Association of Macronutrients Intake with Body Composition and Sarcopenic Obesity in Children and Adolescents: A Population-Based Analysis of the National Health and Nutrition Examination Survey (NHANES) 2011–2018
Abstract
1. Introduction
2. Materials and Methods
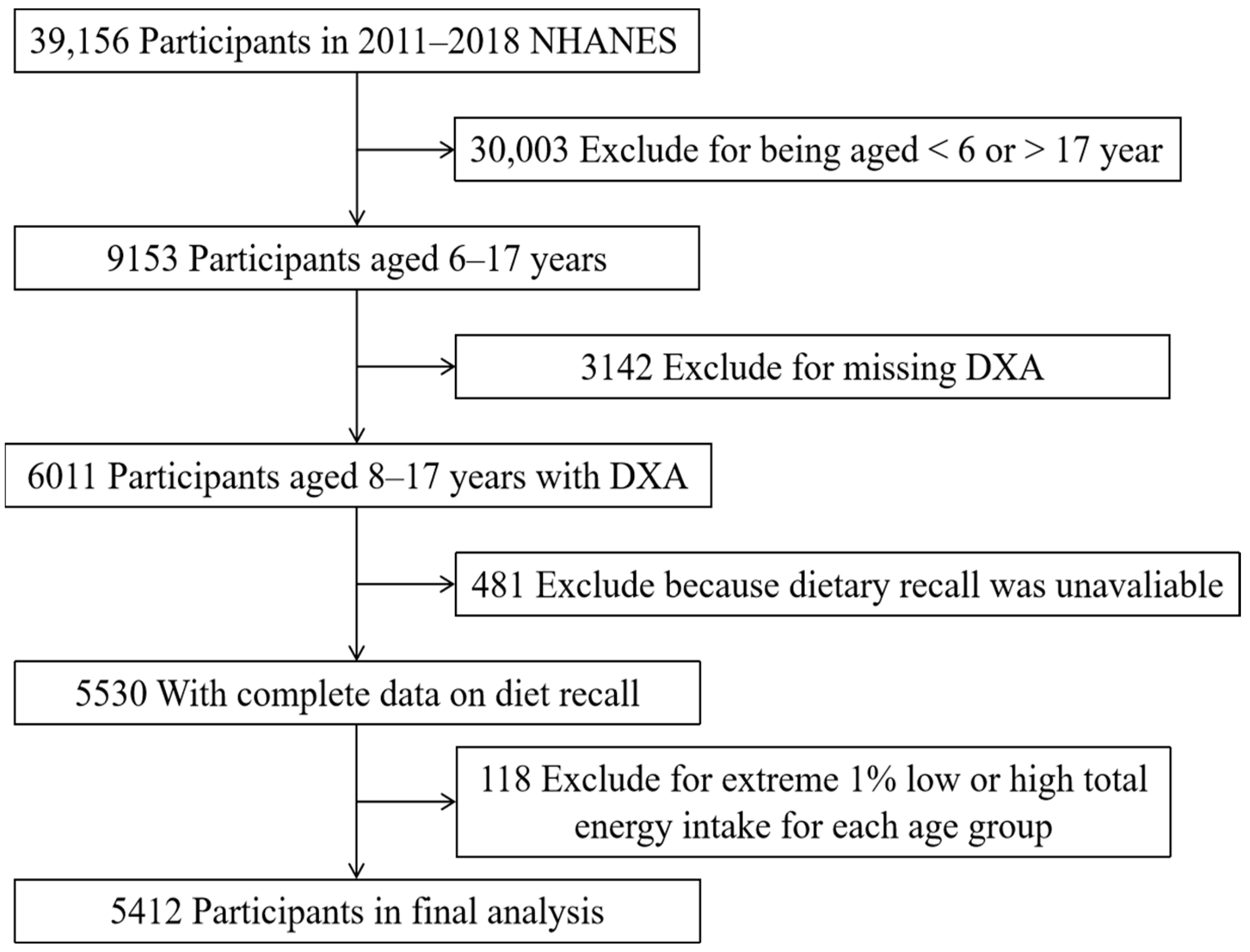
2.1. The Study Design and Study Sample
2.2. Anthropometric Measurements and Whole-Body DXA Scanning
2.3. Assessment of Macronutrients Intake Distribution
2.4. Covariates
2.5. Definitions of Sarcopenia and Obesity
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Associations of Macronutrients Distribution with Body Composition Indicators and Sarcopenic Obesity
3.3. Isocaloric Substitution Analysis Results
4. Discussion
4.1. Comparison with Other Studies
4.2. Mechanism of Sarcopenic Obesity Caused by High Fat Diet
4.3. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benson, A.C.; Torode, M.E.; Singh, M.A. Muscular Strength and Cardiorespiratory FitnessisAssociatedWith Higher Insulin SensitivityinChildren and Adolescents. Int. J. Pediatr. Obes. 2006, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Marcela, Z.; Paweł, M. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022, 13, 914740. [Google Scholar]
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Larsen, T.M.; Dalskov, S.M.; van, B.M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Pihlsgård, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Paineau, D.L.; Beaufils, F.; Boulier, A.; Cassuto, D.A.; Chwalow, J.; Combris, P.; Couet, C.; Jouret, B.; Lafay, L.; Laville, M.; et al. Family dietary coaching to improve nutritional intakes and body weight control: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2008, 162, 34–43. [Google Scholar] [CrossRef]
- Boushey, C.; Ard, J.; Bazzano, L.; Heymsfield, S.; Mayer-Davis, E.; Sabaté, J.; Snetselaar, L.; Horn, L.V.; Schneeman, B.; English, L.K.; et al. Dietary Patterns and Growth, Size, Body composition, and/or Risk of Overweight or Obesity: A Systematic Review; USDA Nutrition Evidence Systematic Review: Alexandria, VA, USA, 2020. [Google Scholar] [CrossRef]
- Davis, J.; Mohebbi, M.; Kim, Y.J.; Collier, F.; Loughman, A.; Staudacher, H.; Shivappa, N.; Hébert, J.; Pasco, J.; Jacka, F. The role of diet quality and dietary patterns in predicting muscle mass and function in men over a 15-year period. Osteoporos. Int. 2021, 32, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.; McKenna, C.; Salvador, A.; Paulussen, K.; Moore, D. Dietary Protein Quantity, Quality, and Exercise Are Key to Healthy Living: A Muscle-Centric Perspective Across the Lifespan. Front. Nutr. 2019, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Morales-Suarez-Varela, M.; Peraita-Costa, I.; Guillamon, E.; Llopis-Morales, A.; Llopis-Gonzalez, A. Total body skeletal muscle mass and diet in children aged 6–8 years: ANIVA Study. Appl. Physiol. Nutr. Metab. 2019, 44, 944–951. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Moon, S.; Yu, J.M.; Chung, H.S. Implication of diet and exercise on the management of age-related sarcopenic obesity in Asians. Geriatr. Gerontol. Int. 2022, 22, 695–704. [Google Scholar] [CrossRef]
- Petroni, M.L.; Caletti, M.T.; Dalle, G.R.; Bazzocchi, A.; Aparisi, M.P.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital. Health Stat. 2013, 161, 1–24. [Google Scholar]
- Dong, H.; Yan, Y.; Liu, J.; Cheng, H.; Zhao, X.; Shan, X.; Huang, G.; Mi, J. Reference centiles for evaluating total body fat development and fat distribution by dual-energy x-ray absorptiometry among children and adolescents aged 3–18 years. Clin. Nutr. 2021, 40, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.M.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.K.; Leslie, W.D. Best Practices for Dual-Energy X-ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 20, 1240–1243. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, H.; Yan, Y.; Liu, J.; Shan, X.; Wang, X.; Mi, J. The associations of muscle mass with glucose and lipid metabolism are influenced by body fat accumulation in children and adolescents. Front. Endocrinol. 2022, 13, 976998. [Google Scholar] [CrossRef]
- Kim, K.; Hong, S.; Kim, E.Y. Reference Values of Skeletal Muscle Mass for Korean Children and Adolescents Using Data From the Korean National Health and Nutrition Examination Survey 2009–2011. PLoS ONE 2016, 11, e0153383. [Google Scholar] [CrossRef]
- Georgia, D.; Mark, S.; Peter, W. Theory and performance of substitution models for estimating relative causal effects in nutritional epidemiology. Am. J. Clin. Nutr. 2022, 116, 1379–1388. [Google Scholar]
- Liu, J.; Yan, Y.; Xi, B.; Huang, G.; Mi, J. Skeletal muscle reference for Chinese children and adolescents. J. Cachexia Sarcopenia Muscle 2019, 10, 155–164. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Sun, J.; Liu, G.; Yan, W.; Xi, B.; Xiong, F.; Ding, W.; Huang, G.; Heymsfield, S.; et al. Bone mineral density reference standards for Chinese children aged 3–18: Cross-sectional results of the 2013–2015 China Child and Adolescent Cardiovascular Health (CCACH) Study. BMJ Open. 2017, 7, e014542. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kong, M.H.; Kim, H.J. Low Muscle Mass and Depressed Mood in Korean Adolescents: A Cross-Sectional Analysis of the Fourth and Fifth Korea National Health and Nutrition Examination Surveys. J. Korean Med. Sci. 2018, 33, e320. [Google Scholar] [CrossRef] [PubMed]
- Gätjens, I.; Schmidt, S.; Plachta-Danielzik, S.; Bosy-Westphal, A.; Müller, M.J. Body Composition Characteristics of a Load-Capacity Model: Age-Dependent and Sex-Specific Percentiles in 5- to 17-Year-Old Children. Obes. Facts. 2021, 14, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Asmaa, A.; Helen, J.M.; Wayne, D.C.; Murray, S.; Carolyn, D.S. Effect of reducing total fat intake on body weight: Systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ 2012, 345, e7666. [Google Scholar]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated fat intake increases body weight and the risk of overweight and obesity among Chinese adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef]
- Yakupova, E.; Bocharnikov, A.D.; Plotnikov, E. Effects of Ketogenic Diet on Muscle Metabolism in Health and Disease. Nutrients 2022, 14, 3842. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. Faseb. J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef]
- Kim, J.E.; O’Connor, L.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef]
- Jen, W.; Karagounis, L.G.; Jaddoe, V.; Franco, O.; Voortman, T. Dietary protein intake in school-age children and detailed measures of body composition: The Generation R Study. Int. J. Obes. 2018, 42, 1715–1723. [Google Scholar] [CrossRef]
- Kathrine, J.V.; Stefan, V.; Amany, K.E.; Eha, N.; Christian, A.D.; Clara, G.G.; Grethe, S.T.; Stein, E.V.; Helga, R. Dietary intake of protein is positively associated with percent body fat in middle-aged and older adults. J. Nutr. 2011, 141, 440–446. [Google Scholar]
- Jen, V.; Braun, K.; Karagounis, L.; Nguyen, A.N.; Jaddoe, V.; Schoufour, J.; Franco, O.; Voortman, T. Longitudinal association of dietary protein intake in infancy and adiposity throughout childhood. Clin. Nutr. 2019, 38, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Duckham, R.L.; Desai, M.; Choy, C.; Sherar, L.; Naseri, T.; Soti-Ulberg, C.; Reupena, M.; Wetzel, A.; Hawley, N. Sex differences in the associations of physical activity and macronutrient intake with child body composition: A crosssectional study of 3- to 7-year-olds in Samoa. Pediatr. Obes. 2020, 15, e12603. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.; Chiriboga, D.; Hebert, J.; Li, Y.; Li, W.; Campbell, M.; Gendreau, K.; Ockene, I. Association between dietary carbohydrates and body weight. Am. J. Epidemiol. 2005, 161, 359367. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.; Bruijne, L.M.; Stolk, R.P.; Peeters, P.H.; Bots, M.L.; Grobbee, D.E.; Schouw, Y.T. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women. J. Am. Coll. Cardiol. 2007, 50, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.; Santos, S.; Braun, K.; Voortman, T. Carbohydrate Intake in Early Childhood and Body Composition and Metabolic Health: Results from the Generation R Study. Nutrients 2020, 12, 1940. [Google Scholar] [CrossRef]
- Wachsmuth, N.B.; Aberer, F.; Haupt, S.; Schierbauer, J.R.; Zimmer, R.T.; Eckstein, M.L.; Zunner, B.; Schmidt, W.; Niedrist, T.; Sourij, H.; et al. The Impact of a High-Carbohydrate/Low Fat vs. Low-Carbohydrate Diet on Performance and Body Composition in Physically Active Adults: A Cross-Over Controlled Trial. Nutrients 2022, 14, 423. [Google Scholar] [CrossRef]
- Nakao, R.; Abe, T.; Yamamoto, S.; Oishi, K. Ketogenic diet induces skeletal muscle atrophy via reducing muscle protein synthesis and possibly activating proteolysis in mice. Sci. Rep. 2019, 9, 19652. [Google Scholar] [CrossRef]
- Vincent, H.K.; Raiser, S.N.; Vincent, K.R. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res. Rev. 2012, 11, 361–373. [Google Scholar] [CrossRef]
- Rinnankoski-Tuikka, R.; Silvennoinen, M.; Torvinen, S.; Hulmi, J.J.; Lehti, M.; Kivelä, R.; Reunanen, H.; Kainulainen, H. Effects of high-fat diet and physical activity on pyruvate dehydrogenase kinase-4 in mouse skeletal muscle. Nutr. Metab. 2012, 9, 53. [Google Scholar] [CrossRef]
- Wende, A.R.; Huss, J.M.; Schaeffer, P.J.; Giguère, V.; Kelly, D.P. PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: A mechanism for transcriptional control of muscle glucose metabolism. Mol. Cell Biol. 2005, 25, 10684–10694. [Google Scholar] [CrossRef]
- Shi, Z.; McEvoy, M.; Luu, J.; Attia, J. Dietary fat and sleep duration in Chinese men and women. Int. J. Obes. 2008, 32, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wu, Y.; Zhu, J.; Wang, W.; Wang, Y. Associations of sleep duration with childhood obesity: Findings from a national cohort study in China. Glob. Health J. 2022, 6, 149–155. [Google Scholar] [CrossRef]
| Total | Normal (N = 3816) | Obesity Alone (N = 156) | Sarcopenia Alone (N = 596) | Sarcopenic Obesity (N = 844) | p | |
|---|---|---|---|---|---|---|
| Age (years) | 12.3 ± 2.9 | 12.4 ± 2.9 | 13.0 ± 2.7 | 11.8 ± 2.6 | 12.2 ± 2.9 | <0.001 |
| Gender | <0.001 | |||||
| Boys | 2780 (51.4) | 1928 (50.5) | 67 (42.9) | 351 (58.9) | 434 (51.4) | |
| Girls | 2632 (48.6) | 1888 (49.5) | 89 (57.1) | 245 (41.1) | 410 (48.6) | |
| Race/ethnicity | <0.001 | |||||
| Non-Hispanic | ||||||
| White | 1486 (27.5) | 1090 (28.6) | 28 (17.9) | 160 (26.8) | 208 (24.6) | |
| Black | 1320 (24.4) | 974 (25.5) | 55 (35.3) | 69 (11.6) | 222 (26.3) | |
| Mexican American | 1152 (21.3) | 700 (18.3) | 40 (25.6) | 186 (31.2) | 226 (26.8) | |
| Other Hispanic | 569 (10.5) | 378 (9.9) | 17 (10.9) | 84 (14.1) | 90 (10.7) | |
| Other Race | 885 (16.3) | 674 (17.7) | 16 (10.3) | 97 (16.3) | 98 (11.6) | |
| BMI (kg/m2) | 21.9 ± 5.8 | 19.5 ± 3.3 | 28.9 ± 3.2 | 22.8 ± 3.0 | 31.2 ± 5.7 | <0.001 |
| MET minutes per week | 827.1 ± 748.2 | 860.8 ± 761.9 | 851.3 ± 740.3 | 637.6 ± 591.7 | 755.8 ± 742.4 | <0.001 |
| Body composition | ||||||
| Muscle mass (kg) | 35.2 ± 12.0 | 33.4 ± 11.3 | 49.9 ± 14.0 | 31.4 ± 8.4 | 43.5 ± 13.8 | <0.001 |
| Fat mass (kg) | 16.8 ± 9.8 | 12.5 ± 5.1 | 25.1 ± 5.6 | 20.2 ± 6.2 | 32.3 ± 10.9 | <0.001 |
| Total energy intake (kcal/d) | 1963.9 ± 779.9 | 1995.7 ± 790.9 | 2007.2 ± 895.5 | 1883.4 ± 675.4 | 1869.2 ± 765.5 | <0.001 |
| Dietary fiber intake (g/d) | 14.4 ± 7.9 | 14.5 ± 8.1 | 14.0 ± 7.8 | 14.2 ± 7.7 | 13.9 ± 7.7 | 0.223 |
| Macronutrients intake | ||||||
| Protein intake (%E) | 14.3 ± 4.7 | 14.2 ± 4.6 | 14.5 ± 5 | 14.6 ± 5 | 14.8 ± 4.8 | 0.052 |
| Carbohydrate intake (%E) | 52.1 ± 9.2 | 52.3 ± 9.1 | 51.3 ± 9 | 51.5 ± 9.4 | 51.0 ± 9.6 | 0.085 |
| Fat intake (%E) | 33.6 ± 7.8 | 33.5 ± 7.7 | 34.2 ± 8 | 33.9 ± 7.7 | 34.4 ± 7.9 | 0.008 |
| Muscle Mass | Fat Mass | |||
|---|---|---|---|---|
| Crude | Model 1 | Crude | Model 1 | |
| Muscle mass index Z-score, β (95% CI) | Fat mass index Z-score, β (95% CI) | |||
| Protein intake (5% increase) | 0.32 (0.24, 0.40) *** | 0.01 (−0.03, 0.06) | 0.23 (0.12, 0.33) *** | −0.01 (−0.06, 0.04) |
| Carbohydrate intake (5% increase) | −0.09 (−0.13, −0.05) *** | 0.02 (−0.01, 0.04) | −0.06 (−0.11, −0.01) * | −0.02 (−0.04, −0.01) * |
| Fat intake (5% increase) | 0.01 (−0.04, 0.05) | −0.03 (−0.06, −0.01) * | 0.01 (−0.05, 0.07) | 0.03 (0.01, 0.06) * |
| Low muscle mass, OR (95% CI) | High fat mass, OR (95% CI) | |||
| Protein intake (5% increase) | 0.96 (0.88, 1.05) | 1.02 (0.85, 1.23) | 1.18 (1.10, 1.27) * | 1.19 (0.91, 1.54) |
| Carbohydrate intake (5% increase) | 1.01 (0.96, 1.05) | 1.00 (0.91, 1.09) | 0.96 (0.92, 0.99) ** | 0.88 (0.76, 0.99) * |
| Fat intake (5% increase) | 1.00 (0.95, 1.05) | 1.01 (0.90, 1.11) | 1.17 (1.01, 1.28) *** | 1.13 (1.02, 1.35) * |
| Sarcopenia and Obesity Status | Protein Intake (5 %E) | Carbohydrate Intake (5 %E) | Fat Intake (5 %E) | |||
|---|---|---|---|---|---|---|
| Crude | Model 1 | Crude | Model 1 | Crude | Model 1 | |
| Normal | Ref | Ref | Ref | Ref | Ref | Ref |
| Obesity alone | 1.06 (0.95, 1.17) | 1.04 (0.98, 1.09) | 0.95 (0.87, 1.03) | 0.95 (0.91, 0.99) * | 1.07 (0.90, 1.26) | 1.10 (1.01, 1.20) * |
| Sarcopenia alone | 0.57 (0.26, 1.27) | 0.99 (0.82, 1.18) | 0.76 (0.44, 1.32) | 0.90 (0.82, 1.10) | 3.69 (1.18, 5.48) * | 3.54 (1.14, 5.08) * |
| Sarcopenic obesity | 0.61 (0.01, 1.33) | 0.85 (0.64, 1.13) | 0.52 (0.03, 1.83) | 0.93 (0.80, 1.08) | 4.38 (1.11, 6.44) * | 4.42 (1.47, 6.59) * |
| Isocaloric Substitution (5 %E) | Muscle Mass | Fat Mass | ||
|---|---|---|---|---|
| Muscle Mass Index Z-Score, β (95% CI) | Low Muscle Mass, OR (95% CI) | Fat Mass Index Z-Score, β (95% CI) | High Fat Mass, OR (95% CI) | |
| Carbohydrate substituting protein | −0.02 (−0.06, 0.03) | 0.99 (0.82, 1.20) | 0.01 (−0.04, 0.06) | 0.81 (0.62, 1.06) |
| Fat substituting protein | −0.05 (−0.10, 0.01) | 0.99 (0.80, 1.24) | 0.04 (−0.02, 0.10) | 0.91 (0.66, 1.26) |
| Fat substituting carbohydrate | −0.03 (−0.06, −0.01) * | 0.99 (0.90, 1.12) | 0.03 (0.01, 0.06) * | 1.12 (1.04, 1.34) * |
| Sarcopenia and Obesity Status | Carbohydrate Substituting Protein | Fat Substituting Protein | Fat Substituting Carbohydrate |
|---|---|---|---|
| Normal | Ref | Ref | Ref |
| obesity alone | 2.26 (0.81, 6.25) | 2.25 (1.53, 4.40) * | 3.66 (1.19, 5.24) * |
| Sarcopenia alone | 1.03 (0.86, 1.24) | 1.21 (1.07, 1.52) * | 1.17 (1.04, 1.32) * |
| Sarcopenic obesity | 1.08 (0.65, 4.98) | 2.36 (1.18, 3.18) * | 3.54 (1.15, 5.87) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Tang, C.; Shi, Z.; Gao, L. Association of Macronutrients Intake with Body Composition and Sarcopenic Obesity in Children and Adolescents: A Population-Based Analysis of the National Health and Nutrition Examination Survey (NHANES) 2011–2018. Nutrients 2023, 15, 2307. https://doi.org/10.3390/nu15102307
Yang B, Tang C, Shi Z, Gao L. Association of Macronutrients Intake with Body Composition and Sarcopenic Obesity in Children and Adolescents: A Population-Based Analysis of the National Health and Nutrition Examination Survey (NHANES) 2011–2018. Nutrients. 2023; 15(10):2307. https://doi.org/10.3390/nu15102307
Chicago/Turabian StyleYang, Bing, Chengjun Tang, Zumin Shi, and Liwang Gao. 2023. "Association of Macronutrients Intake with Body Composition and Sarcopenic Obesity in Children and Adolescents: A Population-Based Analysis of the National Health and Nutrition Examination Survey (NHANES) 2011–2018" Nutrients 15, no. 10: 2307. https://doi.org/10.3390/nu15102307
APA StyleYang, B., Tang, C., Shi, Z., & Gao, L. (2023). Association of Macronutrients Intake with Body Composition and Sarcopenic Obesity in Children and Adolescents: A Population-Based Analysis of the National Health and Nutrition Examination Survey (NHANES) 2011–2018. Nutrients, 15(10), 2307. https://doi.org/10.3390/nu15102307







