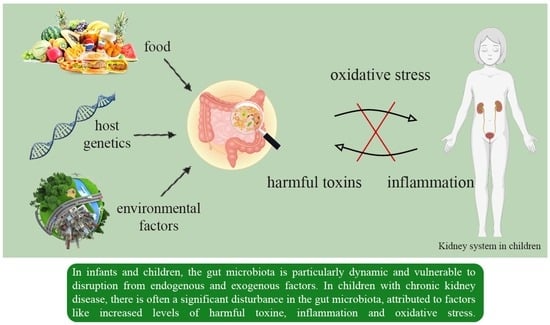
Exploring a Complex Interplay: Kidney–Gut Axis in Pediatric Chronic Kidney Disease
Abstract
:1. Introduction
2. CKD and Gut Microbiota
2.1. Developmental Origins of Health and Disease and CKD
2.2. Nitric Oxide (NO) Prenatal Deficiency and CKD
- Sodium transporters: renal disease and high blood pressure have been linked to increased expression and activity of sodium transporters, leading to higher sodium reabsorption [44,45]. NO has been shown to inhibit the work of certain sodium transporters [46]. Therefore, it is thought that a deficient NO may fail to balance the impaired sodium transporters in the context of early life insults, ultimately contributing to programmed high blood pressure, as illustrated in Figure 1.
- Epigenetic regulation: Epigenetic mechanisms, such as histone alterations, DNA methylation, and RNAs of a non-coding nature play a role in developmental programming [47]. These mechanisms can influence gene expression patterns and contribute to long-term health outcomes. It is possible that NO signaling may impact epigenetic regulation, thereby influencing programming of hypertension and renal disease.
- Gut microbiota: The diversity of the gut microbiota is influenced by various factors, including genetics, comorbidities, and environmental factors like physical exercise, smoking, and medication use. However, it is undeniable that diet, dietary patterns, and specific components of the diet play a significant role in shaping the composition of the gut microbiota. These components refer to microorganisms that are not broken down, but can instead colonize the colon [48]. Moreover, the composition of the diet and the presence or absence of specific nutrients are crucial factors determining the rate at which these bacteria generate and the metabolic effects of the metabolites they produce [49].
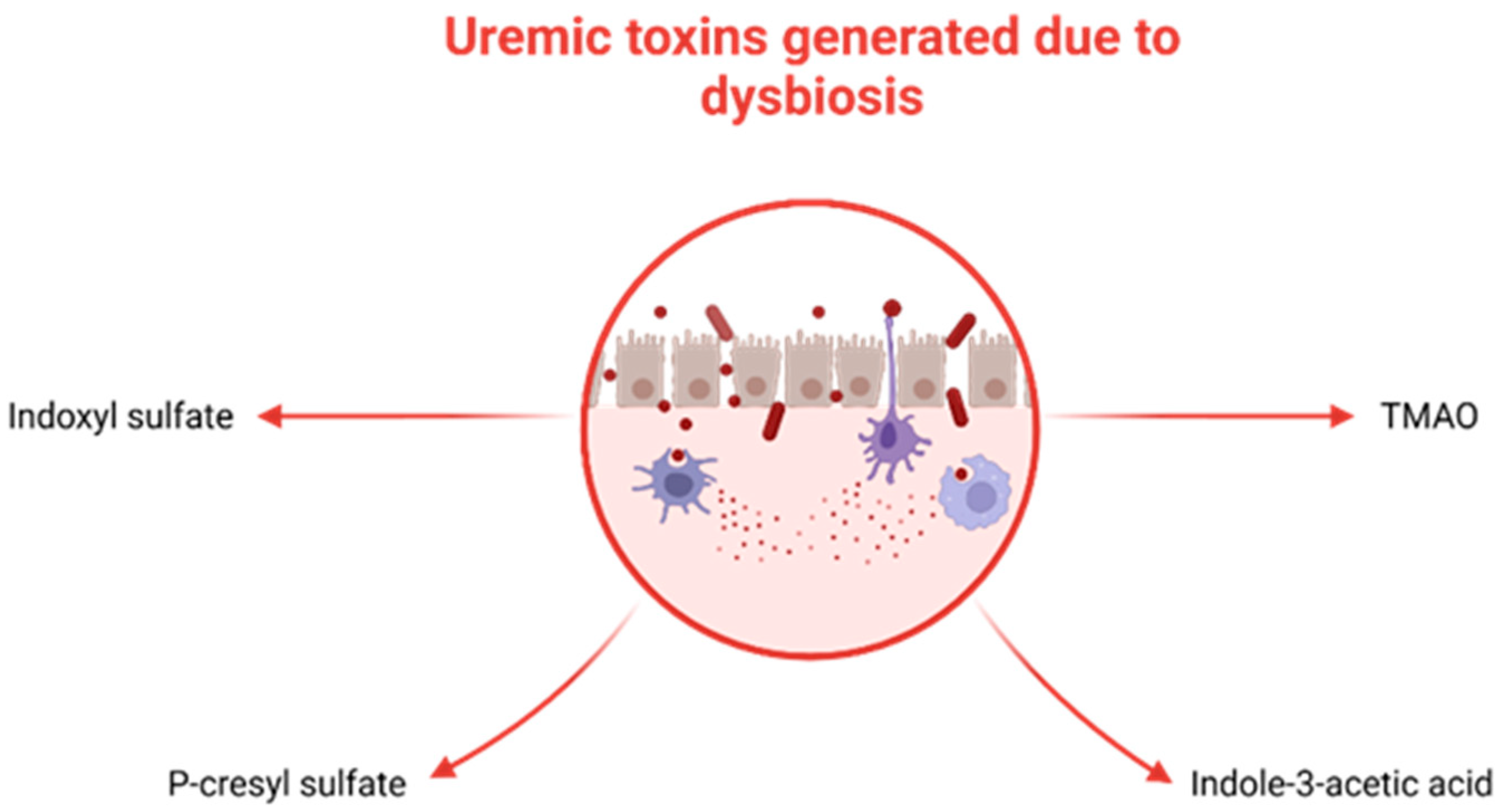
2.3. The Kidney–Gut Axis in CKD
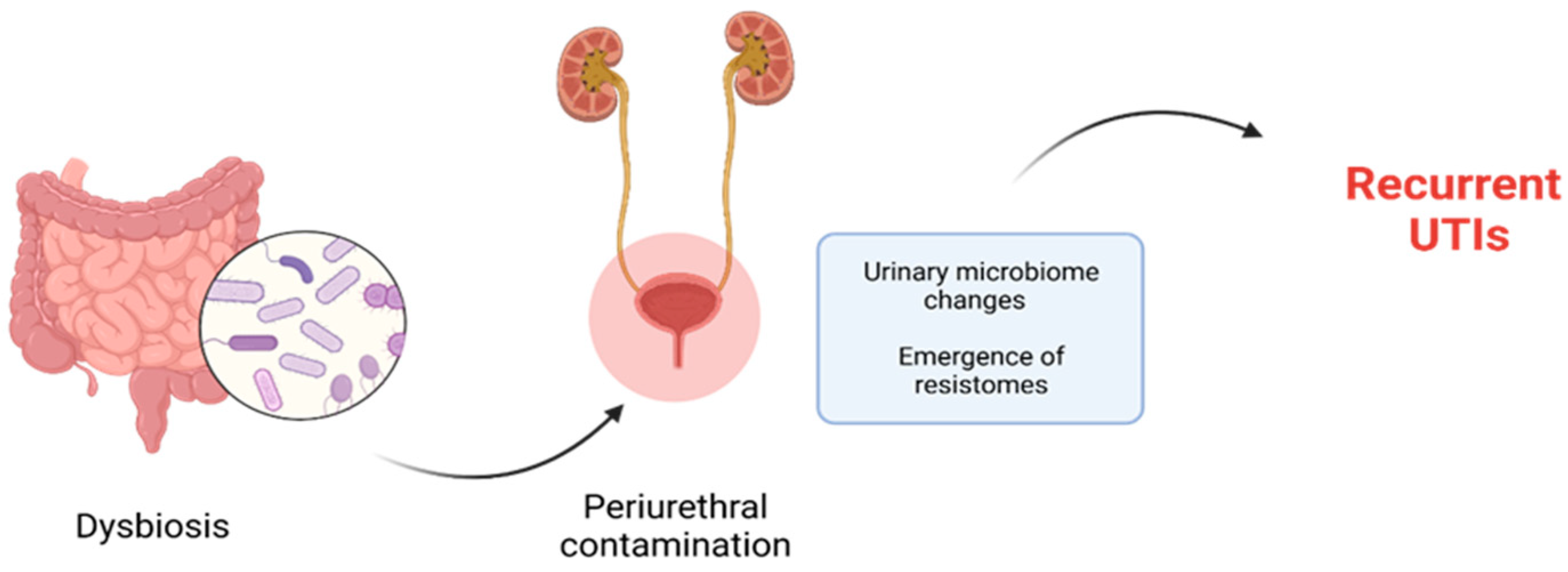
2.4. The Kidney–Gut Axis in Urinary Tract Infections
2.5. The Kidney–Gut Axis in Urinary Lithiasis
2.6. The Kidney–Gut Axis in Kidney Transplantation
2.7. The Kidney–Gut Axis in Other Kidney Diseases
3. Microbiota Modulatory Therapies in CKD Patients
3.1. Diet Intervention in Microbiota Modulation in Pediatric CKD
3.2. Probiotics for Microbiota Modulation in Pediatric CKD
3.3. Prebiotics for Microbiota Modulation in CKD
3.4. Postbiotics for Microbiota Modulation in CKD
3.5. Fecal Microbiota Transplantation for Microbiota Modulation in CKD
3.6. Colon Dialysis for Microbiota Modulation in CKD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | before Christ |
| CKD | chronic kidney disease |
| DNA | deoxyribonucleic acid |
| DOHaD | Developmental Origins of Health and Disease |
| ESKD | end-stage kidney disease |
| FMT | fecal microbiota transplantation |
| IAA | indole-3-acetic acid |
| IgA1 | Imunglobuline a1 |
| IGM | infant gut microbiota |
| IS | indoxyl sulfate |
| LPS | lipopolysaccharides |
| NF-κB | nuclear factor kappa b |
| NO | nitric oxide |
| PCS | p-cresyl sulfate |
| p-CS | p-cresyl sulfate |
| pro-oxidants | promote oxidation |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| RTRs | renal transplant recipients |
| SCFA | short-chain fatty acids |
| TH17 | t helper 17 |
| TLR4 | Toll-like receptor 4 |
| TMAO | trimethylamine n-oxide |
| TNF | tumor necrosis factor |
| Treg | regulatory t cell |
| UTI | urinary tract infection |
References
- Sumida, K.; Kovesdy, C.P. The gut-kidney-heart axis in chronic kidney disease. Physiol. Int. 2019, 106, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, X.; Ghosh, S.; Xie, C.; Chen, J.; Huang, H. Role of gut microbiota-derived metabolites on vascular calcification in CKD. J. Cell. Mol. Med. 2021, 25, 1332–1341. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.; Evenepoel, P.; Anders, H.-J. Intestinal microbiome and fitness in kidney disease. Nat. Rev. Nephrol. 2019, 15, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Crofts, T.S.; Dantas, G. Antibiotics and the developing infant gut microbiota and resistome. Curr. Opin. Microbiol. 2015, 27, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Eggesbø, M.; Moen, B.; Peddada, S.; Baird, D.; Rugtveit, J.; Midtvedt, T.; Bushel, P.R.; Sekelja, M.; Rudi, K. Development of gut microbiota in infants not exposed to medical interventions. Apmis 2011, 119, 17–35. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Moore, A.M.; Patel, S.; Forsberg, K.J.; Wang, B.; Bentley, G.; Razia, Y.; Qin, X.; Tarr, P.I.; Dantas, G. Pediatric Fecal Microbiota Harbor Diverse and Novel Antibiotic Resistance Genes. PLoS ONE 2013, 8, e78822. [Google Scholar] [CrossRef]
- Moore, A.M.; Ahmadi, S.; Patel, S.; Gibson, M.K.; Wang, B.; Ndao, M.I.; Deych, E.; Shannon, W.; Tarr, P.I.; Warner, B.B.; et al. Gut resistome development in healthy twin pairs in the first year of life. Microbiome 2015, 3, 27. [Google Scholar] [CrossRef]
- Greenwood, C.; Morrow, A.L.; Lagomarcino, A.J.; Altaye, M.; Taft, D.H.; Yu, Z.; Newburg, D.S.; Ward, D.V.; Schibler, K.R. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J. Pediatr. 2014, 165, 23–29. [Google Scholar] [CrossRef]
- Dogra, S.K.; Doré, J.; Damak, S. Gut Microbiota Resilience: Definition, Link to Health and Strategies for Intervention. Front. Microbiol. 2020, 11, 572921. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Tain, Y.-L. Chronic Kidney Disease and Gut Microbiota: What Is Their Connection in Early Life? Int. J. Mol. Sci. 2022, 23, 3954. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef]
- Lupu, V.V.; Miron, I.C.; Raileanu, A.A.; Starcea, I.M.; Lupu, A.; Tarca, E.; Mocanu, A.; Buga, A.M.L.; Lupu, V.; Fotea, S. Difficulties in adaptation of the mother and newborn via cesarean section versus natural birth—A narrative review. Life 2023, 13, 300. [Google Scholar] [CrossRef]
- Hajjo, R.; Sabbah, D.A.; Al Bawab, A.Q. Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers. Diagnostics 2022, 12, 1742. [Google Scholar] [CrossRef] [PubMed]
- Bozomitu, L.; Miron, I.; Raileanu, A.A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Ma, K.; Wang, J.; Ding, Z.; Li, Y.; Zhu, S.; Liang, X.; Zhang, Q.; Song, L.; Liu, C. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. J. Immunol. Res. 2021, 2021, 5516035. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. The developmental origins of health and disease. In Early Life Origins of Health and Disease; Springer: New York, NY, USA, 2006; pp. 1–7. [Google Scholar]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A conceptual framework for the developmental origins of health and disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18. [Google Scholar] [CrossRef]
- Charlton, J.R.; Baldelomar, E.J.; Hyatt, D.M.; Bennett, K.M. Nephron number and its determinants: A 2020 update. Pediatr. Nephrol. 2021, 36, 797–807. [Google Scholar] [CrossRef]
- Welham, S.J.; Wade, A.; Woolf, A.S. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 2002, 61, 1231–1242. [Google Scholar] [CrossRef]
- Woods, L.L.; Weeks, D.A.; Rasch, R. Programming of adult blood pressure by maternal protein restriction: Role of nephrogenesis. Kidney Int. 2004, 65, 1339–1348. [Google Scholar] [CrossRef]
- Hoppe, C.C.; Evans, R.G.; Bertram, J.F.; Moritz, K.M. Effects of dietary protein restriction on nephron number in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1768–R1774. [Google Scholar] [CrossRef]
- Lisle, S.J.; Lewis, R.M.; Petry, C.J.; Ozanne, S.E.; Hales, C.N.; Forhead, A.J. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br. J. Nutr. 2003, 90, 33–39. [Google Scholar] [CrossRef]
- Lelievre-Pegorier, M.; Vilar, J.; Ferrier, M.L.; Moreau, E.; Freund, N.; Gilbert, T.; Merlet-Benichou, C. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998, 54, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Mohany, M.; Ashton, N.; Harrath, A.H.; Nyengaard, J.R.; Alomar, S.Y.; Alwasel, S. A new model for fetal programming: Maternal Ramadan-type fasting programs nephrogenesis. J. Dev. Orig. Health Dis. 2018, 9, 287–298. [Google Scholar] [CrossRef]
- Schlender, J.; Behrens, F.; McParland, V.; Müller, D.; Wilck, N.; Bartolomaeus, H.; Holle, J. Bacterial metabolites and cardiovascular risk in children with chronic kidney disease. Mol. Cell. Pediatr. 2021, 8, 17. [Google Scholar] [CrossRef]
- Chong, E.; Yosypiv, I.V. Developmental Programming of Hypertension and Kidney Disease. Int. J. Nephrol. 2012, 2012, 760580. [Google Scholar] [CrossRef]
- Satoh, N.; Nakamura, M.; Suzuki, A.; Tsukada, H.; Horita, S.; Suzuki, M.; Moriya, K.; Seki, G. Effects of Nitric Oxide on Renal Proximal Tubular Na(+) Transport. Biomed. Res. Int. 2017, 2017, 6871081. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Lobach, A.R.; Roberts, A.; Rowland, I.R. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem. Toxicol. 2019, 124, 385–399. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The microbiome and blood pressure: Can microbes regulate our blood pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef]
- Ma, J.; Li, H. The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Role of the Gut Microbiota in Children with Kidney Disease. Children 2023, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Wehedy, E.; Shatat, I.F.; Al Khodor, S. The Human Microbiome in Chronic Kidney Disease: A Double-Edged Sword. Front. Med. 2022, 8, 790783. [Google Scholar] [CrossRef] [PubMed]
- Holle, J.; Bartolomaeus, H.; Löber, U.; Behrens, F.; Bartolomaeus, T.U.; Anandakumar, H.; Wimmer, M.I.; Vu, D.L.; Kuhring, M.; Brüning, U.; et al. Inflammation in Children with CKD Linked to Gut Dysbiosis and Metabolite Imbalance. J. Am. Soc. Nephrol. 2022, 33, 2259–2275. [Google Scholar] [CrossRef]
- Jezierska, M.; Stefanowicz, J. Asymmetric and Symmetric Dimethylarginines as Renal Function Parameters in Paediatric Kidney Diseases: A Literature Review from 2003 to 2022. Children 2022, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Hsu, C.-N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Kesper, M.S.; Marschner, J.A.; Konrad, L.; Ryu, M.; Kumar Vr, S.; Kulkarni, O.P.; Mulay, S.R.; Romoli, S.; Demleitner, J.; et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren. Fail. 2021, 43, 102–112. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2015, 31, 737–746. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients 2019, 11, 496. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef]
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef]
- Vaziri, N.D. CKD impairs barrier function and alters microbial flora of the intestine: A major link to inflammation and uremic toxicity. Curr. Opin. Nephrol. Hypertens. 2012, 21, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Simoes-Silva, L.; Pestana, M.; Araujo, R.; Soares-Silva, I.J. The Role of the Gut Microbiome on Chronic Kidney Disease. Adv. Appl. Microbiol. 2016, 96, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and chronic kidney disease. Kidney Int. 2015, 88, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Pisano, A.; D’Arrigo, G.; Coppolino, G.; Bolignano, D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1224. [Google Scholar] [CrossRef]
- Meštrović, T.; Matijašić, M.; Perić, M.; Čipčić Paljetak, H.; Barešić, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2021, 11, 7. [Google Scholar] [CrossRef]
- Cumpanas, A.A.; Bratu, O.G.; Bardan, R.T.; Ferician, O.C.; Cumpanas, A.D.; Horhat, F.G.; Licker, M.; Pricop, C.; Cretu, O.M. Urinary Microbiota—Are We Ready for Prime Time? A Literature Review of Study Methods’ Critical Steps in Avoiding Contamination and Minimizing Biased Results. Diagnostics 2020, 10, 343. [Google Scholar] [CrossRef]
- Hanage, W.P. Microbiology: Microbiome science needs a healthy dose of scepticism. Nature 2014, 512, 247–248. [Google Scholar] [CrossRef]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef]
- Lupu, V.V.; Ghiciuc, C.M.; Stefanescu, G.; Mihai, C.M.; Popp, A.; Sasaran, M.O.; Bozomitu, L.; Starcea, I.M.; Adam Raileanu, A.; Lupu, A. Emerging role of the gut microbiome in post-infectious irritable bowel syndrome: A literature review. World J. Gastroenterol. 2023, 29, 3241–3256. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Trandafir, L.M.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Starcea, I.M.; Mocanu, A.; Butnariu, L.I.; Stoleriu, G.; Salaru, D.L.; et al. Advances in Understanding the Human Gut Microbiota and Its Implication in Pediatric Celiac Disease—A Narrative Review. Nutrients 2023, 15, 2499. [Google Scholar] [CrossRef] [PubMed]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutiérrez-Fernández, J.; Garcia-Salcedo, J.A. Urinary microbiome: Yin and yang of the urinary tract. Front. Cell. Infect. Microbiol. 2021, 11, 617002. [Google Scholar] [CrossRef]
- Kawalec, A.; Zwolińska, D. Emerging Role of Microbiome in the Prevention of Urinary Tract Infections in Children. Int. J. Mol. Sci. 2022, 23, 870. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Fork, S.D.; Ahmadi, R.; Khameneh, B. Deep insights into urinary tract infections and effective natural remedies. Afr. J. Urol. 2021, 27, 6. [Google Scholar] [CrossRef]
- Xia, J.Y.; Yang, C.; Xu, D.F.; Xia, H.; Yang, L.G.; Sun, G.J. Consumption of cranberry as adjuvant therapy for urinary tract infections in susceptible populations: A systematic review and meta-analysis with trial sequential analysis. PLoS ONE 2021, 16, e0256992. [Google Scholar] [CrossRef]
- González de Llano, D.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry Polyphenols and Prevention against Urinary Tract Infections: Relevant Considerations. Molecules 2020, 25, 3523. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Eisner, B.H.; Lange, D.; Gerlach, R. Current insights into the mechanisms and management of infection stones. Nat. Rev. Urol. 2019, 16, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Wiener, S.V.; Ho, S.P.; Stoller, M.L. Beginnings of nephrolithiasis: Insights into the past, present and future of Randall’s plaque formation research. Curr. Opin. Nephrol. Hypertens. 2018, 27, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Swarte, J.C.; Douwes, R.M.; Hu, S.; Vich Vila, A.; Eisenga, M.F.; van Londen, M.; Gomes-Neto, A.W.; Weersma, R.K.; Harmsen, H.J.M.; Bakker, S.J.L. Characteristics and Dysbiosis of the Gut Microbiome in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, H.; Kyllönen, L.; Madsen, S.; Grave, G.; Solbu, D.; Holdaas, H. Clinicians Underestimate Gastrointestinal Symptoms and Overestimate Quality of Life in Renal Transplant Recipients: A Multinational Survey of Nephrologists. Transplantation 2007, 84, 1052–1054. [Google Scholar] [CrossRef]
- Bunnapradist, S.; Neri, L.; Wong, W.; Lentine, K.L.; Burroughs, T.E.; Pinsky, B.W.; Takemoto, S.K.; Schnitzler, M.A. Incidence and Risk Factors for Diarrhea Following Kidney Transplantation and Association With Graft Loss and Mortality. Am. J. Kidney Dis. 2008, 51, 478–486. [Google Scholar] [CrossRef]
- Lee, J.R.; Magruder, M.; Zhang, L.; Westblade, L.F.; Satlin, M.J.; Robertson, A.; Edusei, E.; Crawford, C.; Ling, L.; Taur, Y.; et al. Gut microbiota dysbiosis and diarrhea in kidney transplant recipients. Am. J. Transplant. 2019, 19, 488–500. [Google Scholar] [CrossRef]
- Tourret, J.; Willing, B.P.; Dion, S.; MacPherson, J.; Denamur, E.; Finlay, B.B. Immunosuppressive Treatment Alters Secretion of Ileal Antimicrobial Peptides and Gut Microbiota, and Favors Subsequent Colonization by Uropathogenic Escherichia coli. Transplantation 2017, 101, 74–82. [Google Scholar] [CrossRef]
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Toussaint, N.C.; Ling, L.; Pamer, E.; Suthanthiran, M. Gut microbial community structure and complications after kidney transplantation: A pilot study. Transplantation 2014, 98, 697–705. [Google Scholar] [CrossRef]
- Ambruzs, J.M.; Larsen, C.P. Renal Manifestations of Inflammatory Bowel Disease. Rheum. Dis. Clin. N. Am. 2018, 44, 699–714. [Google Scholar] [CrossRef]
- Lei, J.; Xie, Y.; Sheng, J.; Song, J. Intestinal microbiota dysbiosis in acute kidney injury: Novel insights into mechanisms and promising therapeutic strategies. Ren. Fail. 2022, 44, 571–580. [Google Scholar] [CrossRef]
- Yang, J.; Kim, C.J.; Go, Y.S.; Lee, H.Y.; Kim, M.-G.; Oh, S.W.; Cho, W.Y.; Im, S.-H.; Jo, S.K. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020, 98, 932–946. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, L.; Sun, L.; Zhou, W.; Zhi, W.; Qing, J.; Saed, Y.A.; Dong, L.; Zhang, X.; Li, Y. Gut Microbes in Immunoglobulin A Nephropathy and Their Potential Therapeutic Applications. Front. Med. 2022, 9, 823267. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int. J. Mol. Sci. 2022, 23, 5354. [Google Scholar] [CrossRef]
- Nhan, J.; Sgambat, K.; Moudgil, A. Plant-based diets: A fad or the future of medical nutrition therapy for children with chronic kidney disease? Pediatr. Nephrol. 2023, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El Amouri, A.; Snauwaert, E.; Foulon, A.; Vande Moortel, C.; Van Dyck, M.; Van Hoeck, K.; Godefroid, N.; Glorieux, G.; Van Biesen, W.; Vande Walle, J.; et al. Dietary fibre intake is associated with serum levels of uraemic toxins in children with chronic kidney disease. Toxins 2021, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sáez, M.J.; Uffing, A.; Leon, J.; Murakami, N.; Watanabe, A.; Borges, T.J.; Sabbisetti, V.S.; Cureton, P.; Kenyon, V.; Keating, L.; et al. Immunological Impact of a Gluten-Free Dairy-Free Diet in Children with Kidney Disease: A Feasibility Study. Front. Immunol. 2021, 12, 624821. [Google Scholar] [CrossRef]
- Lu, P.-H.; Yu, M.-C.; Wei, M.-J.; Kuo, K.-L. The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease. Toxins 2021, 13, 573. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food. In Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; WHO: London, ON, Canada, 2002. [Google Scholar]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.-J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tsuji, S.; Akagawa, S.; Akagawa, Y.; Kino, J.; Yamanouchi, S.; Kimata, T.; Hashiyada, M.; Akane, A.; Kaneko, K. Clinical Significance of Probiotics for Children with Idiopathic Nephrotic Syndrome. Nutrients 2021, 13, 365. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Hsu, C.-N. Perinatal Garlic Oil Supplementation Averts Rat Offspring Hypertension Programmed by Maternal Chronic Kidney Disease. Nutrients 2022, 14, 4624. [Google Scholar] [CrossRef] [PubMed]
- Zółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Lupu, V.V.; Butnariu, L.I.; Fotea, S.; Morariu, I.D.; Badescu, M.C.; Starcea, I.M.; Salaru, D.L.; Popp, A.; Dragan, F.; Lupu, A.; et al. The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice. Nutrients 2023, 15, 3359. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.S.; Nicholson, M.R.; Khoruts, A.; Kahn, S.A. Fecal Microbiota Transplantation across the Lifespan: Balancing Efficacy, Safety, and Innovation. Am. J. Gastroenterol. 2022, 118, 435–439. [Google Scholar] [CrossRef]
- Gu, X.; Chen, Z.-H.; Zhang, S.-C. Fecal microbiota transplantation in childhood: Past, present, and future. World J. Pediatr. 2022, 19, 813–822. [Google Scholar] [CrossRef]
- Lupu, A.; Jechel, E.; Mihai, C.M.; Mitrofan, E.C.; Fotea, S.; Starcea, I.M.; Ioniuc, I.; Mocanu, A.; Ghica, D.C.; Popp, A.; et al. The Footprint of Microbiome in Pediatric Asthma—A Complex Puzzle for a Balanced Development. Nutrients 2023, 15, 3278. [Google Scholar] [CrossRef]
- Sumida, K.; Lau, W.L.; Kalantar-Zadeh, K.; Kovesdy, C.P. Novel intestinal dialysis interventions and microbiome modulation to control uremia. Curr. Opin. Nephrol. Hypertens. 2021, 31, 82–91. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.-M.; Zeinoddini, A.; Heidari, R.; NaserHodjjati, H.; Tourchi, A. A novel alternative for renal replacement therapy: 2-year successful colonic dialysis via a Malone antegrade continent enema stoma. J. Pediatr. Urol. 2014, 10, 511–514. [Google Scholar] [CrossRef]
- Dai, S.; Dai, Y.; Peng, J.; Xie, X.; Ning, J. Simplified colonic dialysis with hemodialysis solutions delays the progression of chronic kidney disease. QJM Int. J. Med. 2019, 112, 189–196. [Google Scholar] [CrossRef]
- Li, Y.; Dai, M.; Yan, J.; Liu, F.; Wang, X.; Lin, L.; Huang, M.; Li, C.; Wen, R.; Qin, J.; et al. Colonic dialysis can influence gut flora to protect renal function in patients with pre-dialysis chronic kidney disease. Sci. Rep. 2021, 11, 12773. [Google Scholar] [CrossRef] [PubMed]
- Zupcic, A.; Slezak, P.; Radloff, J. The Gastrointestinal Microbiota as a Potential Cause and Target in Chronic Kidney Disease Accentuating Treatment and Intervention Strategies. Appl. Sci. 2023, 13, 3212. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocanu, A.; Bogos, R.A.; Lazaruc, T.I.; Trandafir, L.M.; Lupu, V.V.; Ioniuc, I.; Alecsa, M.; Ivanov, A.; Lupu, A.; Starcea, I.M. Exploring a Complex Interplay: Kidney–Gut Axis in Pediatric Chronic Kidney Disease. Nutrients 2023, 15, 3609. https://doi.org/10.3390/nu15163609
Mocanu A, Bogos RA, Lazaruc TI, Trandafir LM, Lupu VV, Ioniuc I, Alecsa M, Ivanov A, Lupu A, Starcea IM. Exploring a Complex Interplay: Kidney–Gut Axis in Pediatric Chronic Kidney Disease. Nutrients. 2023; 15(16):3609. https://doi.org/10.3390/nu15163609
Chicago/Turabian StyleMocanu, Adriana, Roxana Alexandra Bogos, Tudor Ilie Lazaruc, Laura Mihaela Trandafir, Vasile Valeriu Lupu, Ileana Ioniuc, Mirabela Alecsa, Anca Ivanov, Ancuta Lupu, and Iuliana Magdalena Starcea. 2023. "Exploring a Complex Interplay: Kidney–Gut Axis in Pediatric Chronic Kidney Disease" Nutrients 15, no. 16: 3609. https://doi.org/10.3390/nu15163609
APA StyleMocanu, A., Bogos, R. A., Lazaruc, T. I., Trandafir, L. M., Lupu, V. V., Ioniuc, I., Alecsa, M., Ivanov, A., Lupu, A., & Starcea, I. M. (2023). Exploring a Complex Interplay: Kidney–Gut Axis in Pediatric Chronic Kidney Disease. Nutrients, 15(16), 3609. https://doi.org/10.3390/nu15163609