Causal Effect of Chondroitin, Glucosamine, Vitamin, and Mineral Intake on Kidney Function: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
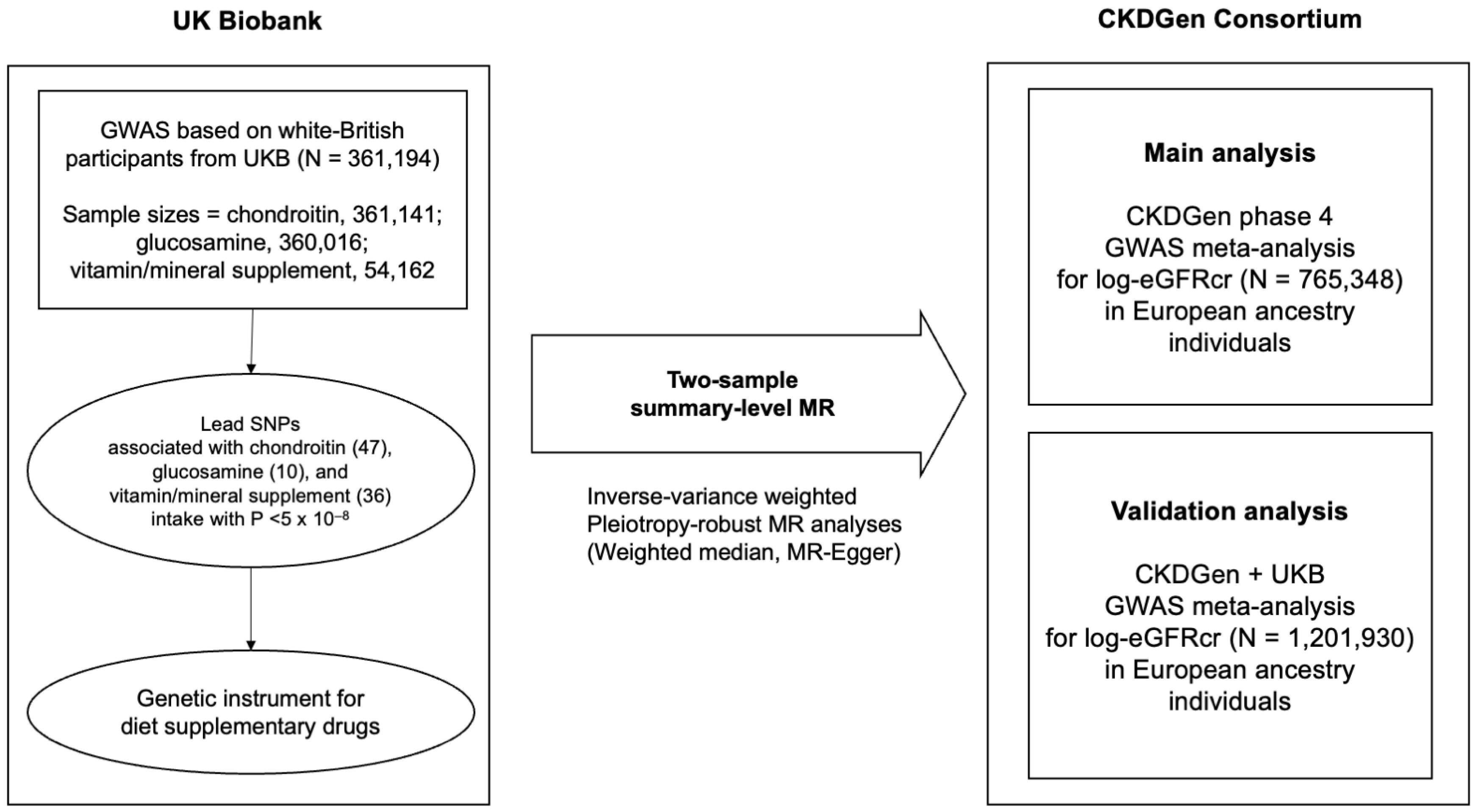
2.2. Study Setting
2.3. Data Sources for Chondroitin, Glucosamine, and Vitamin Supplement Intake
2.4. Data Sources for Kidney Function Traits
2.5. MR Assumptions
2.6. Two-Sample Summary-Level MR Analysis
3. Results
3.1. Characteristics of the Data Sources
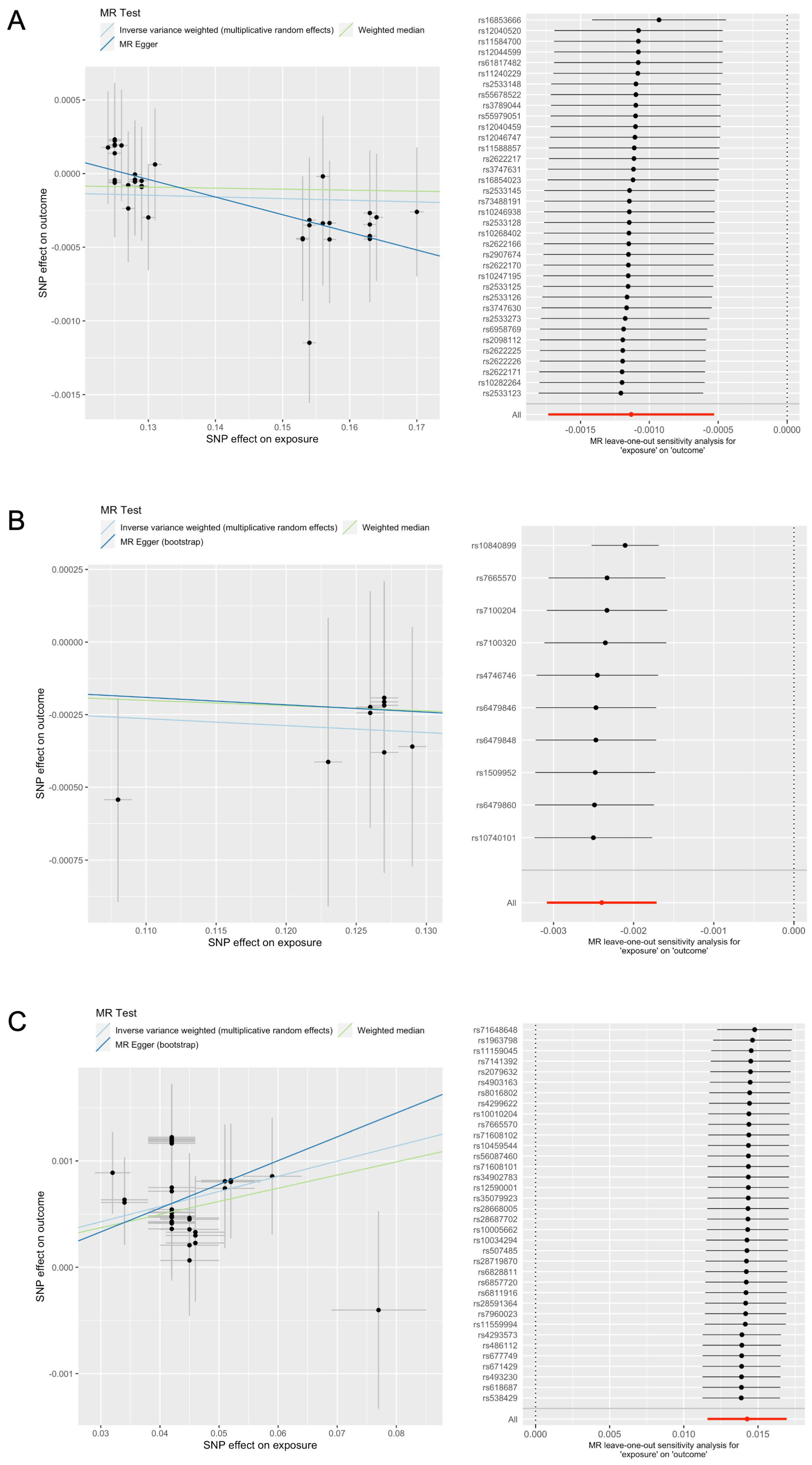
3.2. MR Analysis of Dietary Supplement Intake on Kidney Function
3.3. Sensitivity Analysis for Chondroitin Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perazella, M.A. Pharmacology behind Common Drug Nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef] [Green Version]
- Perazella, M.A. Renal vulnerability to drug toxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 1275–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health Stat. Rep. 2008, 10, 1–23. [Google Scholar]
- Sibbritt, D.; Adams, J.; Lui, C.-W.; Broom, A.; Wardle, J. Who Uses Glucosamine and Why? A Study of 266,848 Australians Aged 45 Years and Older. PLoS ONE 2012, 7, e41540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, K.M.; Arden, N.K.; Doherty, M.; Bannwarth, B.; Bijlsma, J.W.J.; Dieppe, P.; Gunther, K.; Hauselmann, H.; Herrero-Beaumont, G.; Kaklamanis, P.; et al. EULAR Recommendations 2003: An evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 2003, 62, 1145–1155. [Google Scholar] [CrossRef]
- Fransen, M.; Agaliotis, M.; Nairn, L.; Votrubec, M.; Bridgett, L.; Su, S.; Jan, S.; March, L.; Edmonds, J.; Norton, R.; et al. Glucosamine and chondroitin for knee osteoarthritis: A double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann. Rheum. Dis. 2015, 74, 851–858. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Gulin, J.P.; Felson, D.T. Glucosamine and chondroitin for treatment of osteoarthritis: A systematic quality assessment and meta-analysis. JAMA 2000, 283, 1469–1475. [Google Scholar] [CrossRef]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O.; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Bascoul-Colombo, C.; Garaiova, I.; Plummer, S.F.; Harwood, J.L.; Caterson, B.; Hughes, C.E. Glucosamine Hydrochloride but Not Chondroitin Sulfate Prevents Cartilage Degradation and Inflammation Induced by Interleukin-1α in Bovine Cartilage Explants. Cartilage 2016, 7, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.E.; Dacre, J.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef] [PubMed]
- du Souich, P.; García, A.G.; Vergés, J.; Montell, E. Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J. Cell. Mol. Med. 2009, 13, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Olaseinde, O.F.; Owoyele, B.V. Chondroitin and glucosamine sulphate reduced proinflammatory molecules in the DRG and improved axonal function of injured sciatic nerve of rats. Sci. Rep. 2022, 12, 3196. [Google Scholar] [CrossRef]
- King, D.E.; Xiang, J. Glucosamine/Chondroitin and Mortality in a US NHANES Cohort. J. Am. Board Fam. Med. 2020, 33, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, X.; Zhou, T.; Sun, D.; Liang, Z.; Li, Y.; Heianza, Y.; Qi, L. Glucosamine Use, Inflammation, and Genetic Susceptibility, and Incidence of Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes Care 2020, 43, 719–725. [Google Scholar] [CrossRef]
- Ahmad, R. Acute tubulointerstitial nephritis induced by glucosamine. Nephrol. Dial. Transpl. 2007, 22, 282. [Google Scholar] [CrossRef]
- Guillaume, M.-P.; Peretz, A. Possible association between glucosamine treatment and renal toxicity: Comment on the letter by Danao-Camara. Arthritis Rheum. 2001, 44, 2943–2944. [Google Scholar] [CrossRef]
- Gueye, S.; Saint-Cricq, M.; Coulibaly, M.; Goumri, N.; Guilbeau-Frugier, C.; Quentin, H.; Ged, E.; Sidi Aly, A.; Rostaing, L. Chronic tubulointerstitial nephropathy induced by glucosamine: A case report and literature review. Clin. Nephrol. 2016, 86, 106–110. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, S.; Kim, Y.; Cho, S.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; Joo, K.W.; et al. A Mendelian randomization study found causal linkage between telomere attrition and chronic kidney disease. Kidney Int. 2021, 100, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Short or Long Sleep Duration and CKD: A Mendelian Randomization Study. J. Am. Soc. Nephrol. 2020, 31, 2937–2947. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Atrial fibrillation and kidney function: A bidirectional Mendelian randomization study. Eur. Heart J. 2021, 42, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ni, C.; Zhang, Y.; Huang, J.; Hukportie, D.N.; Liang, B.; Tang, S. Association of regular glucosamine use with incident dementia: Evidence from a longitudinal cohort and Mendelian randomization study. BMC Med. 2023, 21, 114. [Google Scholar]
- Staley, J.R.; Blackshaw, J.; Kamat, M.A.; Ellis, S.; Surendran, P.; Sun, B.B.; Paul, D.S.; Freitag, D.; Burgess, S.; Danesh, J.; et al. PhenoScanner: A database of human genotype-phenotype associations. Bioinformatics 2016, 32, 3207–3209. [Google Scholar] [CrossRef] [Green Version]
- Wuttke, M.; Li, Y.; Li, M.; Sieber, K.B.; Feitosa, M.F.; Gorski, M.; Tin, A.; Wang, L.; Chu, A.Y.; Hoppmann, A.; et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 2019, 51, 957–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B.; Wheeler, D.; Levin, A.; Stevens, P.E.; Bilous, R.W.; Lamb, E.J.; Coresh, J.J.K.I. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013, 3, 1–150. [Google Scholar]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiolopy 2016, 40, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Stanzick, K.J.; Li, Y.; Schlosser, P.; Gorski, M.; Wuttke, M.; Thomas, L.F.; Rasheed, H.; Rowan, B.X.; Graham, S.E.; Vanderweff, B.R.; et al. Discovery and prioritization of variants and genes for kidney function in >1.2 million individuals. Nat. Commun. 2021, 12, 4350. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [Green Version]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Bucsi, L.; Poór, G. Efficacy and tolerability of oral chondroitin sulfate as a symptomatic slow-acting drug for osteoarthritis (SYSADOA) in the treatment of knee osteoarthritis. Osteoarthr. Cartil. 1998, 6, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, P.; Chales, G.; Dehais, J.; Delcambre, B.; Kuntz, J.L.; Rozenberg, S. Efficacy and tolerability of chondroitin sulfate 1200 mg/day vs chondroitin sulfate 3 × 400 mg/day vs. placebo. Osteoarthr. Cartil. 1998, 6, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Leonberg-Yoo, A.K.; Johnson, D.; Persun, N.; Bahrainwala, J.; Reese, P.P.; Naji, A.; Trofe-Clark, J. Use of Dietary Supplements in Living Kidney Donors: A Critical Review. Am. J. Kidney Dis. 2020, 76, 851–860. [Google Scholar] [CrossRef]
- Setnikar, I.; Rovati, L.C. Absorption, Distribution, Metabolism and Excretion of Glucosamine Sulfate. A review. Arzneimittelforschung 2001, 51, 699–725. [Google Scholar]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Mazieres, B.; Hucher, M.; Zaim, M.; Garnero, P. Effect of chondroitin sulphate in symptomatic knee osteoarthritis: A multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2007, 66, 639–645. [Google Scholar] [CrossRef]
- Dennis, J.M.; Witting, P.K. Protective role for antioxidants in acute kidney disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Elbassuoni, E.A.; Ragy, M.M.; Ahmed, S.M. Evidence of the protective effect of l-arginine and vitamin D against monosodium glutamate-induced liver and kidney dysfunction in rats. Biomed. Pharmacother. 2018, 108, 799–808. [Google Scholar] [CrossRef]
- Liu, P.; Feng, Y.; Wang, Y.; Zhou, Y.; Zhao, L. Protective effect of vitamin E against acute kidney injury. Biomed. Mater. Eng. 2015, 26, S2133–S2144. [Google Scholar] [CrossRef]
- Xu, F.; Wen, Y.; Hu, X.; Wang, T.; Chen, G. The Potential Use of Vitamin C to Prevent Kidney Injury in Patients with COVID-19. Diseases 2021, 9, 46. [Google Scholar] [CrossRef]
- Salehzadeh, A.; Salehzadeh, A.; Maghsood, A.H.; Heidarisasan, S.; Taheri-Azandaryan, M.; Ghafourikhosroshahi, A.; Abbasalipourkabir, R. Effects of vitamin A and vitamin E on attenuation of amphotericin B-induced side effects on kidney and liver of male Wistar rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 32594–32602. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
| Exposure | Analysis | a Outcome | eGFR Change Beta (%) | Standard Error (%) | p-Value |
|---|---|---|---|---|---|
| Chondroitin | Main | Creatinine-based log-eGFR (CKDGen) | −0.113 | 0.030 | 2 × 10−4 |
| Validation | Creatinine-based log-eGFR (CKDGen + UKB) | −0.283 | 0.016 | 4 × 10−31 | |
| Glucosamine | Main | Creatinine-based log-eGFR (CKDGen) | −0.240 | 0.035 | 6 × 10−12 |
| Validation | Creatinine-based log-eGFR (CKDGen + UKB) | 0.424 | 0.091 | 3 × 10−6 | |
| Vitamin/ mineral supplement intake | Main | Creatinine-based log-eGFR (CKDGen) | 1.426 | 0.136 | 1 × 10−25 |
| Validation | Creatinine-based log-eGFR (CKDGen + UKB) | 1.259 | 0.141 | 4 × 10−19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.-M.; Koh, J.-H.; Kim, S.-G.; Lee, S.; Kim, Y.; Cho, S.; Kim, K.; Kim, Y.-C.; Han, S.-S.; Lee, H.; et al. Causal Effect of Chondroitin, Glucosamine, Vitamin, and Mineral Intake on Kidney Function: A Mendelian Randomization Study. Nutrients 2023, 15, 3318. https://doi.org/10.3390/nu15153318
Cho J-M, Koh J-H, Kim S-G, Lee S, Kim Y, Cho S, Kim K, Kim Y-C, Han S-S, Lee H, et al. Causal Effect of Chondroitin, Glucosamine, Vitamin, and Mineral Intake on Kidney Function: A Mendelian Randomization Study. Nutrients. 2023; 15(15):3318. https://doi.org/10.3390/nu15153318
Chicago/Turabian StyleCho, Jeong-Min, Jung-Hun Koh, Seong-Geun Kim, Soojin Lee, Yaerim Kim, Semin Cho, Kwangsoo Kim, Yong-Chul Kim, Seung-Seok Han, Hajeong Lee, and et al. 2023. "Causal Effect of Chondroitin, Glucosamine, Vitamin, and Mineral Intake on Kidney Function: A Mendelian Randomization Study" Nutrients 15, no. 15: 3318. https://doi.org/10.3390/nu15153318
APA StyleCho, J.-M., Koh, J.-H., Kim, S.-G., Lee, S., Kim, Y., Cho, S., Kim, K., Kim, Y.-C., Han, S.-S., Lee, H., Lee, J.-P., Joo, K.-W., Lim, C.-S., Kim, Y.-S., Kim, D.-K., & Park, S. (2023). Causal Effect of Chondroitin, Glucosamine, Vitamin, and Mineral Intake on Kidney Function: A Mendelian Randomization Study. Nutrients, 15(15), 3318. https://doi.org/10.3390/nu15153318






