In Vivo Toxicity Assessment of the Probiotic Bacillus amyloliquefaciens HTI-19 Isolated from Stingless Bee (Heterotrigona itama) Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Preparation of B. Amyloliquefaciens HTI-19 Culture
2.3. Acute Oral Toxicity Study
2.4. Sub-Acute Oral Toxicity Study
2.5. General Observations
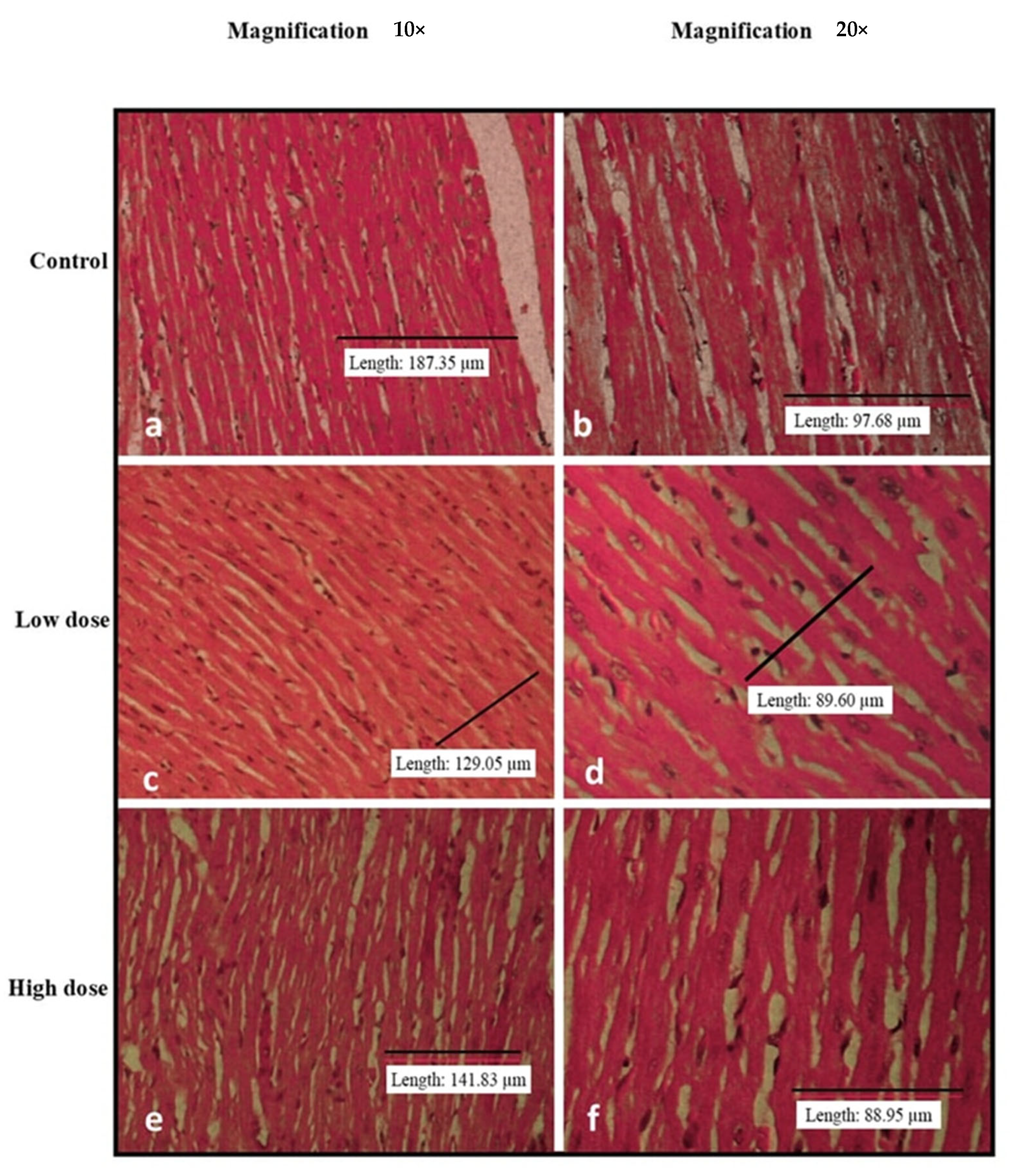
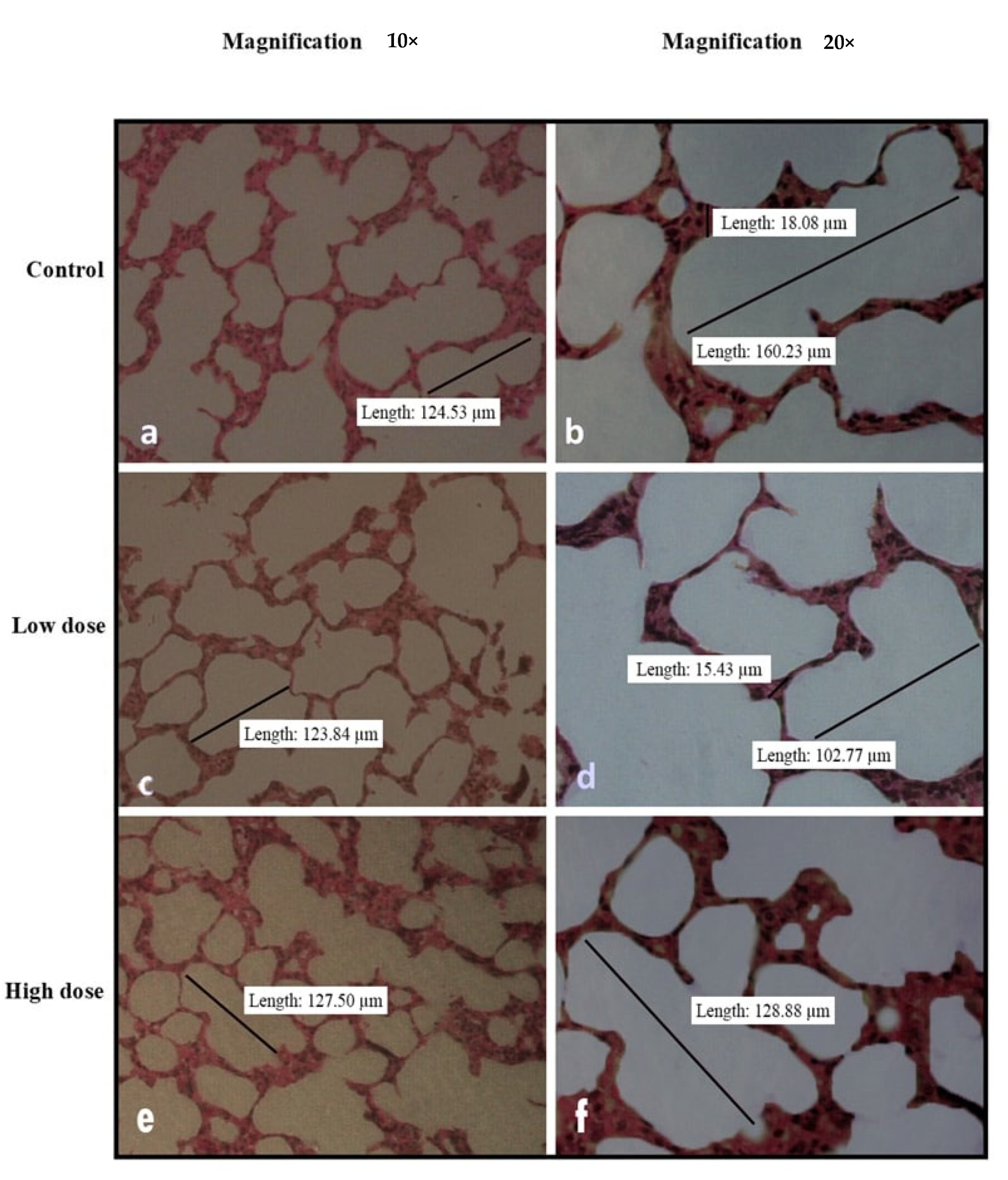
2.6. Relative Organ Weights and Histopathological Analysis
2.7. Serum Biochemistry and Hematological Analyses
2.8. Statistical Analysis
3. Results
3.1. Acute and Sub-Acute Toxicity of B. Amyloliquefaciens HTI-19
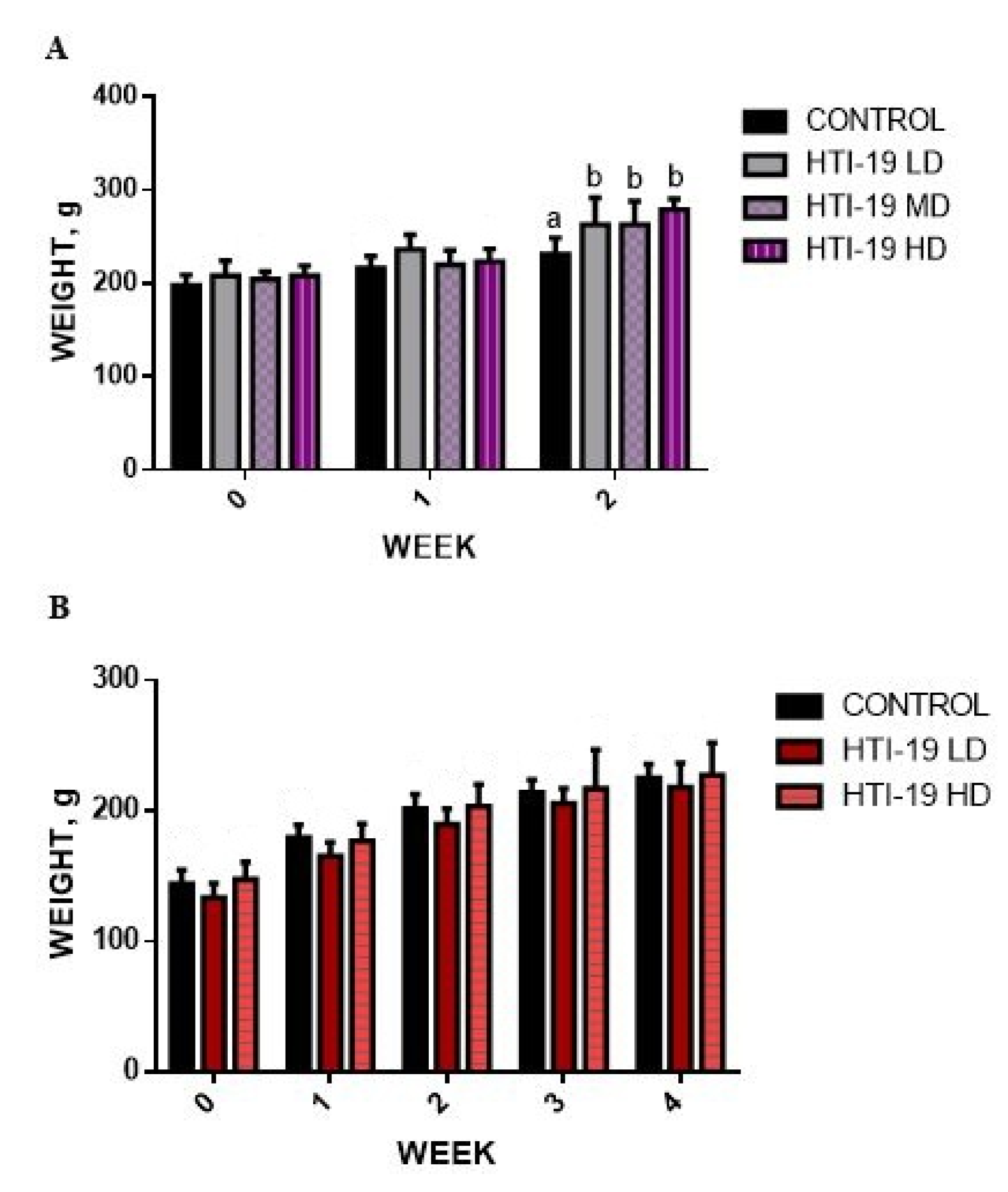
3.2. Effects of Oral Administration of B. Amyloliquefaciens HTI-19 on Body Weight
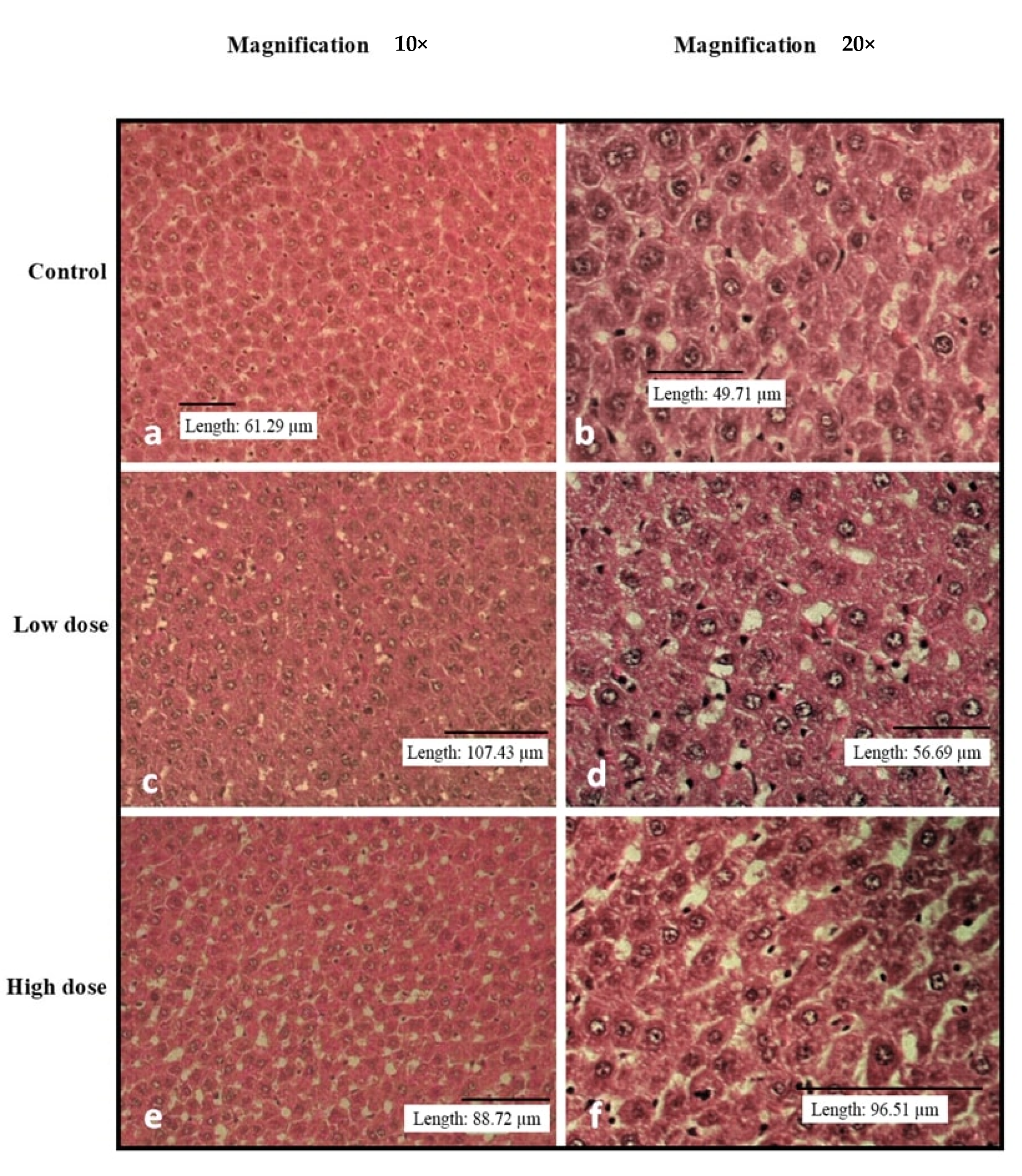
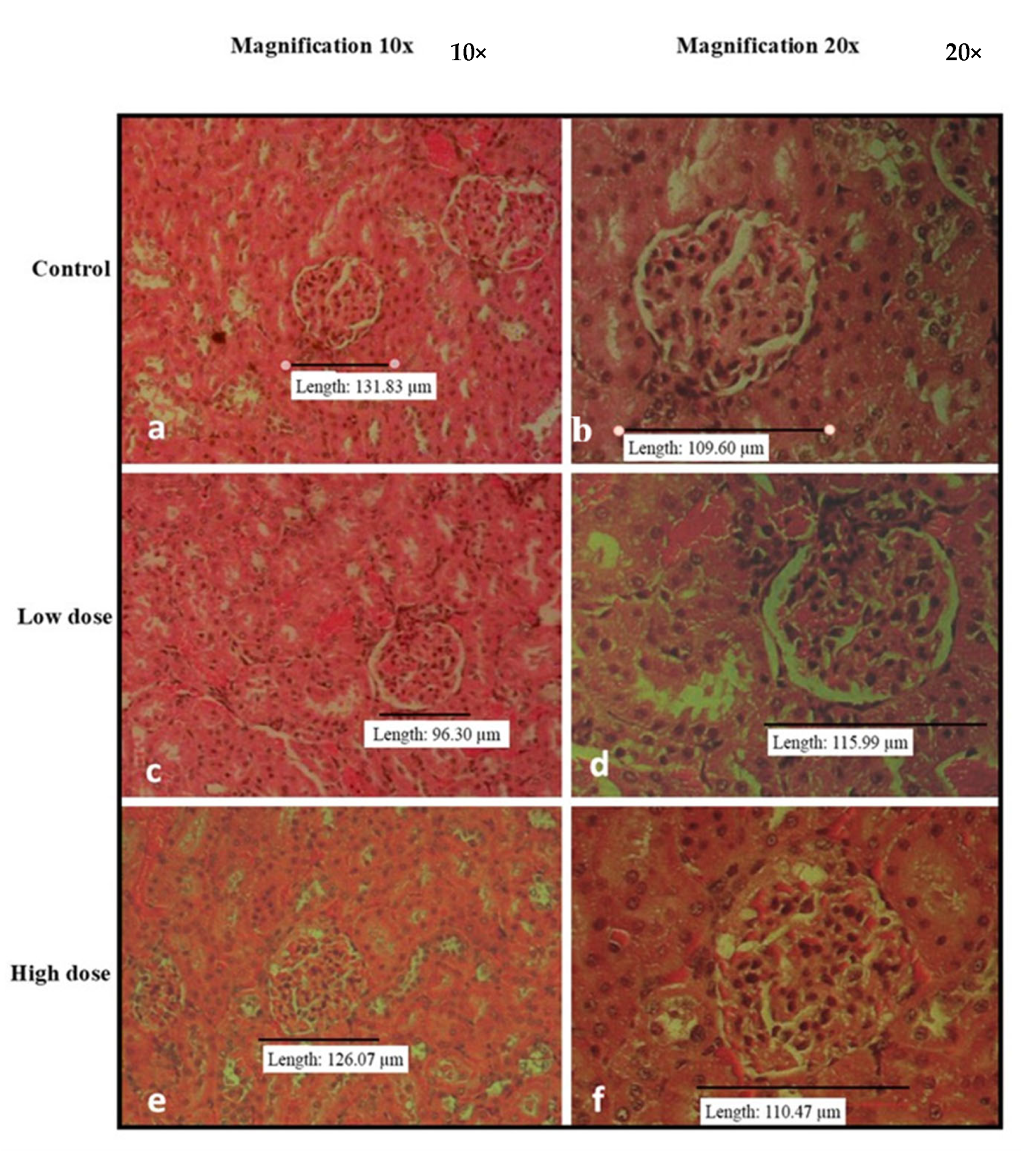
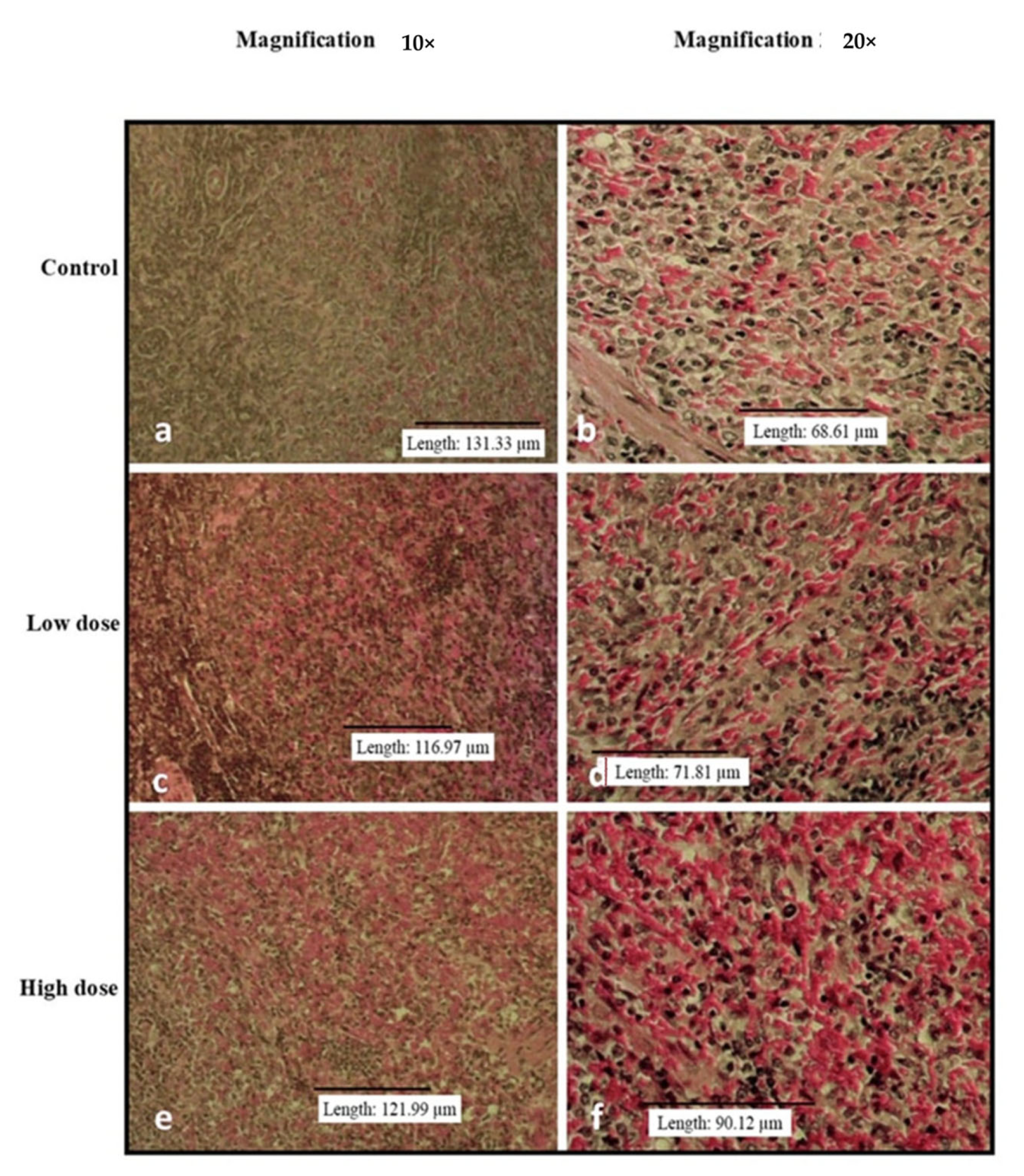
3.3. Effects of Oral Administration of B. Amyloliquefaciens HTI-19 on Relative Organ Weights and Histopathological Examination
3.4. Effects of Oral Administration of B. Amyloliquefaciens HTI-19 on Biochemical Parameters and Complete Blood Count
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current status of probiotic and related health benefits. App. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Narita, M. The Gut Microbiome as a Target for Prevention of Allergic Diseases. Jpn. J. Allerg. 2020, 69, 19–22. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; Hammerman, C.; James, T.; Hörmannsperger, G.; Huys, G.; Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; et al. Safety Assessment of Probiotics for Human Use Safety Assessment of Probiotics for Human Use. Gut Microbes 2010, 1, 37–41. [Google Scholar] [CrossRef]
- Gayathri, L.; Krubha, A. Bacillus Species—Elucidating the Dilemma on Their Probiotic and Pathogenic Traits. In Advances in Probiotics; Dhanasekaran, D., Sankaranarayanan, A., Eds.; Academic Press: Amsterdam, The Netherlands, 2021; pp. 233–245. [Google Scholar] [CrossRef]
- Kotowicz, N.; Bhardwaj, R.K.; Ferreira, W.T.; Hong, H.A.; Olender, A.; Ramirez, J.; Cutting, S.M. Safety and Probiotic Evaluation of Two Bacillus Strains Producing Antioxidant Compounds. Benef. Microbes 2019, 10, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.M.; Colasacco, C.; Emmanouil, E.; Kohlhepp, S.; Harriott, O. Strain-Level Diversity of Commercial Probiotic Isolates of Bacillus, Lactobacillus, and Saccharomyces Species Illustrated by Molecular Identification and Phenotypic Profiling. PLoS ONE 2019, 14, e0213841. [Google Scholar] [CrossRef]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive Approaches for Assessing the Safety of Probiotic Bacteria. Food Control 2020, 108, 106872. [Google Scholar] [CrossRef]
- Islam, M.I.; Seo, H.; Redwan, A.; Kim, S.; Lee, S.; Siddiquee, M.; Song, H.-Y. In Vitro and In Vivo Anti-Clostridioides Difficile Effect of a Probiotic Bacillus amyloliquefaciens Strain. J. Microbiol. Biotechnol. 2022, 32, 46–55. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Raja Abd Rahman, R.N.Z.; Yusof, M.T.; Syahir, A.; Sabri, S. Characterisation of Bacteria Isolated from the Stingless Bee, Heterotrigona Itama, Honey, Bee Bread and Propolis. PeerJ 2019, 7, e7478. [Google Scholar] [CrossRef]
- Zawawi, N.; Zhang, J.; Hungerford, N.L.; Yates, H.S.A.; Webber, D.C.; Farrell, M.; Tinggi, U.; Bhandari, B.; Fletcher, M.T. Unique Physichochemical Properties and Rare Reducing Sugar Trehalulose Mandate New International Regulation for Stingless Bee Honey. Food Chem. 2022, 373 Pt B, 131566. [Google Scholar] [CrossRef]
- Badrulhisham, N.S.R.; Ab Hamid, S.N.P.; Ismail, M.A.H.; Yong, Y.K.; Zakuan, N.M.; Harith, H.H.; Saidi, H.I.; Nurdin, A. Harvested locations influence the total phenolic content, antioxidant levels, cytotoxic, and anti-inflammatory activities of stingless bee honey. J. Asia-Pac. Ent. 2020, 23, 950–956. [Google Scholar] [CrossRef]
- Ismail, N.F.; Maulidiani, M.; Omar, S.; Zulkifli, M.F.; Mohd Radzi, M.N.F.; Ismail, N.; Jusoh, A.Z.; Roowi, S.; Yew, W.M.; Rudiyanto, R.; et al. Classification of stingless bee honey based on species, dehumidification process and geographical origins using physicochemical and ATR-FTIR chemometric approach. J. Food Comp. Anal. 2021, 104, 104126. [Google Scholar] [CrossRef]
- Zulkhairi Amin, F.A.; Sabri, S.; Ismail, M.; Chan, K.W.; Ismail, N.; Mohd Esa, N.; Mohd Lila, M.A.; Zawawi, N. Probiotic Properties of Bacillus Strains Isolated from Stingless Bee (Heterotrigona Itama) Honey Collected across Malaysia. Int. J. Environ. Res. Public Health 2019, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering Probiotic Microorganisms: In Vitro, in Vivo, Genetic and Omics Approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Ngalimat, M.S.; Abd Rahman, R.N.Z.R.; Yusof, M.T.; Amir Hamzah, A.S.; Zawawi, N.; Sabri, S. A Review on the Association of Bacteria with Stingless Bees. Sains Malays. 2020, 49, 1853–1863. [Google Scholar] [CrossRef]
- Zulkhairi Amin, F.A.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic Properties of Stingless Bee Honey in Comparison with European Bee Honey. Adv. Pharmacol. Sci. 2018, 2018, 6179596. [Google Scholar] [CrossRef]
- Avila, S.; Beux, M.R.; Ribani, R.H.; Zambiazi, R.C. Stingless Bee Honey: Quality Parameters, Bioactive Compounds, Health-Promotion Properties and Modification Detection Strategies. Trends in Food Sci. Tech. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Wahed, A.A.A.; Al Naggar, Y.; Saeed, A.; Xiao, J.; Ullah, H.; Musharraf, S.G.; Boskabady, M.H.; Cao, W.; Guo, Z.; et al. Insights into the Role of Natural Products in the Control of the Honey Bee Gut Parasite (Nosema spp.). Animals 2022, 12, 3062. [Google Scholar] [CrossRef]
- Salama, S.; Shou, Q.; Abd El-Wahed, A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K.; et al. Royal Jelly: Beneficial Properties and Synergistic Effects with Chemotherapeutic Drugs with Particular Emphasis in Anticancer Strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee Pollen: Clinical Trials and Patent Applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- El-Wahed, A.A.A.; Farag, M.A.; Eraqi, W.A.; Mersal, G.A.M.; Zhao, C.; Khalifa, S.A.M.; El-Seedi, H.R. Unravelling the beehive air volatiles profile as analysed via solid-phase microextraction (SPME) and chemometrics. J. King Saud Univ. Sci. 2021, 33, 101449–101456. [Google Scholar] [CrossRef]
- OECD. Acute Oral Toxicity—Fixed Dose Procedure (Chptr). In OECD Guideline for Testing of Chemical; OECD Publishing: Paris, France, 2001. [Google Scholar]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2008; Section 4. [Google Scholar]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Kalavathy, R.; Sieo, C.C.; Ho, Y.W. Safety Assessment of Two New Lactobacillus Strains as Probiotic for Human Using a Rat Model. PLoS ONE 2016, 11, e0159851. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Su, G.; Liu, K.; Zhang, F.; Jiang, Y.; Gao, J.; Liu, L.; Jiang, Z.; Jin, M.; Xie, H. Sex-Specific Reference Intervals of Hematologic and Biochemical Analytes in Sprague-Dawley Rats Using the Nonparametric Rank Percentile Method. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.-Z.; Xu, H.-D.; Kim, K.-H.; Ahn, T.-H.; Bae, J.-S.; Lee, J.-Y.; Gil, K.-H.; Lee, J.-Y.; Woo, S.-J.; Yoo, H.-J.; et al. Reference Data of the Main Physiological Parameters in Control Sprague-Dawley Rats from Pre-Clinical Toxicity Studies. Lab Anim. Res. 2010, 26, 153. [Google Scholar] [CrossRef]
- Nithya, V.; Muthukumar, S.P.; Halami, P.M. Safety Assessment of Bacillus licheniformis Me1 Isolated from Milk for Probiotic Application. Int. J. Toxicol. 2012, 31, 228–237. [Google Scholar] [CrossRef]
- Delwatta, S.L.; Gunatilake, M.; Baumans, V.; Seneviratne, M.D.; Dissanayaka, M.L.B.; Batagoda, S.S.; Udagedara, A.H.; Walpola, P.B. Reference Values for Selected Hematological, Biochemical and Physiological Parameters of Sprague-Dawley Rats at the Animal House, Faculty of Medicine, University of Colombo, Sri Lanka. Anim. Model Exp. Med. 2018, 1, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, V.; Melnikov, V.; Chikindas, M.L.; Khutsishvili, M.; Chagelishvili, A.; Bren, A.; Kostina, N.; Cavera, V.; Elisashvili, V. Poultry-Beneficial Solid-State Bacillus amyloliquefaciens B-1895 Fermented Soybean Formulation. Biosci. Microbiota Food Health 2015, 34, 25–28. [Google Scholar] [CrossRef]
- Hong, Y.; Cheng, Y.; Li, Y.; Li, X.; Zhou, Z.; Shi, D.; Li, Z.; Xiao, Y. Preliminary Study on the Effect of Bacillus amyloliquefaciens TL on Cecal Bacterial Community Structure of Broiler Chickens. Biomed. Res. Int. 2019, 2019, 5431354. [Google Scholar] [CrossRef]
- An, B.K.; Cho, B.L.; You, S.J.; Paik, H.D.; Chang, H.I.; Kim, S.W.; Yun, C.W.; Kang, C.W. Growth Performance and Antibody Response of Broiler Chicks Fed Yeast Derived β-Glucan and Single-Strain Probiotics. Asian-Australas. J. Anim. Sci. 2008, 21, 1027–1032. [Google Scholar] [CrossRef]
- Balogun, S.O.; da Silva, I.F.; Colodel, E.M.; de Oliveira, R.G.; Ascêncio, S.D.; Martins, D.T.D.O. Toxicological Evaluation of Hydroethanolic Extract of Helicteres sacarolha A. J. Ethnopharmacol. 2014, 157, 285–291. [Google Scholar] [CrossRef]
- Peters, J.M.; Boyd, E.M. Organ Weights and Water Levels of the Rat Following Reduced Food Intake. J. Nutr. 1966, 90, 354–360. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Leu, S.-F.; Huang, Q.-R.; Chou, L.-C.; Huang, C.-C. Safety Evaluation of Multiple Strains of Lactobacillus plantarum and Pediococcus pentosaceus in Wistar Rats Based on the Ames Test and a 28-Day Feeding Study. Sci. World J. 2014, 2014, 928652. [Google Scholar] [CrossRef] [PubMed]
- Abotsi, W.K.M.; Ainooson, G.K.; Gyasi, E.B. Acute and Sub-Acute Toxicity Studies of the Ethanolic Extract of the Aerial Parts of the Aerial Parts of Hilleria latifolia (Lam.) H. walt (Phytolaccaceae) in Rodents. West Afr. Pharm. 2011, 22, 27–35. [Google Scholar]
- AACC. American Association for Clinical Chemistry. Lab Tests Online. American Association for Clinical Chemistry. Available online: https://www.labtestsonline.org (accessed on 13 January 2019).
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W. Bacillus amyloliquefaciens B10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin B1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, H.; Meng, F.; Zhou, L.; Lu, Z.; Lu, Y. Surfactin alleviated hyperglycaemia in mice with type 2 diabetes induced by a high-fat diet and streptozotocin. Food Sci. Hum. Wellness 2023, 12, 2095–2110. [Google Scholar] [CrossRef]
- Szabo, N.J.; Dolan, L.C.; Burdock, G.A.; Shibano, T.; Sato, S.; Suzuki, H.; Uesugi, T.; Yamahira, S.; Toba, M.; Ueno, H. Safety Evaluation of Lactobacillus Pentosus Strain B240. Food Chem. Toxicol. 2011, 49, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Shu, Q.; Rutherfurd, K.J.; Prasad, J.; Birtles, M.J.; Gopal, P.K.; Gill, H.S. Safety Assessment of Potential Probiotic Lactic Acid Bacterial Strains Lactobacillus rhamnosus HN001, Lb. Acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c Mice. Int. J. Food Microbiol. 2000, 56, 87–96. [Google Scholar] [CrossRef]
- Endres, J.R.; Clewell, A.; Jade, K.A.; Farber, T.; Hauswirth, J.; Schauss, A.G. Safety Assessment of a Proprietary Preparation of a Novel Probiotic, Bacillus Coagulans, as a Food Ingredient. Food Chem. Toxicol. 2009, 47, 1231–1238. [Google Scholar] [CrossRef]
| Animal Grouping | Treatment |
|---|---|
| Group 1 | Normal group (N)—Normal rat, receive a normal diet and 10% UHT milk. |
| Group 2 | Low Dose (LD)—receive a normal diet and probiotic B. amyloliquefaciens culture (1 × 109 CFU·mL−1) in 10% UHT milk. |
| Group 3 | Medium Dose (LD)—receive a normal diet and probiotic B. amyloliquefaciens culture (3 × 109 CFU·mL−1) in 10% UHT milk. |
| Group 4 | High Dose (HD)—receive a normal diet and probiotic B. amyloliquefaciens culture (1 × 1010 CFU·mL−1) in 10% UHT milk. |
| Animal Grouping | Treatment |
|---|---|
| Group 1 | Normal group (N)—Normal rat, receive a normal diet and 10% UHT milk. |
| Group 2 | Low Dose (LD)—receive a normal diet and probiotic B. amyloliquefaciens culture (1 × 109 CFU·mL−1) in 10% UHT milk. |
| Group 3 | High Dose (HD)—receive a normal diet and probiotic B. amyloliquefaciens culture (1 × 1010 CFU·mL−1) in 10% UHT milk. |
| Treatment, CFU·mL−1 | ||||
|---|---|---|---|---|
| Organ | Acute Tocixity | |||
| Control | B. amyloliquefaciens HTI-19 | |||
| 1 × 109 (LD) | 3.0 × 109 (MD) | 1 × 1010 (HD) | ||
| Lung | 0.53 0.12 | 0.50 0.16 | 0.59 0.17 | 0.46 0.23 |
| Heart | 0.39 0.10 | 0.34 0.09 | 0.29 0.08 | 0.53 0.35 |
| Liver | 4.20 0.25 | 3.66 0.51 | 3.62 0.47 | 4.00 0.73 |
| Spleen | 0.26 0.04 | 0.25 0.06 | 0.26 0.07 | 0.21 0.05 |
| Right kidney | 0.42 0.06 | 0.38 0.08 | 0.39 0.12 | 0.44 0.11 |
| Left Kidney | 0.39 0.05 | 0.37 0.06 | 0.37 0.07 | 0.42 0.15 |
| Treatment, CFU·mL−1 | |||
|---|---|---|---|
| Organ | Acute Tocixity | ||
| Control | B. amyloliquefaciens HTI-19 | ||
| 1 × 109 (LD) | 1 × 1010 (HD) | ||
| Lung | 0.55 ± 0.03 a | 0.63 ± 0.10 a | 0.71 ± 0.07 b |
| Heart | 0.34 ± 0.03 a | 0.40 ± 0.45 b | 0.40 ± 0.04 b |
| Liver | 4.35 ± 0.28 a | 4.54 ± 0.50 a | 4.94 ± 0.40 a |
| Spleen | 0.22 ± 0.04 a | 0.27 ± 0.02 a | 0.30 ± 0.05 b |
| Right kidney | 0.39 ± 0.04 a | 0.41 ± 0.04 a | 0.48 ± 0.10 a |
| Left Kidney | 0.36 ± 0.02 a | 0.39 ± 0.03 a | 0.45 ± 0.07 b |
| Parameter | Normal Range [25,26,27] | Treatment, CFU·mL−1 | |||
|---|---|---|---|---|---|
| Acute Tocixity | |||||
| Control | B. amyloliquefaciens HTI-19 | ||||
| 1 × 109 (LD) | 3.0 × 109 (MD) | 1 × 1010 (HD) | |||
| ALP, U/L | 59.00–196.00 | 148.20 ± 28.01 a | 97.63 ± 41.13 a | 123.13 ± 43.9 a | 60.83 ± 13.93 b |
| ALT, U/L | 19.20–48.70 | 44.00 ± 3.87 | 33.00 ± 10.04 | 44.00 ± 17.89 | 33.00 ± 31.26 |
| AST, U/L | 67.30–166.00 | 114.33 ± 14.84 | 132.50 ± 34.73 | 141.50 ± 55.91 | 104.17 ± 45.91 |
| ALB, g/L | 26.85–34.55 | 38.20 ± 2.86 a | 34.70 ± 9.81 a | 30.59 ± 8.15 a | 25.00 ± 7.71 b |
| TP, g/L | 49.70–73.00 | 71.90 ± 4.54 a | 63.80 ± 19.24 a | 58.21 ± 17.11 a | 44.80 ± 13.26 b |
| Creat, umol/L | 29.00–63.00 | 45.00 ± 2.40 a | 46.00 ± 11.03 a | 42.00 ± 11.78 a | 29.00 ± 6.05 b |
| Parameter | Normal Range [25,26,27] | Treatment, CFU·mL−1 | ||
|---|---|---|---|---|
| Sub-Acute Tocixity | ||||
| Control | B. amyloliquefaciens HTI-19 | |||
| 1 × 109(LD) | 1 × 1010 (HD) | |||
| ALP, U/L | 59.00–196.00 | 30.62 | 15.93 | 35.16 |
| ALT, U/L | 19.20–48.70 | 26.43 | 8.04 | 11.47 |
| AST, U/L | 67.30–166.00 | 52.82 | 24.99 | 51.64 |
| ALB, g/L | 26.85–34.55 | 2.66 | 1.96 | 4.11 |
| TBil, umol/L | 1.20–8.40 | 0.50 | 0.59 | 0.58 |
| TP, g/L | 49.70–73.00 | 5.12 | 4.00 | 7.82 |
| Creat, umol/L | 29.00–63.00 | 4.05 | 3.51 | 4.38 |
| Urea, mmol/L | 5.56–12.67 | 1.23 | 0.80 | 1.90 |
| Parameter | Unit | Normal Range, [25] | Treatment, CFU·mL−1 | ||
|---|---|---|---|---|---|
| Sub-Acute Tocixity | |||||
| Control | B. amyloliquefaciens HTI-19 | ||||
| 1 × 109(LD) | 1 × 1010 (HD) | ||||
| RBC | ×1012/L | 2.9–6.8 | 0.90 a | 1.20 a | 5.7038 ± 0.74 a |
| Hemoglobin | g/L | 86.00–153.80 | 18.31 a | 25.07 a | 142.38 ± 14.94 a |
| Packed cell volume | L/L | 0.10–0.47 | 0.05 a | 0.06a | 0.4 ± 0.04 a |
| Mean corpuscular volume (MCV) | fL | 15.15–119.44 | 6.38 a | 3.23 a | 70.58 ± 5.33 a |
| Mean corpuscular hemoglobin concentration (MCHC) | g/L | 211.60–950.00 | 25.41 a | 18.34 a | 355.80 ± 16.26 a |
| WBC | x109/L | 3.60–14.50 | 2.54 a | 4.11 a | 10.36 ± 6.75 a |
| Neutrophils | % | 13.00–61.00 | 2.62 a | 3.024 a | 18.75 ± 25.75 b |
| Lymphocytes | % | 55.00–86.00 | 3.16 a | 3.38 a | 74.9 ± 7.019 b |
| Monocytes | % | 0.00–1.00 | 0.71 a | 0.74 a | 4.50 ± 1.20 a |
| Eosinophils | % | 0.00–8.00 | 0.46 a | 0.835 a | 0.88 ± 0.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkhairi Amin, F.A.; Shafiq Cheng, M.Z.; Sabri, S.; Ismail, N.; Chan, K.W.; Mohd Esa, N.; Mohd Lila, M.A.; Nur-Fazila, S.H.; Khalifa, S.A.M.; El-Seedi, H.R.; et al. In Vivo Toxicity Assessment of the Probiotic Bacillus amyloliquefaciens HTI-19 Isolated from Stingless Bee (Heterotrigona itama) Honey. Nutrients 2023, 15, 2390. https://doi.org/10.3390/nu15102390
Zulkhairi Amin FA, Shafiq Cheng MZ, Sabri S, Ismail N, Chan KW, Mohd Esa N, Mohd Lila MA, Nur-Fazila SH, Khalifa SAM, El-Seedi HR, et al. In Vivo Toxicity Assessment of the Probiotic Bacillus amyloliquefaciens HTI-19 Isolated from Stingless Bee (Heterotrigona itama) Honey. Nutrients. 2023; 15(10):2390. https://doi.org/10.3390/nu15102390
Chicago/Turabian StyleZulkhairi Amin, Fatin Aina, Mohamad Zulhafiz Shafiq Cheng, Suriana Sabri, Norsharina Ismail, Kim Wei Chan, Norhaizan Mohd Esa, Mohd Azmi Mohd Lila, Saulol Hamid Nur-Fazila, Shaden A. M. Khalifa, Hesham R. El-Seedi, and et al. 2023. "In Vivo Toxicity Assessment of the Probiotic Bacillus amyloliquefaciens HTI-19 Isolated from Stingless Bee (Heterotrigona itama) Honey" Nutrients 15, no. 10: 2390. https://doi.org/10.3390/nu15102390
APA StyleZulkhairi Amin, F. A., Shafiq Cheng, M. Z., Sabri, S., Ismail, N., Chan, K. W., Mohd Esa, N., Mohd Lila, M. A., Nur-Fazila, S. H., Khalifa, S. A. M., El-Seedi, H. R., & Zawawi, N. (2023). In Vivo Toxicity Assessment of the Probiotic Bacillus amyloliquefaciens HTI-19 Isolated from Stingless Bee (Heterotrigona itama) Honey. Nutrients, 15(10), 2390. https://doi.org/10.3390/nu15102390











