G.N.Y.; Klein Wolterink, R.G.J.; Van Elssen, C.H.M.J.; Wieten, L.; Germeraad, W.T.V.; Bos, G.M.J. Influence of Vitamin C on Lymphocytes: An Overview. Antioxidants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-M.; Kim, J.-H.; Kang, J.S.; Lee, W.J.; Hwang, Y.-I. Vitamin C is taken up by human T cells via sodium-dependent vitamin C transporter 2 (SVCT2) and exerts inhibitory effects on the activation of these cells in vitro. Anat. Cell Biol. 2016, 49, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, P.; Amitai, G.; Kehrl, J.; Dhariwal, K.R.; Klein, H.G.; Levine, M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J. Biol. Chem. 1990, 265, 2584–2587. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Rossi, A.; Pierro, C.; Avigliano, L.; Catani, M.V. SVCT1 and SVCT2: Key proteins for vitamin C uptake. Amino Acids 2007, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hasselholt, S.; Tveden-Nyborg, P.; Lykkesfeldt, J. Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br. J. Nutr. 2015, 113, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Canoy, D.; Wareham, N.; Welch, A.; Bingham, S.; Luben, R.; Day, N.; Khaw, K.-T. Plasma ascorbic acid concentrations and fat distribution in 19 068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am. J. Clin. Nutr. 2005, 82, 1203–1209. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Fain, O.; Pariés, J.; Jacquart Bt Le Moël, G.; Kettaneh, A.; Stirnemann, J.; Héron, C.; Sitbon, M.; Taleb, C.; Letellier, E. Hypovitaminosis C in hospitalized patients. Eur. J. Intern. Med. 2003, 14, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in criti-cally ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Metnitz, P.G.; Bartens, C.; Fischer, M.; Fridrich, P.; Steltzer, H.; Druml, W. Antioxidant status in patients with acute respiratory dis-tress syndrome. Intensive Care Med. 1999, 25, 180–185. [Google Scholar] [CrossRef]
- Schorah, C.J.; Downing, C.; Piripitsi, A.; Gallivan, L.; Al-Hazaa, A.H.; Sanderson, M.J.; Bodenham, A. Total vitamin C, ascorbic acid, and dehy-droascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996, 63, 760–765. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.M.; Pei, S.F.; Chong, Y.Z.; Guo, Y.; Gao, X.L.; Tang, Q.Y.; Li, Y.; Feng, F.M. CRP, SAA, LDH, and DD predict poor prognosis of coronavirus disease (COVID-19): A meta-analysis from 7739 patients. Scand. J. Clin. Lab. Investig. 2021, 81, 679–686. [Google Scholar] [CrossRef]
- Putzu, A.; Daems, A.-M.; Lopez-Delgado, J.C.; Giordano, V.F.; Landoni, G. The Effect of Vitamin C on Clinical Outcome in Critically Ill Patients: A Systematic Review With Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2019, 47, 774–783. [Google Scholar] [CrossRef]
- Zhang, M.; Jativa, D.F. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 2050312118807615. [Google Scholar] [CrossRef]
- Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose Ascorbic acid on vasopressor′s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100. [Google Scholar]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 2013, CD000980. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; on behalf of theHLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Ströhle, A.; Wolters, M.; Hahn, A. Micronutrients at the interface between inflammation and infection ascorbic acid and calciferol. Part 1: General overview with a focus on ascorbic acid. Inflamm. Allergy-Drug Targets 2011, 10, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Bozonet, S.; Pullar, J.; Spencer, E.; Rosengrave, P.; Shaw, G. Neutrophils Isolated from Septic Patients Exhibit Elevated Uptake of Vitamin C and Normal Intracellular Concentrations despite a Low Vitamin C Milieu. Antioxidants 2021, 10, 1607. [Google Scholar] [CrossRef]
- NHCotPsRo. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment, 7th Edition 2020. China. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed on 1 November 2021).
- Yang, Z.; Hu, Q.; Huang, F.; Xiong, S.; Sun, Y. The prognostic value of the SOFA score in patients with COVID-19: A retrospective, observational study. Medicine 2021, 100, e26900. [Google Scholar] [CrossRef] [PubMed]
- van Gorkom, G.; Gijsbers, B.; Ververs, E.-J.; El Molla, A.; Sarodnik, C.; Riess, C.; Wodzig, W.; Bos, G.; Van Elssen, C. Easy-to-Use HPLC Method to Measure Intra-cellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma. Antioxidants 2022, 11, 134. [Google Scholar] [CrossRef]
- German Nutrition Society. New Reference Values for Vitamin C Intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum Levels of Vitamin C and Vitamin D in a Cohort of Critically Ill COVID-19 Patients of a North American Community Hospital Intensive Care Unit in May 2020: A Pilot Study. Med. Drug Discov. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Patterson, T.; Isales, C.M.; Fulzele, S. Low level of Vitamin C and dysregulation of Vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2021, 12, 14–26. [Google Scholar] [CrossRef]
- Tomasa-Irriguible, T.M.; Bielsa-Berrocal, L. COVID-19: Up to 82% critically ill patients had low Vitamin C values. Nutr. J. 2021, 20, 66. [Google Scholar] [CrossRef]
- Galan, P.; Viteri, F.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef]
- McCall, S.J.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T.; Myint, P.K. Plasma Vitamin C Levels: Risk Factors for Deficiency and Association with Self-Reported Functional Health in the European Prospective Investigation into Cancer-Norfolk. Nutrients 2019, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Hyder Pottoo, F.; Abu-Izneid, T.; Mohammad Ibrahim, A.; Noushad Javed, M.; AlHajri, N.; Hamrouni, A.M. Immune system re-sponse during viral Infections: Immunomodulators, cytokine storm (CS) and Immunotherapeutics in COVID-19. Saudi. Pharm. J. 2021, 29, 173–187. [Google Scholar] [CrossRef]
- Mueller, S.N.; Rouse, B.T. Immune responses to viruses. Clin. Immunol. 2008, 421–431. [Google Scholar] [CrossRef]
- Praveen, D.; Puvvada, R.C. Association of vitamin C status in diabetes mellitus: Prevalence and predictors of vitamin C defi-ciency. Future J. Pharm. Sci. 2020, 6, 30. [Google Scholar]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Basmaji, J.; Fernando, S.M.; Ge, F.Z.; Xiao, Y.; Faisal, H.; Honarmand, K.; Hylands, M.; Lau, V.; Lewis, K.; et al. Parenteral Vitamin C in Patients with Severe Infection: A Systematic Review. NEJM Evid. 2022, 1, e33989. [Google Scholar] [CrossRef]
- Rawat, D.; Roy, A.; Maitra, S.; Gulati, A.; Khanna, P.; Baidya, D.K. Vitamin C and COVID-19 treatment: A systematic review and me-ta-analysis of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102324. [Google Scholar] [CrossRef]
- Ran, L.; Zhao, W.; Wang, J.; Wang, H.; Zhao, Y.; Tseng, Y.; Bu, H. Extra Dose of Vitamin C Based on a Daily Supplementation Shortens the Common Cold: A Meta-Analysis of 9 Randomized Controlled Trials. BioMed Res. Int. 2018, 2018, 1837634. [Google Scholar] [CrossRef]


| Characteristic | n = 70 (Mean ± SD or Median (IQR)) |
|---|---|
| Age (years) | 70 ± 12 |
| Gender (n, %) | |
| Male | 45 (64%) |
| Female | 25 (36%) |
| Vitals upon admission | |
| Oxygen suppletion (L/min) | 2 (1–5) |
| Oxygen saturation (%) | 93 ± 3 |
| Comorbidities (n, %) | |
| None | 12 (17%) |
| 1 | 15 (21%) |
| 2 | 22 (31%) |
| 3+ | 21 (30%) |
| Body mass index (BMI) | 27.8 (24.4–31.6) |
| Diabetes mellitus (n, %) | 20 (29%) |
| (Hematologic) cancer (n, %) | 3 (4%) |
| Medication (n,%) | |
| None | 20 (29%) |
| Immunosuppression | 9 (13%) |
| Anticoagulation therapeutic | 14 (20%) |
| Anticoagulation prophylactic | 24 (34%) |
| Antibiotics | 12 (20%) |
| Chemotherapy | 1 (1%) |
| Abnormal chest X-ray (n, %) | 40 (57%) |
| CT-value SARS-CoV-2 PCR | 22 ± 6 |
| Time since first symptoms (days) | 6.5 (2.3–9.0) |
| SOFA score | 2 (2-3) |
| Maximal clinical COVID-19 severity classification * (n, %) | |
| Mild | 1 (1%) |
| Moderate | 8 (11%) |
| Severe | 42 (60%) |
| Critically ill (ICU indication) | 19 (27%) |
| Duration of hospital admission (days) | 9 (4–14) |
| Actual ICU admission (n, %) | 5 (7%) |
| Duration ICU admission (days) | 7 (4–29) |
| Death (n, %) | 11 (16%) |
| Vitamin C Serum Day 1 (n = 66) | Vitamin C Serum Day 5 (n = 26) | Vitamin C Intracellular Day 1 (n = 58) | Vitamin C Intracellular Day 5 (n = 22) | |||||
|---|---|---|---|---|---|---|---|---|
| r(s) | p-Value | r(s) | p-Value | r(s) | p-Value | r(s) | p-Value | |
| Maximal clinical COVID-19 severity classification * | −0.341 | 0.005 | −0.169 | 0.407 | −0.082 | 0.542 | −0.142 | 0.528 |
| Improving Disease (n = 29) | Progressive Disease (n = 11) | p-Value | |
|---|---|---|---|
| Serum vitamin C day 1 | 32.1 ± 19.8 | 19.8 ± 14.4 | 0.108 |
| Intracellular vitamin C day 1 | 5.2 ± 6.5 | 5.4 ± 3.7 | 0.920 |
| Vitamin C Serum Day 1 | Vitamin C Serum Day 5 | Vitamin C Intracellular Day 1 | Vitamin C Intracellular Day 5 | |||||
|---|---|---|---|---|---|---|---|---|
| r(s) | p-Value | r(s) | p-Value | r(s) | p-Value | r(s) | p-Value | |
| Duration hospital stay (days) | −0.201 | 0.105 | 0.206 | 0.313 | 0.008 | 0.952 | −0.032 | 0.886 |
| Duration of ICU stay (days) | 0.029 | 0.957 | - | - | 0.143 | 0.787 | - | - |
| SOFA score | −0.021 | 0.1 | 0.156 | 0.467 | −0.017 | 0.904 | −0.240 | 0.354 |
| CRP | LDH | |||
|---|---|---|---|---|
| r(s) | p-Value | r(s) | p-Value | |
| Maximal clinical COVID-19 severity classification | 0.26 | 0.030 | 0.11 | 0.374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boerenkamp, L.S.; Gijsbers, B.L.M.G.; Ververs, E.-J.; Pijpers, E.M.S.; Spaetgens, B.; de Coninck, A.; Germeraad, W.T.V.; Wodzig, W.K.W.H.; Wieten, L.; van Gorkom, G.N.Y.; et al. Low Levels of Serum and Intracellular Vitamin C in Hospitalized COVID-19 Patients. Nutrients 2023, 15, 3653. https://doi.org/10.3390/nu15163653
Boerenkamp LS, Gijsbers BLMG, Ververs E-J, Pijpers EMS, Spaetgens B, de Coninck A, Germeraad WTV, Wodzig WKWH, Wieten L, van Gorkom GNY, et al. Low Levels of Serum and Intracellular Vitamin C in Hospitalized COVID-19 Patients. Nutrients. 2023; 15(16):3653. https://doi.org/10.3390/nu15163653
Chicago/Turabian StyleBoerenkamp, Lara S., Birgit L. M. G. Gijsbers, Erik-Jan Ververs, Eva M. S. Pijpers, Bart Spaetgens, Aniek de Coninck, Wilfred T. V. Germeraad, Will K. W. H. Wodzig, Lotte Wieten, Gwendolyn N. Y. van Gorkom, and et al. 2023. "Low Levels of Serum and Intracellular Vitamin C in Hospitalized COVID-19 Patients" Nutrients 15, no. 16: 3653. https://doi.org/10.3390/nu15163653
APA StyleBoerenkamp, L. S., Gijsbers, B. L. M. G., Ververs, E.-J., Pijpers, E. M. S., Spaetgens, B., de Coninck, A., Germeraad, W. T. V., Wodzig, W. K. W. H., Wieten, L., van Gorkom, G. N. Y., & van Elssen, C. H. M. J. (2023). Low Levels of Serum and Intracellular Vitamin C in Hospitalized COVID-19 Patients. Nutrients, 15(16), 3653. https://doi.org/10.3390/nu15163653






