Attenuation of Oxidative Stress and Regulation of AKT Signaling by Vanillic Acid during Bovine Pre-Implantation Embryo Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. In Vitro Maturation (IVM) of Oocytes
2.3. In Vitro Fertilization (IVF)
2.4. In Vitro Culture (IVC)
2.5. Trophectoderm (TE) and Inner Cell Mass (ICM) Counting
2.6. Quantification of Intracellular ROS Levels and Cytoplasmic Lipid Content
2.7. Estimation of Mitochondrial Membrane Potential (ΔΨm)
2.8. RT-qPCR
2.9. Immunofluorescence
2.10. Statistical Analysis
3. Results
3.1. Effect of VA Administration on Oocyte and Pre-Implantation Embryo Development
3.2. Beneficial Effects of VA on the Quality of Blastocysts
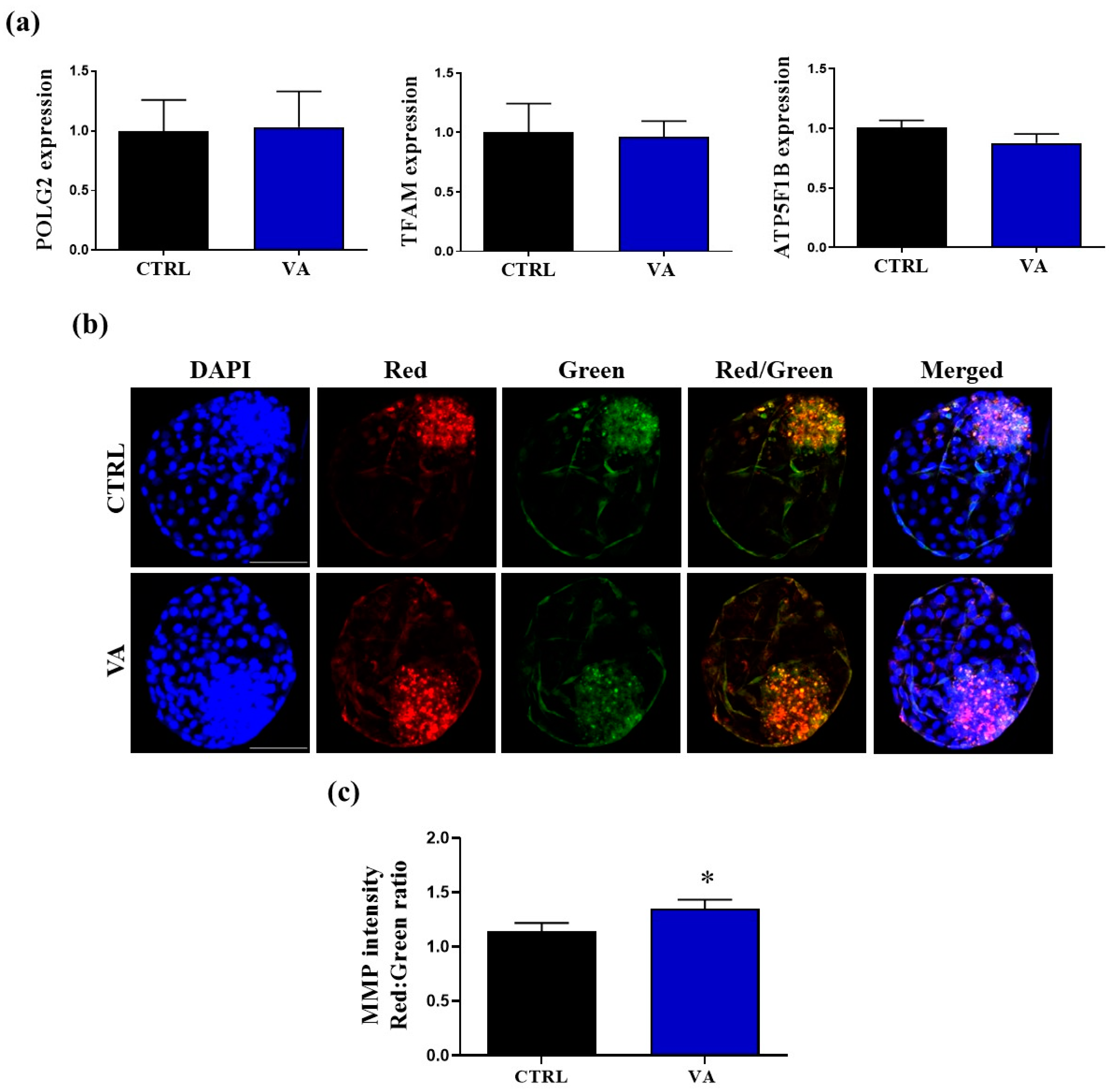
3.3. Effect of VA on Embryonic Mitochondrial Function
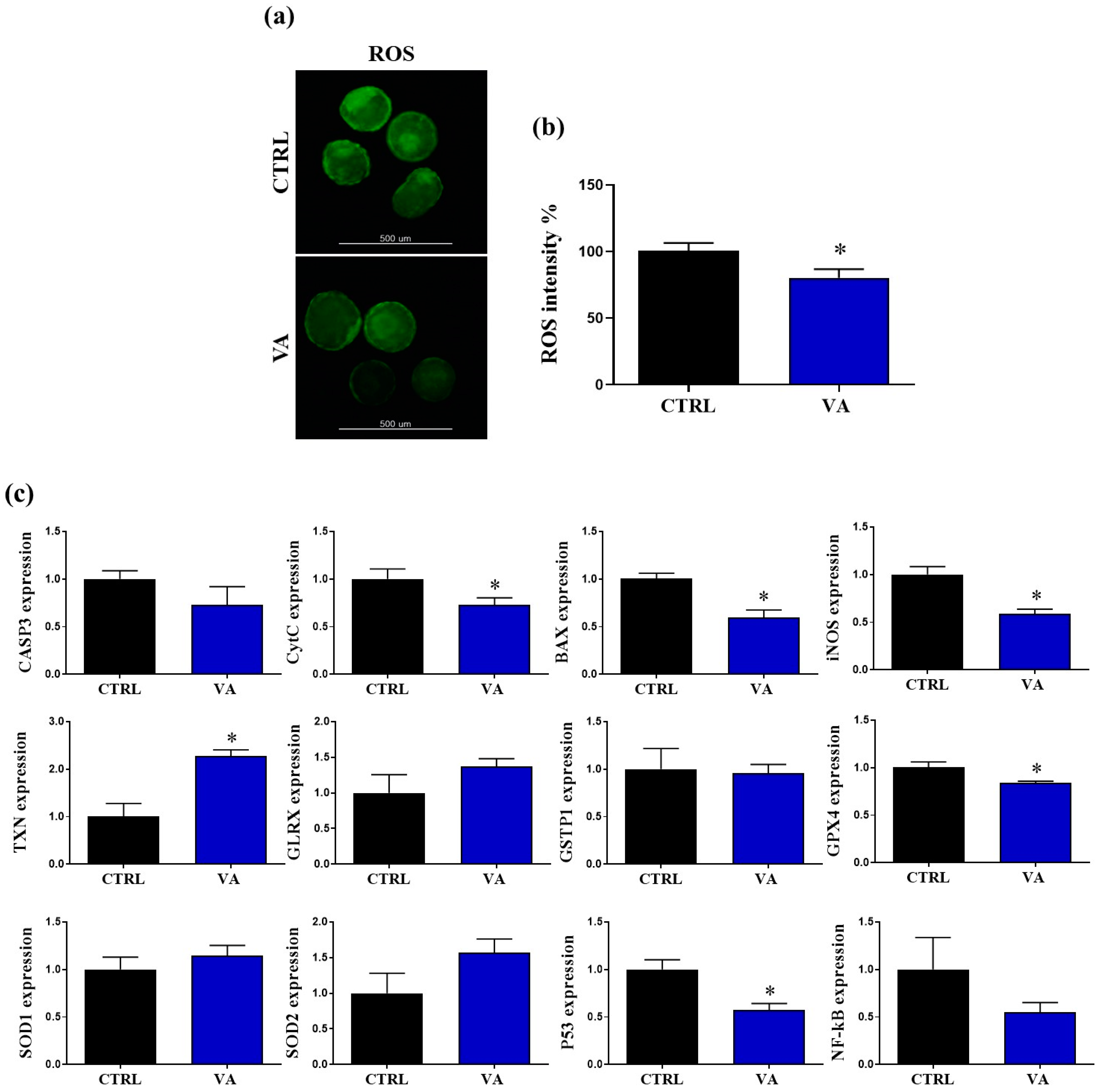
3.4. Regulation of Redox Homeostasis and Apoptosis in VA-Treated Embryos
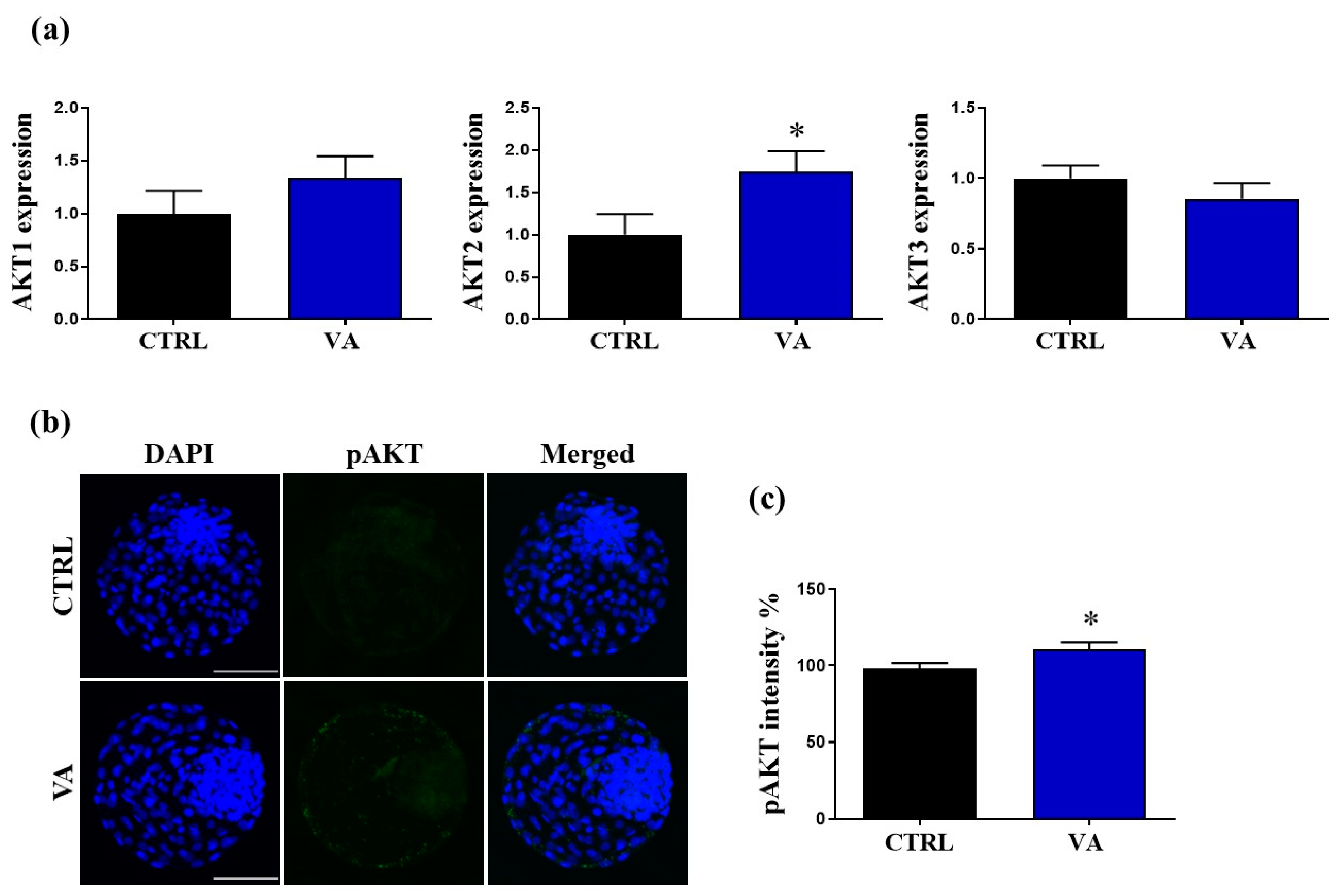
3.5. Regulation of AKT Signaling in VA-Treated Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hardy, M.L.M.; Day, M.L.; Morris, M.B. Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed In Vivo and In Vitro. Int. J. Environ. Res. Public Health 2021, 18, 11374. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L. Oxidative Stress in Oocytes and Embryo Development: Implications for In Vitro Systems. Antioxid. Redox. Signal 2020, 34, 1394–1406. [Google Scholar] [CrossRef]
- Surai, P.F.; Earle-Payne, K. Antioxidant Defences and Redox Homeostasis in Animals. Antioxidants 2022, 11, 1012. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef]
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef]
- Tatone, C.; Heizenrieder, T.; Di Emidio, G.; Treffon, P.; Amicarelli, F.; Seidel, T.; Eichenlaub-Ritter, U. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum. Reprod. 2011, 26, 1843–1859. [Google Scholar] [CrossRef]
- Gu, L.; Liu, H.; Gu, X.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic control of oocyte development: Linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Bellacosa, A.; Testa, J.R.; Staal, S.P.; Tsichlis, P.N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 1991, 254, 274–277. [Google Scholar]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: An update. J. Mol. Endocrinol. 2014, 53, R103–R118. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.L.; Kong, I.K. Melatonin Abrogates the Anti-Developmental Effect of the AKT Inhibitor SH6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef] [PubMed]
- El Sheikh, M.; Mesalam, A.A.; Idrees, M.; Sidrat, T.; Mesalam, A.; Lee, K.L.; Kong, I.K. Nicotinamide Supplementation during the In Vitro Maturation of Oocytes Improves the Developmental Competence of Preimplantation Embryos: Potential Link to SIRT1/AKT Signaling. Cells 2020, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFkappaB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Sindhu, G.; Nishanthi, E.; Sharmila, R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: A biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, J.; Kim, H.L.; Sim, J.E.; Youn, D.H.; Kang, J.; Lim, S.; Jeong, M.Y.; Yang, W.M.; Lee, S.G.; et al. Vanillic acid attenuates obesity via activation of the AMPK pathway and thermogenic factors in vivo and in vitro. FASEB J. 2018, 32, 1388–1402. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A.; Singh, H.; Kaur, S.; Arora, S.; Singh, B. Protective effect of vanillic acid against diabetes and diabetic nephropathy by attenuating oxidative stress and upregulation of NF-kappaB, TNF-alpha and COX-2 proteins in rats. Phytother. Res. 2022, 36, 1338–1352. [Google Scholar] [CrossRef]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Abeta1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef]
- Taqvi, S.; Ahmed Bhat, E.; Sajjad, N.; Sabir, J.S.M.; Qureshi, A.; Rather, I.A.; Rehman, S. Protective effect of vanillic acid in hydrogen peroxide-induced oxidative stress in D.Mel-2 cell line. Saudi J. Biol. Sci. 2021, 28, 1795–1800. [Google Scholar] [CrossRef]
- Kang, J.I.; Choi, Y.K.; Koh, Y.S.; Hyun, J.W.; Kang, J.H.; Lee, K.S.; Lee, C.M.; Yoo, E.S.; Kang, H.K. Vanillic Acid Stimulates Anagen Signaling via the PI3K/Akt/ β-Catenin Pathway in Dermal Papilla Cells. Biomolecules. Ther. 2020, 28, 354–360. [Google Scholar] [CrossRef]
- Chuang, H.-W.; Wei, I.H.; Lin, F.-Y.; Li, C.-T.; Chen, K.-T.; Tsai, M.-H.; Huang, C.-C. Roles of Akt and ERK in mTOR-Dependent Antidepressant Effects of Vanillic Acid. ACS Omega 2020, 5, 3709–3716. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.A.; Song, S.H.; Ko, J.; Kong, I.K. Melatonin Alleviates the Toxicity of High Nicotinamide Concentrations in Oocytes: Potential Interaction with Nicotinamide Methylation Signaling. Oxidative Med. Cell. Longev. 2021, 2021, 5573357. [Google Scholar] [CrossRef]
- Fouladi-Nashta, A.A.; Alberio, R.; Kafi, M.; Nicholas, B.; Campbell, K.H.; Webb, R. Differential staining combined with TUNEL labelling to detect apoptosis in preimplantation bovine embryos. Reprod. Biomed Online 2005, 10, 497–502. [Google Scholar] [CrossRef]
- Bonnefont, J.P.; Djouadi, F.; Prip-Buus, C.; Gobin, S.; Munnich, A.; Bastin, J. Carnitine palmitoyltransferases 1 and 2: Biochemical, molecular and medical aspects. Mol. Asp. Med. 2004, 25, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Mesalam, A.A.; El-Sheikh, M.; Joo, M.D.; Khalil, A.A.K.; Mesalam, A.; Ahn, M.J.; Kong, I.K. Induction of Oxidative Stress and Mitochondrial Dysfunction by Juglone Affects the Development of Bovine Oocytes. Int. J. Mol. Sci. 2021, 22, 168. [Google Scholar] [CrossRef]
- Guo, J.; Kim, N.H.; Cui, X.S. Inhibition of Fatty Acid Synthase Reduces Blastocyst Hatching through Regulation of the AKT Pathway in Pigs. PLoS ONE 2017, 12, e0170624. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.; Khalil, A.A.K.; Idrees, M.; Ahn, M.J.; Mesalam, A.A.; Kong, I.K. Downregulation of PI3K/AKT/mTOR Pathway in Juglone-Treated Bovine Oocytes. Antioxidants 2023, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.; Lopes, F.G.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.A.; Kang, S.-M.; Joo, M.-D.; Soliman, S.S.; Khalil, A.A.K.; Ahn, M.-J.; Kong, I.K. Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes. Animals 2023, 13, 1475. [Google Scholar] [CrossRef]
- Ashry, M.; Rajput, S.K.; Folger, J.K.; Knott, J.G.; Hemeida, N.A.; Kandil, O.M.; Ragab, R.S.; Smith, G.W. Functional role of AKT signaling in bovine early embryonic development: Potential link to embryotrophic actions of follistatin. Reprod. Biol. Endocrinol. 2018, 16, 1. [Google Scholar] [CrossRef]
- Kumar, P.; Ammani, K.; Mahammad, A.; Gosala, J. Vanillic acid induces oxidative stress and apoptosis in non-small lung cancer cell line. Int. J. Recent Sci. Res. 2013, 4, 1077–1083. [Google Scholar]
- Ma, W.F.; Duan, X.C.; Han, L.; Zhang, L.L.; Meng, X.M.; Li, Y.L.; Wang, M. Vanillic acid alleviates palmitic acid-induced oxidative stress in human umbilical vein endothelial cells via Adenosine Monophosphate-Activated Protein Kinase signaling pathway. J. Food Biochem. 2019, 43, e12893. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Ding, H.Y.; Hung, W.J.; Liang, C.H. Antioxidative characteristics and inhibition of alpha-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Derm. 2010, 19, 742–750. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Silveira, J.C.; Bruemmer, J.E.; Bouma, G.J.; Carnevale, E.M.; Clay, C.M. Caspase 3 Expression in Equine Granulosa Cells and Oocytes in Young and Old Mares. Biol. Reprod. 2010, 83, 694. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Li, R.; Peng, H.; Hua, S.; Li, Q.; Quan, F.; Guo, Z.; Zhang, Y. Oocytes selected using BCB staining enhance nuclear reprogramming and the in vivo development of SCNT embryos in cattle. PLoS ONE 2012, 7, e36181. [Google Scholar] [CrossRef]
- Dhali, A.; Anchamparuthy, V.M.; Butler, S.P.; Pearson, R.E.; Mullarky, I.K.; Gwazdauskas, F.C. Gene expression and development of mouse zygotes following droplet vitrification. Theriogenology 2007, 68, 1292–1298. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Wu, X.; Cao, S.; Zhou, L.; Wang, Y.; Chen, X.; Lu, J.; Zhao, C.; Chen, M.; et al. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single-blastocyst transfer cycle in a Chinese population. J. Assist. Reprod. Genet. 2014, 31, 1475–1481. [Google Scholar] [CrossRef]
- Wydooghe, E.; Vandaele, L.; Beek, J.; Favoreel, H.; Heindryckx, B.; De Sutter, P.; Van Soom, A. Differential apoptotic staining of mammalian blastocysts based on double immunofluorescent CDX2 and active caspase-3 staining. Anal. Biochem. 2011, 416, 228–230. [Google Scholar] [CrossRef]
- Van Soom, A.; Vandaele, L.; Goossens, K.; de Kruif, A.; Peelman, L. Gamete origin in relation to early embryo development. Theriogenology 2007, 68 (Suppl. S1), S131–S137. [Google Scholar] [CrossRef]
- Brill, A.; Torchinsky, A.; Carp, H.; Toder, V. The role of apoptosis in normal and abnormal embryonic development. J. Assist. Reprod. Genet. 1999, 16, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.C.; Kakalij, R.M.; Kshirsagar, R.P.; Kumar, B.H.; Komakula, S.S.; Diwan, P.V. Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharm. Biol. 2015, 53, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Kim, J.; Seli, E. Mitochondria as a biomarker for IVF outcome. Reproduction 2019, 157, R235–R242. [Google Scholar] [CrossRef]
- Magata, F.; Shimizu, T. Effect of lipopolysaccharide on developmental competence of oocytes. Reprod. Toxicol. 2017, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Iwase, A.; Mawatari, M.; Wang, J.; Yamashita, M.; Kikkawa, F. Mitochondrial membrane potential in 2-cell stage embryos correlates with the success of preimplantation development. Reproduction 2014, 147, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Li, Z.; Zhang, Y.; Huang, Y.; Wu, Q.; Ru, G.; Chen, M.; Wang, B. Response of Mouse Zygotes Treated with Mild Hydrogen Peroxide as a Model to Reveal Novel Mechanisms of Oxidative Stress-Induced Injury in Early Embryos. Oxidative Med. Cell. Longev. 2016, 2016, 1521428. [Google Scholar] [CrossRef]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE 2014, 9, e87327. [Google Scholar] [CrossRef]
- Sudano, M.J.; Paschoal, D.M.; Rascado Tda, S.; Magalhaes, L.C.; Crocomo, L.F.; de Lima-Neto, J.F.; Landim-Alvarenga Fda, C. Lipid content and apoptosis of in vitro-produced bovine embryos as determinants of susceptibility to vitrification. Theriogenology 2011, 75, 1211–1220. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef]
- Tang, M.; Dong, X.; Xiao, L.; Tan, Z.; Luo, X.; Yang, L.; Li, W.; Shi, F.; Li, Y.; Zhao, L.; et al. CPT1A-mediated fatty acid oxidation promotes cell proliferation via nucleoside metabolism in nasopharyngeal carcinoma. Cell Death Dis. 2022, 13, 331. [Google Scholar] [CrossRef] [PubMed]





| Names | Primer Sequences | |
|---|---|---|
| Forward | Reverse | |
| P53 | CTATGAGATGTTCCGAGAGC | CTCTCTCTTGAGCATTGGTT |
| BAX | CACCAAGAAGCTGAGCGAGTGT | TCGGAAAAAGACCTCTCGGGGA |
| iNOS | CGAGCTTCTACCTCAAGCTATC | CTGGCCAGATGTTCCTCTATTT |
| Caspase-3 | CCCAAGTGTGACCACTGAAC | CCATTAGGCCACACTCACTG |
| Cytc | CCAGGTAGCCAAGGATGTGT | CTTTCGGCTCTTGAGGACTG |
| BCL2 | TGGATGACCGAGTACCTGAA | CAGCCAGGAGAAATCAAACA |
| GSTP1 | CTCACGCTGTACCAGTCCAA | GCAGCGAAGGTCCTCTACAC |
| GLRX | CAGAACGGTACCTCGGGTCT | TGCCATCCTATCCCCAAGGG |
| GPX4 | GCACGAATTTTCAGCCAAGG | AAACCACACTCGGCGTATCG |
| NF-kB | TGGCGGAATTACCTTCCATAC | CATCACTCTTGCCACAACTTTC |
| SOD1 | CCATCCACTTCGAGGCAAAG | TCTCCAAACTGATGGACGTGG |
| SOD2 | GGGAGAATGTAACTGCACGA | ACAACAGAGCAGCGTACTGG |
| TXN | TCGGATCCGTGTCCATCGAT | CACGTGGCTGAGAAGTCGAC |
| SIRT1 | CAACGGTTTCCATTCGTGTG | GTTCGAGGATCTGTGCCAAT |
| POLG2 | CTTCTGGGAAACTACGGGAGAAC | GTAGCCTCTTGTTTACCAGATCCA |
| TFAM | CTGGTCAGTGCTTTGTCTGC | CTAAAGGGATAGCGCAGTCG |
| ATP5F1B | TGCTTTATTGGGCAGAATCC | GATCCGTCAAGTCATCAGCA |
| AKT1 | AAAAGGAAGTGGTGTACAGG | GAAGTCGGTGATCTTGATGT |
| AKT2 | CGACTATCTCAAACTCCTGG | ATCTTCATGGCATAGTAGCG |
| AKT3 | AGCTGTTTTTCCATTTGTCG | TGTAGATAGTCCAAGGCAGA |
| GADPH | CCCAGAATATCATCCCTGCT | CTGCTTCACCACCTTCTTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheikh, M.; Mesalam, A.; Joo, M.-D.; Sidrat, T.; Mesalam, A.A.; Kong, I.-K. Attenuation of Oxidative Stress and Regulation of AKT Signaling by Vanillic Acid during Bovine Pre-Implantation Embryo Development. Nutrients 2023, 15, 2257. https://doi.org/10.3390/nu15102257
El-Sheikh M, Mesalam A, Joo M-D, Sidrat T, Mesalam AA, Kong I-K. Attenuation of Oxidative Stress and Regulation of AKT Signaling by Vanillic Acid during Bovine Pre-Implantation Embryo Development. Nutrients. 2023; 15(10):2257. https://doi.org/10.3390/nu15102257
Chicago/Turabian StyleEl-Sheikh, Marwa, Ayman Mesalam, Myeong-Don Joo, Tabinda Sidrat, Ahmed Atef Mesalam, and Il-Keun Kong. 2023. "Attenuation of Oxidative Stress and Regulation of AKT Signaling by Vanillic Acid during Bovine Pre-Implantation Embryo Development" Nutrients 15, no. 10: 2257. https://doi.org/10.3390/nu15102257
APA StyleEl-Sheikh, M., Mesalam, A., Joo, M.-D., Sidrat, T., Mesalam, A. A., & Kong, I.-K. (2023). Attenuation of Oxidative Stress and Regulation of AKT Signaling by Vanillic Acid during Bovine Pre-Implantation Embryo Development. Nutrients, 15(10), 2257. https://doi.org/10.3390/nu15102257








