Impact of Food-Based Weight Loss Interventions on Gut Microbiome in Individuals with Obesity: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias
3. Results
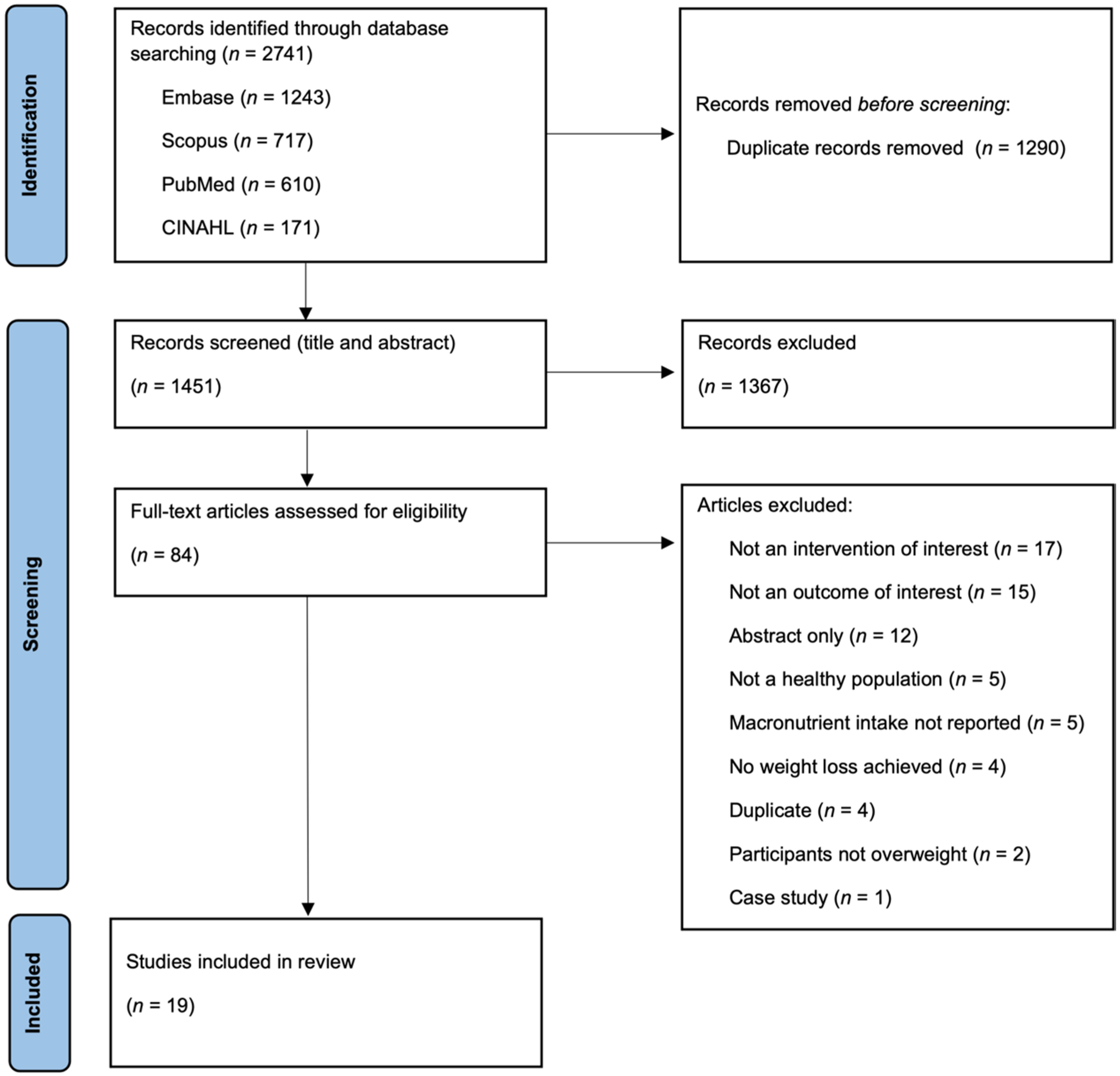
3.1. Study Selection
3.2. General Study Characteristics
3.3. Dietary Intervention Characteristics
3.4. Weight Loss
3.5. Changes in α-Diversity
3.6. Changes in β-Diversity
3.7. Changes in Relative Bacterial Abundance
3.8. Changes in Faecal SCFAs
3.9. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2022).
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Alter, D.A. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Thaker, V.V. Genetic and epigenetic causes of obesity. Adolesc. Med. State Art Rev. 2017, 28, 379. [Google Scholar] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.; Busse, J.W.; Thorlund, K.; Guyatt, G. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA 2014, 312, 923–933. [Google Scholar] [CrossRef]
- Christiansen, T.; Bruun, J.M.; Madsen, E.L.; Richelsen, B. Weight loss maintenance in severely obese adults after an intensive lifestyle intervention: 2-to 4-year follow-up. Obesity 2007, 15, 413–420. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Bendtsen, L.Q.; Blædel, T.; Holm, J.B.; Lorenzen, J.K.; Mark, A.B.; Kiilerich, P.; Kristiansen, K.; Astrup, A.; Larsen, L.H. High intake of dairy during energy restriction does not affect energy balance or the intestinal microflora compared with low dairy intake in overweight individuals in a randomized controlled trial. Appl. Physiol. Nutr. Metab. 2018, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Hess, A.L.; Krautbauer, S.; Liebisch, G.; Christensen, L.; Hjorth, M.F.; Larsen, T.M.; Sanz, Y. Sex, Food, and the Gut Microbiota: Disparate Response to Caloric Restriction Diet with Fiber Supplementation in Women and Men. Mol. Nutr. Food Res. 2021, 65, e2000996. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Romo-Hualde, A.; Aranaz, P.; Goni, L.; Cuervo, M.; Martínez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. Diet- and sex-related changes of gut microbiota composition and functional profiles after 4 months of weight loss intervention. Eur. J. Nutr. 2021, 60, 3279–3301. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; McCormack, L.; Dey, M. Association of the Gut Microbiota with Weight-Loss Response within a Retail Weight-Management Program. Microorganisms 2020, 8, 1246. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. A High Protein Calorie Restriction Diet Alters the Gut Microbiome in Obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Fragiadakis, G.K.; Wastyk, H.C.; Robinson, J.L.; Sonnenburg, E.D.; Sonnenburg, J.L.; Gardner, C.D. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 2020, 111, 1127–1136. [Google Scholar] [CrossRef]
- Gratz, S.W.; Hazim, S.; Richardson, A.J.; Scobbie, L.; Johnstone, A.M.; Fyfe, C.; Holtrop, G.; Lobley, G.E.; Russell, W.R. Dietary carbohydrate rather than protein intake drives colonic microbial fermentation during weight loss. Eur. J. Nutr. 2019, 58, 1147–1158. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Molina-Vega, M.; Bernal-López, M.R.; Garrido-Sánchez, L.; García-Almeida, J.M.; Sajoux, I.; Moreno-Indias, I.; Tinahones, F.J. Different Weight Loss Intervention Approaches Reveal a Lack of a Common Pattern of Gut Microbiota Changes. J. Pers. Med. 2021, 11, 109. [Google Scholar] [CrossRef]
- Jaagura, M.; Viiard, E.; Karu-Lavits, K.; Adamberg, K. Low-carbohydrate high-fat weight reduction diet induces changes in human gut microbiota. Microbiologyopen 2021, 10, e1194. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Kelly, J.; Ryan, S.; Romero-Gonzalez, R.; McKinnon, H.; Fyfe, C.; Naslund, E.; Lopez-Nicolas, R.; Bosscher, D.; Bonnema, A.; et al. Nondigestible Carbohydrates Affect Metabolic Health and Gut Microbiota in Overweight Adults after Weight Loss. J. Nutr. 2020, 150, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Nowak, A.; Agnello, M.; Chutkan, R.; Holubkov, R.; Barnard, N.D. Weight Loss is Associated with Changes in Gut Microbiome: A Randomized, Cross-Over Trial Comparing a Mediterranean and a Low-Fat Vegan Diet in Overweight Adults. J. Obes. Weight Loss Ther. 2021, 11, 443. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Y.; Sun, L.; Liu, X.; Zeng, R.; Lin, X.; Li, Y. Effects of gut microbiota and fatty acid metabolism on dyslipidemia following weight-loss diets in women: Results from a randomized controlled trial. Clin. Nutr. 2021, 40, 5511–5520. [Google Scholar] [CrossRef]
- Nogacka, A.M.; de Los Reyes-Gavilán, C.G.; Martínez-Faedo, C.; Ruas-Madiedo, P.; Suarez, A.; Mancabelli, L.; Ventura, M.; Cifuentes, A.; León, C.; Gueimonde, M.; et al. Impact of Extreme Obesity and Diet-Induced Weight Loss on the Fecal Metabolome and Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, e2000030. [Google Scholar] [CrossRef]
- Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Frank, D.N.; Borengasser, S.J.; Ostendorf, D.M.; Ir, D.; Jambal, P.; Bing, K.; Wayland, L.; Siebert, J.C.; Bessesen, D.H.; et al. The Gut Microbiota during a Behavioral Weight Loss Intervention. Nutrients 2021, 13, 3248. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, P.; Tian, Y.; Liu, B.; Huang, L.; Liu, Z.; Lin, N.; Xu, N.; Ruan, Y.; Zhang, Z.; et al. Gut Microbiota Serves a Predictable Outcome of Short-Term Low-Carbohydrate Diet (LCD) Intervention for Patients with Obesity. Microbiol. Spectr. 2021, 9, e0022321. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut Microbes 2022, 14, 2020068. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American Gut: An Open Platform for Citizen Science Microbiome Research. mSystems 2018, 3, e00031-18. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e785. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Koren, O.; Knights, D.; Gonzalez, A.; Waldron, L.; Segata, N.; Knight, R.; Huttenhower, C.; Ley, R.E. A Guide to Enterotypes across the Human Body: Meta-Analysis of Microbial Community Structures in Human Microbiome Datasets. PLoS Comput. Biol. 2013, 9, e1002863. [Google Scholar] [CrossRef]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J. Transl. Med. 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Nehra, V.; Mailing, L.; Holscher, H.D.; Van Dyke, C.T.; Edens, K.; Swanson, K.S.; Boardman, L.A.; Jensen, M.D.; Murray, J.A.; et al. Gut microbiota: Predictor of success in a comprehensive of lifestyle modification program for obesity. Gastroenterology 2017, 152, S626–S627. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Watanabe, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Vuholm, S.; Roager, H.M.; Nielsen, D.S.; Krych, L.; Kristensen, M.; Astrup, A.; Hjorth, M.F. Prevotella Abundance Predicts Weight Loss Success in Healthy, Overweight Adults Consuming a Whole-Grain Diet Ad Libitum: A Post Hoc Analysis of a 6-Wk Randomized Controlled Trial. J. Nutr. 2019, 149, 2174–2181. [Google Scholar] [CrossRef]
- Cuevas Sierra, A.; Riezu Boj, J.; Guruceaga, E.; Barceló, A.; Cuervo, M.; Milagro, F.; Martínez, A. Metagenomic biomarker in precision treatment for obesity: Microbiota composition change between before and after a nutrition intervention. Obes. Rev. 2020, 21, e13115. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Mitra, S.; Schollenberger, A.E.; Kramer, K.M.; Meile, T.; Königsrainer, A.; Huson, D.H.; Bischoff, S.C. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed. Res. Int. 2015, 2015, 806248. [Google Scholar] [CrossRef]
- Dao, M.C.; Sokolovska, N.; Brazeilles, R.; Affeldt, S.; Pelloux, V.; Prifti, E.; Chilloux, J.; Verger, E.O.; Kayser, B.D.; Aron-Wisnewsky, J.; et al. A Data Integration Multi-Omics Approach to Study Calorie Restriction-Induced Changes in Insulin Sensitivity. Front. Physiol. 2018, 9, 1958. [Google Scholar] [CrossRef]
- Di Rosa, C.; Lattanzi, G.; Taylor, S.F.; Manfrini, S.; Khazrai, Y.M. Very low calorie ketogenic diets in overweight and obesity treatment: Effects on anthropometric parameters, body composition, satiety, lipid profile and microbiota. Obes. Res. Clin. Pract. 2020, 14, 491–503. [Google Scholar] [CrossRef]
- Diener, C.; Qin, S.; Zhou, Y.; Patwardhan, S.; Tang, L.; Lovejoy, J.C.; Magis, A.T.; Price, N.D.; Hood, L.; Gibbons, S.M. Baseline Gut Metagenomic Functional Gene Signature Associated with Variable Weight Loss Responses following a Healthy Lifestyle Intervention in Humans. mSystems 2021, 6, e00964-21. [Google Scholar] [CrossRef]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. The Intestinal Microbiome Predicts Weight Loss on a Calorie-Restricted Diet and Is Associated With Improved Hepatic Steatosis. Front. Nutr. 2021, 8, 718661. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F.; Umar, S.; Ahmed, I.; Befort, C.A.; Nydegger, J.L.; Kreutzjans, A.L.; Powers, K.R.; Klemp, J.R.; Spaeth, K.R.; et al. Changes in the gut microbiome of post-menopausal women 2 weeks after initiating a structured weight loss intervention. Cancer Res. 2017, 77, P4-13-03. [Google Scholar] [CrossRef]
- Fangmann, D.; Heinsen, F.A.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Müller, N.; Lieb, W.; Baines, J.F.; Schreiber, S.; et al. Dietary and weight loss effects on human gut microbiome diversity and metabolism. Diabetol. Stoffwechs. 2016, 11, P166. [Google Scholar] [CrossRef]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutr. Health 2020, 26, 79–85. [Google Scholar] [CrossRef]
- Gardner, C.D.; Hauser, M.; Gobbo, L.D.; Trepanowski, J.; Rigdon, J.; Ioannidis, J.; King, A.; Desai, M. Neither insulin secretion nor genotype pattern modify 12-month weight loss effects of healthy low-fat vs. healthy low-carbohydrate diets among adults with obesity. Circulation 2017, 135. [Google Scholar] [CrossRef]
- Gratz, S.W.; Scobbie, L.; Richardson, A.J.; Zhang, X.; Fyfe, C.; Farquharson, F.M.; Duncan, G.; Filipe, J.; Zhu, W.Y.; Johnstone, A.M.; et al. Comparison of meat versus soya based high-protein diets on faecal microbiota and microbial metabolites. Proc. Nutr. Soc. 2020, 79, E781. [Google Scholar] [CrossRef]
- Grembi, J.A.; Nguyen, L.H.; Haggerty, T.D.; Gardner, C.D.; Holmes, S.P.; Parsonnet, J. Gut microbiota plasticity is correlated with sustained weight loss on a low-carb or low-fat dietary intervention. Sci. Rep. 2020, 10, 1405. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Sun, D.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Changes in gut microbiota metabolites and successful weight-loss in response to weight-loss diets: The POUNDS lost trial. Circulation 2017, 136, A14459. [Google Scholar] [CrossRef]
- Heianza, Y.; Sun, D.; Ma, W.; Zheng, Y.; Champagne, C.M.; Bray, G.A.; Sacks, F.M.; Qi, L. Gut-microbiome-related LCT genotype and 2-year changes in body composition and fat distribution: The POUNDS Lost Trial. Int. J. Obes. 2018, 42, 1565–1573. [Google Scholar] [CrossRef]
- Heinsen, F.A.; Fangmann, D.; Müller, N.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Lieb, W.; Baines, J.F.; Schreiber, S.; et al. Beneficial Effects of a Dietary Weight Loss Intervention on Human Gut Microbiome Diversity and Metabolism Are Not Sustained during Weight Maintenance. Obes. Facts 2016, 9, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Woo, S.L.; Lee, R.P.; Huang, J.; Rasmusen, A.; Carpenter, C.L.; Thames, G.; Gilbuena, I.; Tseng, C.H.; et al. Hass Avocado Inclusion in a Weight-Loss Diet Supported Weight Loss and Altered Gut Microbiota: A 12-Week Randomized, Parallel-Controlled Trial. Curr. Dev. Nutr. 2019, 3, nzz068. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Blædel, T.; Bendtsen, L.Q.; Lorenzen, J.K.; Holm, J.B.; Kiilerich, P.; Roager, H.M.; Kristiansen, K.; Larsen, L.H.; Astrup, A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: Results from a post-hoc analysis. Int. J. Obes. 2019, 43, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Christensen, L.; Larsen, T.M.; Roager, H.M.; Krych, L.; Kot, W.; Nielsen, D.S.; Ritz, C.; Astrup, A. Pretreatment Prevotella-to-Bacteroides ratio and salivary amylase gene copy number as prognostic markers for dietary weight loss. Am. J. Clin. Nutr. 2020, 111, 1079–1086. [Google Scholar] [CrossRef]
- Holt, P.R.; Aleman, J.O.; Bokulich, N.A.; Swann, J.R.; Dannenberg, A.J.; Blaser, M.J.; Hudis, C.A.; Breslow, J. Vlcd-induced rapid weight loss is accompanied by enhanced lipolysis, altered adipose tissues with concomitant changes in the fecal microbiome and plasma metabolome. Gastroenterology 2016, 150, S9. [Google Scholar] [CrossRef]
- Hric, I.; Ugrayová, S.; Penesová, A.; Rádiková, Ž.; Kubáňová, L.; Šardzíková, S.; Baranovičová, E.; Klučár, Ľ.; Beke, G.; Grendar, M.; et al. The Efficacy of Short-Term Weight Loss Programs and Consumption of Natural Probiotic Bryndza Cheese on Gut Microbiota Composition in Women. Nutrients 2021, 13, 1753. [Google Scholar] [CrossRef]
- Jie, Z.; Yu, X.; Liu, Y.; Sun, L.; Chen, P.; Ding, Q.; Gao, Y.; Zhang, X.; Yu, M.; Liu, Y.; et al. The Baseline Gut Microbiota Directs Dieting-Induced Weight Loss Trajectories. Gastroenterology 2021, 160, 2029–2042.e2016. [Google Scholar] [CrossRef]
- Karl, J.P.; Xueyan, F.; Xiaoxin, W.; Yufeng, Z.; Jian, S.; Chenhong, Z.; Wolfe, B.E.; Saltzman, E.; Liping, Z.; Booth, S.L. Fecal menaquinone profiles of overweight adults are associated with gut microbiota composition during a gut microbiota-targeted dietary intervention. Am. J. Clin. Nutr. 2015, 102, 84–93. [Google Scholar] [CrossRef]
- Kayser, B.D.; Prifti, E.; Lhomme, M.; Belda, E.; Dao, M.C.; Aron-Wisnewsky, J.; Kontush, A.; Zucker, J.D.; Rizkalla, S.W.; Dugail, I.; et al. Elevated serum ceramides are linked with obesity-associated gut dysbiosis and impaired glucose metabolism. Metabolomics 2019, 15, 140. [Google Scholar] [CrossRef]
- Kong, L.; Wuillemin, P.; Hajduch, F.; Bastard, J.; Fellahi, S.; Basdevant, A.; Zucker, J.; Doré, J.; Rizkalla, S.; Clément, K. Insulinemia and inflammatory markers might predict different responses of obese subjects under the same hypocaloric diet intervention. Ann. Nutr. Metab. 2011, 58, 75–76. [Google Scholar] [CrossRef]
- Kong, L.C.; Hajduch, F.; Wuillemin, P.H.; Bastard, J.P.; Fellahi, S.; Bonnefont-Rousselot, D.; Bittar, R.; Basdevant, A.; Zucker, J.D.; Doré, J.; et al. Plasma insulin and inflammatory markers prior to weight loss can predict dietary responders. Diabetes 2011, 60, A517. [Google Scholar] [CrossRef][Green Version]
- Lee, C.; Florea, L.; Potter, J.; Sears, C.; Durkin, N.; Scudder, M.; Maruthur, N.M.; Schweitzer, M.; Magnuson, T.; Steele, K.; et al. Changes in gut microbiome after medical vs. surgical weight loss in a randomized trial. Diabetes 2016, 65, A508. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Sun, D.; Zhou, T.; Heianza, Y.; Bray, G.; Sacks, F.; Qi, L. Changes in Gut microbiota metabolite TMAO are related to changes in hepatic and visceral fat in weightloss diet interventions: The POUNDS Lost trial. Circulation 2019, 139. [Google Scholar] [CrossRef]
- Lin, B.Y.; Lin, W.D.; Huang, C.K.; Hsin, M.C.; Lin, W.Y.; Pryor, A.D. Changes of gut microbiota between different weight reduction programs. Surg. Obes. Relat. Dis. 2019, 15, 749–758. [Google Scholar] [CrossRef]
- Kong, L.C.; Wuillemin, P.-H.; Bastard, J.-P.; Sokolovska, N.; Gougis, S.; Fellahi, S.; Darakhshan, F.; Bonnefont-Rousselot, D.; Bittar, R.; Doré, J.; et al. Insulin resistance and inflammation predict kinetic body weight changes in response to dietary weight loss and maintenance in overweight and obese subjects by using a Bayesian network approach1-4. Am. J. Clin. Nutr. 2013, 98, 1385–1394. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef]
- MacHado, A.D.; Förster-Fromme, K.; Mitra, S.; Friedrich, A.; Kramer, K.; Huson, D.H.; Bischoff, S.C. Competence network obesity: An integrated multi-omics approach to study the guest-host metabolic interaction during restrictive obesity intervention. Obes. Facts 2012, 5, 4–5. [Google Scholar] [CrossRef]
- Mahadzir, M.D.A.; Shyam, S.; Barua, A.; Krishnappa, P.; Ramamurthy, S. Effect of probiotic microbial cell preparation (MCP) on fasting blood glucose, body weight, waist circumference, and faecal short chain fatty acids among overweight Malaysian adults: A pilot randomised controlled trial of 4 weeks. Malays. J. Nutr. 2017, 23, 329–341. [Google Scholar]
- Mueller, N.T.; Differding, M.K.; Maruthur, N.; Juraschek, S.; Miller, E.R.; Yeh, H.C. Effects of metformin and behavioral weight loss on the gut microbiome. Circulation 2020, 141. [Google Scholar] [CrossRef]
- Muñiz-Pedrogo, D.A.; Schmidt, B.A.; Jensen, M.D.; Murray, J.A.; Kashyap, P.C.; Nehra, V. Individualized responses to lifestyle interventions can be predicted by gut microbiota. United Eur. Gastroenterol. J. 2016, 4, A337–A338. [Google Scholar] [CrossRef]
- Muñiz-Pedrogo, D.A.; Jensen, M.D.; Van Dyke, C.T.; Murray, J.A.; Woods, J.A.; Chen, J.; Kashyap, P.C.; Nehra, V. Gut Microbial Carbohydrate Metabolism Hinders Weight Loss in Overweight Adults Undergoing Lifestyle Intervention With a Volumetric Diet. Mayo Clin. Proc. 2018, 93, 1104–1110. [Google Scholar] [CrossRef]
- Muralidharan, J.; Moreno-Indias, I.; Bulló, M.; Lopez, J.V.; Corella, D.; Castañer, O.; Vidal, J.; Atzeni, A.; Fernandez-García, J.C.; Torres-Collado, L.; et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-Plus Study. Am. J. Clin. Nutr. 2021, 114, 1148–1158. [Google Scholar] [CrossRef]
- Oh, B.; Kim, J.S.; Kweon, M.; Kim, B.S.; Huh, I.S. Six-week Diet Correction for Body Weight Reduction and Its Subsequent Changes of Gut Microbiota: A Case Report. Clin. Nutr. Res. 2016, 5, 137–140. [Google Scholar] [CrossRef]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Soto-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1700484. [Google Scholar] [CrossRef]
- Remely, M.; Tesar, I.; Hippe, B.; Gnauer, S.; Rust, P.; Haslberger, A.G. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef. Microbes 2015, 6, 431–439. [Google Scholar] [CrossRef]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Kaplan, A.; Zelicha, H.; Rubin, E.; Koren, O.; Shai, I. Autologous fecal microbiota transplantation can retain the metabolic achievements of dietary interventions. Eur. J. Intern. Med. 2021, 92, 17–23. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Alcoholado, L.; Gutiérrez Repiso, C.; Alcaide, J.; García Fuentes, E.; Bernal López, R.; Tinahones, F.; Moreno Indias, I. Gut microbiota is differentially affected by distinct weight loss strategies. Obes. Facts 2019, 12, 193–194. [Google Scholar] [CrossRef]
- Siebert, J.C.; Stanislawski, M.A.; Zaman, A.; Ostendorf, D.M.; Konigsberg, I.R.; Jambal, P.; Ir, D.; Bing, K.; Wayland, L.; Scorsone, J.J.; et al. Multiomic Predictors of Short-Term Weight Loss and Clinical Outcomes During a Behavioral-Based Weight Loss Intervention. Obesity 2021, 29, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.D.; Maukonen, J.; Scott, K.P.; Virtanen, K.A.; Pietiläinen, K.H.; Saarela, M. Impact of a very low-energy diet on the fecal microbiota of obese individuals. Eur. J. Nutr. 2014, 53, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- von Schwartzenberg, R.J.; Bisanz, J.E.; Lyalina, S.; Spanogiannopoulos, P.; Ang, Q.Y.; Cai, J.; Dickmann, S.; Friedrich, M.; Liu, S.Y.; Collins, S.L.; et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature 2021, 595, 272–277. [Google Scholar] [CrossRef]
- Yoriko, H.; Dianjianyi, S.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Lu, Q.; Heianza, Y.; Sun, D.; Qi, L. Changes in Gut Microbiota-Related Metabolites and Long-term Successful Weight Loss in Response to Weight-Loss Diets: The POUNDS Lost Trial. Diabetes Care 2018, 41, 413–419. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, W.; Wang, H.; Wu, W.; Zhou, Q.; Chen, Y.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. A multiphase dietetic protocol incorporating an improved ketogenic diet enhances weight loss and alters the gut microbiome of obese people. Int. J. Food Sci. Nutr. 2021, 73, 238–250. [Google Scholar] [CrossRef]
- Zhou, T.; Heianza, Y.; Chen, Y.; Li, X.; Sun, D.; DiDonato, J.A.; Pei, X.; LeBoff, M.S.; Bray, G.A.; Sacks, F.M.; et al. Circulating Gut Microbiota Metabolite Trimethylamine N-Oxide (TMAO) and Changes in Bone Density in Response to Weight Loss Diets: The POUNDS Lost Trial. Diabetes Care 2019, 42, 1365–1371. [Google Scholar] [CrossRef]
- Zolotarevsky, M.V.; Zolotarevsky, E.; DeBenedet, A.; Duda, J.; Velarde, M.; Lyons, D.; Montagano, J.; Gunaratnam, N.T. A multifacted approach to weight loss is effective in obese NASH and GERD patients in a community GI practice: An interim analysis. Gastroenterology 2018, 154, S-431. [Google Scholar] [CrossRef]
- Zou, H.; Wang, D.; Ren, H.; Cai, K.; Chen, P.; Fang, C.; Shi, Z.; Zhang, P.; Wang, J.; Yang, H.; et al. Effect of Caloric Restriction on BMI, Gut Microbiota, and Blood Amino Acid Levels in Non-Obese Adults. Nutrients 2020, 12, 631. [Google Scholar] [CrossRef]
| Trial, Country | n (Female) | Age, Years | BMI, kg/m2 | Intervention Protocol | Duration | Microbiota Analysis Method |
|---|---|---|---|---|---|---|
| Bendsten 2018, Denmark [12] | 40 (35) | 42 (1) | 31.5 (0.4) | High-dairy diet: 18% P, 52% C, 30% F, 500 kcal/day deficit, 1500 mg calcium/day | 24 weeks | 16S rRNA (V3–V4) |
| 40 (34) | 45 (2) | 30.8 (0.4) | Low-dairy diet: 18% P, 52% C, 30% F, 500 kcal/day deficit, 600 mg calcium/day | 24 weeks | 16S rRNA (V3–V4) | |
| Benítez-Páez 2021, Denmark [13] | 59 (39) | 48.9 (8.6) | 32.8 (3.9) | Calorie-restricted diet + fibre: 18–20% P, 52–53% C, 32–33% F, 500 kcal/day deficit, 14–22 g/day fibre + 20 g/day prebiotic fibre supplement (10 g inulin + 10 g resistant maltodextrin) | 12 weeks | Shotgun metagenomics |
| 57 (37) | 48.4 (8.3) | 34.4 (4.4) | Calorie-restricted diet + placebo: 18–20% P, 52–53% C, 32–33% F, 500 kcal/day deficit, 14–22 g/day fibre + placebo supplement (maltodextrin) | 12 weeks | Shotgun metagenomics | |
| Cuevas-Sierra 2021, Spain [14] | 82 (54) | NR | M: 31.9 (3.2) F: 30.9 (3.2) | Moderately high-protein diet: 30% P, 40% C, 30% F, 30% energy restriction | 16 weeks | 16S rRNA (V3–V4) |
| 97 (70) | NR | M: 32.1 (3.5) F: 31.9 (3.9) | Low-fat diet: 18% P, 60% C, 22% F, 30% energy restriction | 16 weeks | 16S rRNA (V3–V4) | |
| Dhakal 2020, USA [15] | 58 (44) | 45.7 (15.8) | 34.6 (7.2) | Retail weight reduction program | 12 weeks | 16S rRNA (V4) |
| Dong 2020, USA [16] | 43 (10) | 55.9 (10.1) | 34.9 (4.5) | High-protein diet: 30% P, 40% C, 30% F, 2 weeks ad libitum then 6 weeks 500 kcal/day deficit | 8 weeks | 16S rRNA (V4) |
| 37 (8) | 55.7 (11.4) | 34.6 (5.1) | Normal-protein diet: 15% P, 55% C, 30% F, 2 weeks ad libitum then 6 weeks 500 kcal/day deficit | 8 weeks | 16S rRNA (V4) | |
| Duncan 2008, Scotland [17] | 23 (0) | NR | >30 | High-protein, moderate-carbohydrate, non-ketogenic diet: 30% P, 35% C, 35% F, <8.5 MJ/day, 164 g/d CHO, 12.2 g/day non-starch polysaccharide | 4 weeks | 16S rRNA-based quantitative FISH |
| Fragiadakis 2020, USA [18] | 25 (20) | 42.6 (5.8) | 32.8 (3.9) | Low-carbohydrate diet | 12 months | 16S rRNA (V4) |
| 24 (19) | 39.2 (5.5) | 33.7 (3.5) | Low-fat diet | 12 months | 16S rRNA (V4) | |
| Gratz 2019, Scotland [19] | 18 (0) | 49 (12) | 36.6 (5.8) | Participants followed a 7-day weight maintenance diet followed by three 10-day weight loss diets in a randomized crossover design without washout: 1. Normal-protein diet: 15% P, 55% C, 30% F, energy = 1 × BMR 2. Normal-protein diet enriched with free amino acids and moderate amounts of carbohydrate: 15% P, 15% free amino acids, 40% C, 30% F, energy = 1 × BMR 3. High-protein diet containing moderate amounts of carbohydrate: 30% P, 40% C, 30% F, energy = 1 × BMR | 37 days | None |
| Gutiérrez-Repiso 2021, Spain [20] | 21 (11) | 64.0 (4.7) | 33.4 (3.3) | Mediterranean diet: 20% P, 40–45% C, 35–40% F, 8–10% SFA, 600 kcal/day deficit, 150 min/week walking | 6 months | 16S rRNA (NR) |
| Jaagura 2021, Estonia [21] | 27 (NR) | NR | 28.9–44.4 | Low-carbohydrate, high-fat weight loss diet: 30 ± 10% energy deficit | 4 weeks | 16S rRNA (V3–V4) |
| Johnstone 2020, UK [22] | 24 (16) | 20–62 | 32.8 (4.07) | Weight loss diet: 30% P, 40% C, 30% F, 25 g/day fibre, energy intake = RMR | 3 weeks | qPCR, 16S rRNA (NR) |
| Kahleova 2020, USA [23] | 84 (69) | 52.9 (11.7) | 32.6 (3.7) | Low-fat vegan diet: 20–30 g/day fat, high in vegetables, grains, legumes, and fruit, instructed to avoid animal products and added oil, vitamin B12 supplemented (500 μg/day) | 16 weeks | 16S rRNA (V4) |
| Kahleova 2021, USA [24] | 62 (48) | 57.4 (NR) | 34.0 (NR) | Low-fat vegan diet: consisted of fruits, vegetables, grains, and legumes. Animal products and added fats were excluded. Vitamin B12 was supplemented (500 μg/day) | 16 weeks | 16S rRNA (V4) |
| Ley 2006, USA [7] | 6 (4) | 53.7 (NR) | >30 | Fat-restricted diet: 30% F, 10–15 g/day fibre, 1200–1500 kcal/day for women, 1500–1800 kcal/day for men | 12 months | 16S rRNA (NR) |
| 6 (5) | 42.0 (NR) | >30 | Carbohydrate-restricted diet: 25% C, 10–15 g/d fibre, 1200–1500 kcal/day for women, 1500–1800 kcal/day for men | 12 months | 16S rRNA (NR) | |
| Ma 2021, China [25] | 25 (25) | NR | 26.6 (0.5) | Low-carbohydrate diet: 20 g/day carbohydrates in the first week, then 10 g/day extra weekly until reaching 120 g/day at the end of the intervention | 12 weeks | Shotgun metagenomics |
| 25 (25) | NR | 26.9 (0.4) | Calorie-restricted diet: 1200 kcal/day, 20% P, 55% C, 25% F, 10% SFA, 300 mg/day cholesterol | 12 weeks | Shotgun metagenomics | |
| Nogacka 2021, Spain [26] | 9 (4) | 49.67 (7.81) | >40 | Hypocaloric diet: 15% P, 55% C, 30% F, <10% SFA, 20–25 g/day fibre, 20 kcal/kg body weight (~1800–2000 kcal/day) | 6–8 months | 16S rRNA (NR) |
| Pisanu 2020, Italy [27] | 23 (20) | 53 (9) | 35.2 (4.3) | Mediterranean diet: 20% P, 55% C, 25% F, ≥25 g/day fibre, energy = BMR (±10%) | 3 months | 16S rRNA (V3–V4) |
| Stanislawksi 2021, USA [28] | 71 (NR) | 40.7 (9.8) | 33.1 (4.4) | Energy-restricted diet: 34% weekly energy deficit achieved through either daily caloric restriction or intermittent fasting (80% energy deficit on 3 non-consecutive days each week). Moderate intensity physical activity: 300 min per week. | 12 weeks | 16S rRNA (V3–V4) |
| Zhang 2021, China [29] | 26 (22) | 36.58 (8.70) | 30.44 (3.38) | Low-carbohydrate diet: 10–25% C, no energy restriction | 12 weeks | 16S rRNA (V3–V4) |
| Trial | Reported Dietary Intake | Weight Loss, kg | α-Diversity | β-Diversity | Relative Bacterial Abundance |
|---|---|---|---|---|---|
| Bendsten 2018 [12] | High-dairy diet: 1649 kcal, 21% P, 47% C, 31% F, 20 g fibre | 6.6 (1.3) | ↔ Shannon | ↔ UniFrac | ↔ |
| Low-dairy diet: 1585 kcal, 19% P, 46% C, 32% F, 22 g fibre | 7.9 (1.5) | ↔ Shannon | ↔ UniFrac | ↓ Veillonella | |
| Benítez-Páez 2021 [13] | Calorie-restricted diet + fibre: 1642 kcal, 21% P, 47% C, 31% F, 18 g fibre | 6.1 (NR) | ↔ Simpson | ↔ B–C | NR |
| Calorie-restricted diet + placebo: 1730 kcal, 21% P, 46% C, 32% F, 18 g fibre | 5.5 (NR) | ↔ Simpson | ↔ B–C | NR | |
| Cuevas-Sierra 2021 [14] | Moderately high-protein diet: M: 33% P, 50% C, 17% F: 34% P, 49% C, 17% F | M: 10.3 (NR) F: 8.9 (NR) | M: ↔ Shannon F: ↔ Shannon | M: ↔ B–C F: ↔ B–C | ↑ Granulicatella ↓ Phascolarctobacterium, Dielma |
| Low-fat diet: M: 25% P, 61% C, 14% FF: 24% P, 63% C, 13% F | M: 11.0 (NR) F: 8.6 (NR) | M: ↑ Shannon F: ↔ Shannon | M: ↑↓ B–C F: ↔ B–C | ↔ | |
| Dhakal 2020 [15] | Retail weight reduction program: 1818 kcal, 24% P, 38% C, 38% F, 18 g fibre | 10.2 (NR) | ↑ OTU richness ↔ Shannon | NR | ↑ Tenericutes, Euryarchaeota ↓ Firmicutes, p_Actinobacteria |
| Dong 2020 [16] | High-protein diet: NR | 3.5 (NR) | ↑ Shannon | ↔ Aitchison | ↑ Akkermansia, Bifidobacterium ↓ Prevotella_9 |
| Normal-protein diet: NR | 2.8 (NR) | ↔ Shannon | ↔ Aitchison | ↑ Akkermansia, Bifidobacterium ↓ Prevotella_9 | |
| Duncan 2008 [17] | High-protein, moderate-carbohydrate, non-ketogenic diet: NR | 4.6 (NR) | NR | NR | ↑ Clostridium coccoides-related bacteria (other than Roseburia + Eubacterium rectale) ↓ Total bacterial number, Roseburia + Eubacterium rectale, Bifidobacterium |
| Fragiadakis 2020 [18] | Low-carbohydrate diet: 426 kcal/d deficit, 22% P, 32% C, 43% F, 18 g fibre | 5.1 (6.7) | ↔ Observed ASVs | 3 months: ↓ B–C 6 months: ↔ B–C 12 months: ↔ B–C | 3 m: ↑ Bacteroidetes, Bacteroides, Parabacteroides, Sutterella, Bilophila, Desulfovibrio, Butyricimonas, Lachnospira, Oscillospira 12 m: ↔ |
| Fragiadakis 2020 [18] | Low-fat diet: 484 kcal/d deficit, 21% P, 48% C, 29% F, 20 g fibre | 5.6 (5.7) | ↔ Observed ASVs | 3 months: ↔ B–C 6 months: ↔ B–C 12 months: ↔ B–C | 3 m: ↑ Bacteroidetes, Bacteroides, Parabacteroides 3 m: ↓ Actinobacteria, Firmicutes, Bifidobacterium, Dorea, Blautia, Ruminococcus 12 m: ↔ |
| Gutiérrez- Repiso 2021 [20] | Mediterranean diet: NR | 7.8 (1.9) | ↑ Observed ASVs ↑ Shannon ↑ Faith ↑ Pielou | ↔ UniFrac | ↑ Faecalibacterium |
| Jaagura 2021 [21] | Low-carbohydrate, high-fat weight loss diet: 25% P, 23% C, 50% F, 12 g fibre/1000 kcal | 7.7 (2.5) | ↔ Observed species ↔ Shannon | ↓ B–C | ↑ Alistipes, Butyricimonas, Odoribacter, Ruminococcus_1 ↓ Bifidobacterium, Collinsella, Dorea |
| Johnstone 2020 [22] | Weight loss diet: 1930 kcal, 29% P, 40% C, 30% F, 10% SFA, 25 g fibre, 15 g insoluble fibre, 5 g soluble fibre, 7 g resistant starch | 2.8 (NR) | ↔ Chao1 ↔ Shannon | NR | ↔ |
| Kahleova 2020 [23] | Low-fat vegan diet: 1294 kcal, 43 g P (13%), 236 g C (73%), 24.3 g F (17%), 33 g fibre, 9 g soluble fibre, 25 g insoluble fibre | 6.4 (NR) | ↔ AWPD | NR | ↑ Faecalibacterium ↓ Proteobacteria ↔ Bacteroidetes:Firmicutes, butyrate producing bacteria |
| Kahleova 2021 [24] | Low-fat vegan diet: 1315 kcal, 12% P, 69% C, 17% F, 33 g fibre, 9 g soluble fibre, 24 g insoluble fibre | 6.0 (NR) | ↓ AWPD | NR | ↑ Eubacterium ↓ Bacteroidetes, Proteobacteria ↔ Bacteroidetes:Firmicutes, butyrate-producing bacteria |
| Ley 2006 [7] | Fat-restricted diet: NR | 15.4 (NR) | ↔ Shannon | NR | ↑ Bacteroidetes ↓ Firmicutes |
| Carbohydrate-restricted diet: NR | 8.0 (NR) | ↔ Shannon | NR | ↑ Bacteroidetes ↓ Firmicutes | |
| Ma 2021 [25] | Low-carbohydrate diet: 1195 kcal, 26% P, 36% C, 38% F, 10 g fibre | 5.3 (NR) | ↔ Shannon | ↓ B–C | ↑ Bacteroidetes:Firmicutes |
| Calorie-restricted diet: 1355 kcal, 18% P, 51% C, 31% F, 11 g fibre | 5.1 (NR) | ↔ Shannon | ↔ B–C | ↔ Bacteroidetes:Firmicutes | |
| Nogacka 2021 [26] | Hypocaloric diet: NR | Group 1: <5% BW (n = 5) Group 2: >5% BW (n = 4) | Group 2 vs. total at baseline: ↔ Chao1 ↔ Shannon | NR | Group 2 vs. total at baseline: ↑ Clostridum sensu stricto 1 ↓ Parabacteroides |
| Pisanu 2020 [27] | Mediterranean diet: 1341 kcal, 19% P, 50% C, 29% F, 17 g fibre | 6.7 (NR) | ↔ Shannon | ↑↓ B–C | ↑ Catenibacterium, Caldilinea, Parabacteroides, Sphingobacterium, Veillonella ↓ Proteobacteria, Megamonas, Roseburia, Ruminococcus, Streptococcus, Sutterella ↔ Bacteroidetes:Firmicutes |
| Stanislawksi 2021 [28] | Energy-restricted diet: 1276 kcal, 21% P, 42% C, 35% F | 5.8 (3.8) | ↑ Observed OTUs ↑ Evenness ↑ Shannon ↑ Faith | ↑↓ UniFrac | ↑ Parabacteroides, Alistipes, Bacteroides ↓ Subdoligranulum, Collinsella |
| Zhang 2021 [29] | Low-carbohydrate diet: 1470 kcal, 34% P, 16% C, 50% F | 2.2 (1.2) kg/m2 | ↔ Shannon ↔ Simpson ↔ Richness (genus level) | ↔ B–C | ↔ (phylum level) |
| Trial | Reported Dietary Intake | Weight Loss, kg | Total SCFAs | Butyrate | Propionate | Acetate |
|---|---|---|---|---|---|---|
| Benítez-Páez 2021 [13] | Calorie-restricted diet + fibre: 1642 kcal, 21% P, 47% C, 31% F, 18 g fibre | 6.1 (NR) | NR | ↔ | ↔ | ↔ |
| Calorie-restricted diet + placebo: 1730 kcal, 21% P, 46% C, 32% F, 18 g fibre | 5.5 (NR) | NR | ↔ | ↔ | ↔ | |
| Gratz 2019 [19] | Normal-protein weight loss diet: 2154 kcal, 80 g P (15%), 309 g C (57%), 73 g F (31%), 29 g fibre | 3.9 (NR) | ↔ | ↔ | ↔ | ↔ |
| Normal-protein weight loss diet enriched with free amino acids and moderate amounts of carbohydrate: 2143 kcal, 156 g P (29%), 219 g C (41%), 73 g F (31%), 20 g fibre | 4.3 (NR) | ↔ | ↔ | ↔ | ↔ | |
| High-protein weight loss diet containing moderate amounts of carbohydrate: 2106 kcal, 153 g P (29%), 219 g C (42%), 72 g F (31%), 18 g fibre | 4.0 (NR) | ↔ | ↓ | ↔ | ↔ | |
| Johnstone 2020 [22] | Weight loss diet: 1930 kcal, 29% P, 40% C, 30% F, 10% SFA, 25 g fibre, 15 g insoluble fibre, 5 g soluble fibre, 7 g resistant starch | 2.8 (NR) | NR | ↔ (% of total SCFA) | ↔ (% of total SCFA) | ↔ (% of total SCFA) |
| Nogacka 2021 [26] | Hypocaloric diet: NR | Group 1: <5% BW (n = 5) Group 2: >5% BW (n = 4) | ↔ (Group 2 vs. total at baseline) | ↔ (Group 2 vs. total at baseline) | ↔ (Group 2 vs. total at baseline) | ↔ (Group 2 vs. total at baseline) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bliesner, A.; Eccles-Smith, J.; Bates, C.; Hayes, O.; Ho, J.Y.; Martins, C.; Truby, H.; Nitert, M.D. Impact of Food-Based Weight Loss Interventions on Gut Microbiome in Individuals with Obesity: A Systematic Review. Nutrients 2022, 14, 1953. https://doi.org/10.3390/nu14091953
Bliesner A, Eccles-Smith J, Bates C, Hayes O, Ho JY, Martins C, Truby H, Nitert MD. Impact of Food-Based Weight Loss Interventions on Gut Microbiome in Individuals with Obesity: A Systematic Review. Nutrients. 2022; 14(9):1953. https://doi.org/10.3390/nu14091953
Chicago/Turabian StyleBliesner, Aleisha, Jade Eccles-Smith, Claire Bates, Olivia Hayes, Jet Yee Ho, Catia Martins, Helen Truby, and Marloes Dekker Nitert. 2022. "Impact of Food-Based Weight Loss Interventions on Gut Microbiome in Individuals with Obesity: A Systematic Review" Nutrients 14, no. 9: 1953. https://doi.org/10.3390/nu14091953
APA StyleBliesner, A., Eccles-Smith, J., Bates, C., Hayes, O., Ho, J. Y., Martins, C., Truby, H., & Nitert, M. D. (2022). Impact of Food-Based Weight Loss Interventions on Gut Microbiome in Individuals with Obesity: A Systematic Review. Nutrients, 14(9), 1953. https://doi.org/10.3390/nu14091953









