Development of the Hedonic Overeating–Questionnaire (HEDO–Q)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Hedonic Overeating–Questionnaire
2.2.2. Eating Disorder Examination–Questionnaire
2.2.3. Patient Health Questionnaire
2.2.4. Measures for Discriminant Validation
2.3. Data Analytic Plan
3. Results
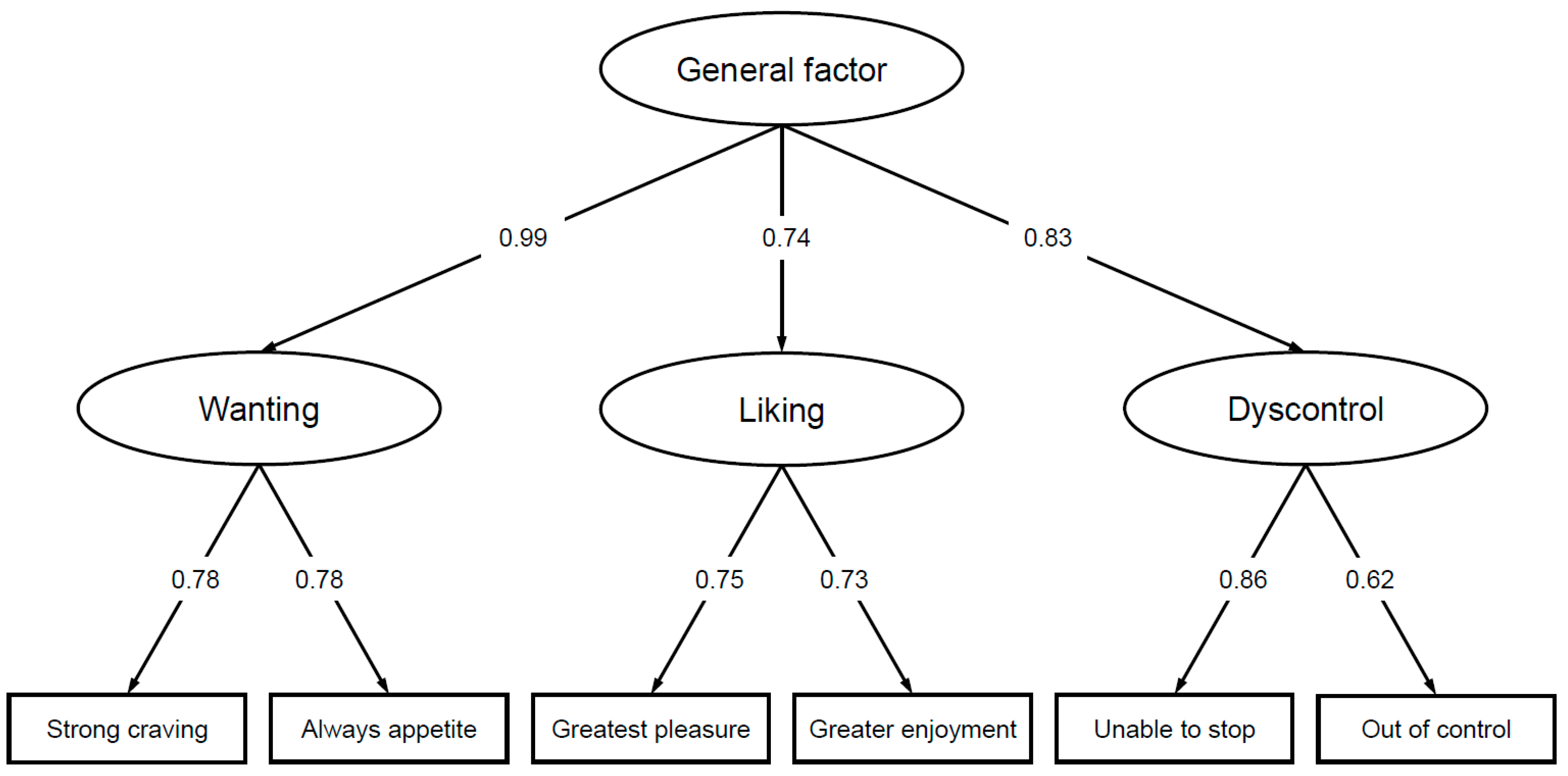
3.1. Factor Structure
3.2. Item Characteristics
3.3. Internal Consistency
3.4. Measurement Invariance
3.5. Norms
3.6. Construct Validity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Obesity and Overweight—Key Facts. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 29 March 2022).
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. The NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Vandevijvere, S.; Jaacks, L.M.; Monteiro, C.A.; Moubarac, J.; Girling-Butcher, M.; Lee, A.C.; Pan, A.; Bentham, J.; Swinburn, B. Global trends in ultraprocessed food and drink product sales and their association with adult body mass index trajectories. Obes. Rev. 2019, 20, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Mariani, M.; Sarti, E. Is the development of obesogenic food environments a self-reinforcing process? Evidence from soft drink consumption. Glob. Health 2021, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gearhardt, A.N.; Schulte, E.M. Is food addictive? A review of the science. Annu. Rev. Nut. 2021, 41, 387–410. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV-TR); American Psychiatric Publishing: Arlington, TX, USA, 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5); American Psychiatric Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- Schulte, E.M.; Wadden, T.A.; Allison, K.C. An evaluation of food addiction as a distinct psychiatric disorder. Int. J. Eat. Disord. 2020, 53, 1610–1622. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Hebebrand, J. The concept of “food addiction” helps inform the understanding of overeating and obesity: Debate consensus. Am. J. Clin. Nutr. 2021, 113, 274–276. [Google Scholar] [CrossRef]
- Hebebrand, J.; Albayrak, Ö.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menizies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, E.; Tavares, H.; von Ranson, K.M. Moving beyond the “eating addiction” versus “food addiction” debate: Comment on Schulte et al. (2017). Appetite 2018, 130, 286–292. [Google Scholar] [CrossRef]
- Schulte, E.M.; Potenza, M.N.; Gearhardt, A.N. Specific theoretical considerations and future research directions for evaluating addictive-like eating as a substance-based, food addiction: Comment on Lacroix et al. (2018). Appetite 2018, 130, 293–295. [Google Scholar] [CrossRef]
- Finlayson, G. Food addiction and obesity: Unnecessary medicalization of hedonic overeating. Nat. Rev. Endocrinol. 2017, 13, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meule, A.; Gearhardt, A.N. Ten years of the Yale Food Addiction Scale: A review of version 2.0. Curr. Addict. Rep. 2019, 6, 218–228. [Google Scholar] [CrossRef]
- Ziauddeen, H.; Fletcher, P.C. Is food addiction a valid and useful concept? Obes. Rev. 2013, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Westwater, M.L.; Fletcher, P.C.; Ziauddeen, H. Sugar addiction: The state of the science. Eur. J. Nutr. 2016, 55, 55–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasi, D.; Volkow, N.D. Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, H.; Farooqi, I.S.; Fletcher, P.C. Obesity and the brain: How convincing is the addiction model? Nat. Rev. Neurosci. 2012, 13, 279–286. [Google Scholar] [CrossRef]
- Ruddock, H.K.; Hardman, C.A. Food addiction beliefs amongst the lay public: What are the consequences for eating behaviour? Curr. Addict. Rep. 2017, 4, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Ruddock, H.K.; Field, M.; Hardman, C.A. Exploring food reward and calorie intake in self-perceived food addicts. Appetite 2017, 115, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Smeal, J.K.; Lewis, J.; Gearhardt, A.N. Development of the Highly Processed Food Withdrawal Scale. Appetite 2018, 131, 148–154. [Google Scholar] [CrossRef]
- Davis, C. Compulsive overeating as an addictive behavior: Overlap between food addiction and binge eating disorder. Curr. Obes. Rep. 2013, 2, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Gearhardt, A.N. Associations of food addiction in a sample recruited to be nationally representative of the United States. Eur. Eat. Disord. Rev. 2018, 26, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, H.K.; Christiansen, P.; Halford, J.C.G.; Hardman, C.A. The development and validation of the Addiction-like Eating Behaviour Scale. Int. J. Obes. 2017, 41, 1710–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, E.M.; Potenza, M.N.; Gearhardt, A.N. How much does the Addiction-Like Eating Behavior Scale add to the debate regarding food versus eating addictions? Int. J. Obes. 2018, 42, 946. [Google Scholar] [CrossRef]
- Ruddock, H.K.; Christiansen, P.; Halford, J.C.G.; Hardman, C.A. Response to ‘Jangle fallacy epidemic in obesity research: A comment on Ruddock et al. (2017)’. Int. J. Obes. 2018, 42, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, K.C. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996, 20, 1–25. [Google Scholar] [CrossRef]
- Mela, D.J. Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite 2006, 47, 10–17. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [Green Version]
- Morales, I.; Berridge, K.C. ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef]
- Arumäe, K.; Kreegipuu, K.; Vainik, U. Assessing the overlap between three measures of food reward. Front. Psychol. 2019, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; King, N.; Blundell, J.E. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol. Behav. 2007, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Bordes, I.; Griffioen-Roose, S.; de Graaf, C.; Blundell, J.E. Susceptibility to overeating affects the impact of savory or sweet drinks on satiation, reward, and food intake in nonobese women. J. Nutr. 2012, 142, 125–130. [Google Scholar] [CrossRef] [PubMed]
- French, S.A.; Mitchell, N.R.; Wolfson, J.; Finlayson, G.; Blundell, J.E.; Jeffery, R.W. Questionnaire and laboratory measures of eating behavior. Associations with energy intake and BMI in a community sample of working adults. Appetite 2014, 72, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Finlayson, G.; Arlotti, A.; Dalton, M.; King, N.; Blundell, J.E. Implicit wanting and explicit liking are markers for trait binge eating. A susceptible phenotype for overeating. Appetite 2011, 57, 722–728. [Google Scholar] [CrossRef]
- Geiser, C.; Götz, T.; Preckel, F.; Freund, P.A. States and traits. Theories, models, and assessment. Eur. J. Psychol. Ass. 2017, 33, 219–223. [Google Scholar] [CrossRef]
- Cepeda-Benito, A.; Gleaves, D.H.; Williams, T.L.; Erath, S.A. The development and validation of the State and Trait Food-Cravings Questionnaires. Behav. Res. Ther. 2000, 31, 151–173. [Google Scholar] [CrossRef]
- Meule, A.; Hermann, T.; Kübler, A. A short version of the Food Cravings Questionnaire-Trait: The FCQ-T-reduced. Front. Psychol. 2014, 5, 190. [Google Scholar] [CrossRef] [Green Version]
- Lowe, M.R.; Butryn, M.L.; Didie, E.R.; Annunziato, R.A.; Thomas, J.G.; Crerand, C.E.; Ochner, C.; Coletta, M.C.; Bellace, D.; Wallaert, M.; et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 2009, 53, 114–118. [Google Scholar] [CrossRef]
- Epel, E.S.; Tomiyama, A.J.; Mason, A.E.; Laraia, B.A.; Hartman, W.; Ready, K.; Acree, M.; Adam, T.C.; Jeor, S.S.; Kessler, D. The Reward-based Eating Drive Scale: A self-report index of reward-based eating. PLoS ONE 2014, 9, e101350. [Google Scholar] [CrossRef] [Green Version]
- Vainik, U.; Eun Han, J.; Epel, E.S.; Tomiyama, J.; Dagher, A.; Mason, A.E. Rapid assessment of reward-related eating: The RED-X5. Obesity 2019, 27, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Yokum, S. Neural vulnerability factors that increase risk for future weight gain. Psychol. Bull. 2016, 142, 447–471. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.; Lacroix, E.; von Ranson, K.M. Conceptualizing addictive-like eating: A qualitative analysis. Appetite 2019, 141, 104326. [Google Scholar] [CrossRef] [PubMed]
- Saruco, E.; Pleger, B. A systematic review of obesity and binge eating associated impairment of the cognitive inhibition system. Front. Nutr. 2021, 8, 609012. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Cross, L.; O’Doherty, J.P. Elucidating the underlying components of food valuation in the human orbitofrontal cortex. Nat. Neurosci. 2017, 20, 1780–1786. [Google Scholar] [CrossRef] [Green Version]
- Michaud, A.; Vainik, U.; Garcia-Garcia, I.; Dagher, A. Overlapping neural endophenotypes in addiction and obesity. Front. Endocrinol. 2017, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Kalon, E.; Hong, J.Y.; Tobin, C.; Schulte, T. Psychological and neurobiological correlates of food addiction. Int. Rev. Neurobiol. 2016, 129, 85–110. [Google Scholar] [CrossRef] [Green Version]
- Boateng, G.O.; Neilands, T.B.; Frongillo, E.A.; Melgar-Quiñonez, H.R.; Young, S.L. Best practices for developing and validating scales for health, social and behavioral research: A primer. Front. Public Health 2018, 11, 149. [Google Scholar] [CrossRef]
- Simms, L.J. Classical and modern methods of psychological scale construction. Soc. Personal. Psychol. Compass 2008, 2, 149. [Google Scholar] [CrossRef]
- Mason, A.E.; Vainik, U.; Acree, M.; Tomiyama, A.J.; Dagher, A.; Epel, E.S.; Hecht, F.M. Improving assessment of the spectrum of reward-related eating: The RED-13. Front. Psychol. 2017, 8, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreeva, E.; Neumann, M.; Nöhre, M.; Brähler, E.; Hilbert, A.; de Zwaan, M. Validation of the German version of the Power of Food Scale in a general population sample. Obes. Facts. 2019, 12, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliemann, N.; Beeken, R.J.; Wardle, J.; Johnson, F. Development and validation of the Self-Regulation of Eating Behaviour Questionnaire for adults. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmalbach, I.; Schmalbach, B.; Zenger, M.; Petrowski, K.; Beutel, M.; Hilbert, A.; Brähler, E. Psychometric properties of the German version of the Self-Regulation of Eating Behavior Questionnaire. Front. Psychol. 2021, 12, 649867. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The Three-factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Pudel, V.; Westenhoefer, J. Fragebogen zum Essverhalten; Hogrefe: Göttingen, Germany, 1989. [Google Scholar]
- Fairburn, C.G.; Beglin, S.J. Eating Disorder Examination-Questionnaire (Edition 6.0). 2008. Available online: http://www.credo-oxford.com/7.2.html (accessed on 29 March 2022).
- Hilbert, A.; Tuschen-Caffier, B. Eating Disorder Examination-Questionnaire. Deutschsprachige Übersetzung, 2nd ed.; Dgvt-Verlag: Tübingen, Germany, 2016. [Google Scholar]
- Kliem, S.; Mößle, T.; Zenger, M.; Strauß, B.; Brähler, E.; Hilbert, A. The Eating Disorder Examination-Questionnaire 8: A brief measure of eating disorder psychopathology (EDE–Q8). Int. J. Eat. Disord. 2016, 49, 613–616. [Google Scholar] [CrossRef] [Green Version]
- Löwe, B.; Wahl, I.; Rose, M.; Spitzer, C.; Glaesmer, H.; Wingenfeld, K.; Schneider, A.; Brähler, E. A 4-item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J. Affect. Disord. 2010, 122, 86–95. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA 1999, 282, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.M.; Grilo, C.M.; Sinha, R. Food cravings, binge eating, and eating disorder psychopathology: Exploring the moderating roles of gender and race. Eat. Behav. 2016, 21, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Hilbert, A.; de Zwaan, M.; Braehler, E. How frequent are eating disturbances in the population? Norms of the Eating Disorder Examination-Questionnaire. PLoS ONE 2012, 7, e29125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann. Transl. Med. 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Soft. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Browne, M.W.; Cudeck, R. Alternative ways of assessing model fit. Sage Focus Ed. 1993, 21. [Google Scholar] [CrossRef]
- Chen, F.F. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct. Equ. Model. A Multidiscip. J. 2007, 14, 464–504. [Google Scholar] [CrossRef]
- Chen, F.F.; Sousa, K.H.; West, S.G. Teacher’s corner: Testing measurement invariance of second-order factor models. Struct. Equ. Model. A Multidiscip. J. 2005, 12, 471–492. [Google Scholar] [CrossRef]
- Byrne, B.M.; Shavelson, R.J.; Muthén, B.O. Testing for the equivalence of factor covariance and mean structures: The issue of partial measurement invariance. Psychol. Bull. 1989, 105, 456–466. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Soft. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Eisinga, R.; Grotenhuis, M.T.; Pelzer, B. The reliability of a two-item scale: Pearson, Cronbach or Spearman-Brown? Int. J. Public Health 2013, 58, 637–642. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 29 March 2022).
- Moosbrugger, H.; Kelava, A. Testtheorie und Fragebogenkonstruktion (3. Überarbeitete Auflage); Springer: Berlin, Germany, 2020. [Google Scholar]
- Darby, A.; Hay, P.; Mond, J.; Rodgers, B.; Owen, C. Disordered eating behaviours and cognitions in young women with obesity: Relationship with psychological status. Int. J. Obes. 2007, 31, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedemann, A.A.; Carr, M.M.; Ivezaj, V.; Barnes, R.D. Examining the construct validity of food addiction severity specifiers. Eat. Weight. Disord. 2021, 26, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Dolan, S.C.; Khindri, R.; Franko, D.L.; Thomas, J.J.; Reilly, E.E.; Eddy, K.T. Anhedonia in eating disorders: A meta-analysis and systematic review. Int. J. Eat. Disord. 2022, 55, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Sandman, C.F.; Craske, M.G. Psychological Treatments for Anhedonia, Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–23. [Google Scholar] [CrossRef]
- German National Cohort Consortium. The German National Cohort: Aims, study design and organization. Eur. J. Epidemiol. 2014, 29, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Connor Gorber, S.; Tremblay, M.; Moher, D.; Gorber, B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: A systematic review. Obes. Rev. 2007, 8, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Pursey, K.; Burrows, T.L.; Stanwell, P.; Collins, C.E. How accurate is web-based self-reported height, weight, and body mass index in young adults? J. Med. Internet. Res. 2014, 16. [Google Scholar] [CrossRef]
- Mensink, G.B.; Schienkiewitz, A.; Haftenberger, M.; Lampert, T.; Ziese, T.; Scheidt-Nave, C. Übergewicht und Adipositas in Deutschland. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 786–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federal Statistical Office. Einkommens- und Verbrauchsstichprobe: Einnahmen und Ausgaben Privater Haushalte 2018; Federal Statistical Office: Wiesbaden, Germany, 2020. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Einkommen-Konsum-Lebensbedingungen/Einkommen-Einnahmen-Ausgaben/Publikationen/Downloads-Einkommen/evs-einnahmen-ausgaben-privater-haushalte-2152604189004.pdf?__blob=publicationFile (accessed on 29 March 2022).
- Federal Statistical Office. Bevölkerung nach. Nationalität und Geschlecht; Federal Statistical Office: Wiesbaden, Germany, 2019. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Bevoelkerungsstand/Tabellen/zensus-geschlecht-staatsangehoerigkeit-2019.html (accessed on 29 March 2022).
- de Araujo, I.E.; Schatzker, M.; Small, D.M. Rethinking food reward. Annu. Rev. Psychol. 2020, 71, 139–164. [Google Scholar] [CrossRef]
- Sherman, J.W.; Klein, S.A.W. The four deadly sins of implicit attitude research. Front. Psychol. 2021, 11, 604340. [Google Scholar] [CrossRef]
- Havermans, R.C. “You say it’s liking, I say it’s wanting …” On the difficulty of disentangling food reward in man. Appetite 2011, 57, 286–294. [Google Scholar] [CrossRef]
- Finlayson, G.; Dalton, M. Current progress in the assessment of ‘liking’ vs. ‘wanting’ food in human appetite. Comment on ‘“You say it’s liking, I say it’s wanting..”. On the difficulty of disentangling food reward in man’. Appetite 2012, 58, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Oustric, P.; Beaulieu, K.; Casanova, N.; O’Connor, D.; Gibbons, C.; Hopkins, M.; Blundell, J.; Finlayson, G. Food liking but not wanting decreases after controlled intermittent or continuous energy restriction to ≥5% weight loss in women with overweight/obesity. Nutrients 2021, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Oustric, P.; Gibbons, C.; Beaulieu, K.; Blundell, J.; Finlayson, G. Changes in food reward during weight management interventions—A systematic review. Obes. Rev. 2018, 19, 1642–1658. [Google Scholar] [CrossRef] [PubMed]
- Hardman, C.A.; Jones, A.; Burton, S.; Duckwoth, J.J.; McGale, L.S.; Mead, B.R.; Roberts, C.A.; Field, M.; Werthmann, J. Food-related attentional bias and its associations with appetitive motivation and body weight: A systematic review and meta-analysis. Appetite 2021, 157, 104986. [Google Scholar] [CrossRef] [PubMed]
- Stott, N.; Fox, J.R.E.; Williams, M.O. Attentional bias in eating disorders: A meta-review. Int. J. Eat. Disord. 2021, 54, 1377–1399. [Google Scholar] [CrossRef]
- Boswell, R.G.; Kober, H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes. Rev. 2016, 17, 159–177. [Google Scholar] [CrossRef] [PubMed]
| Total | Women | Men | |
|---|---|---|---|
| (N = 2531) | (N = 1350) | (N = 1181) | |
| Age (years) | |||
| Mean (SD) | 48.4 (17.9) | 49.1 (17.8) | 47.7 (17.9) |
| Median [min, max] | 50.0 [14.0, 95.0] | 50.0 [14.0, 90.0] | 49.0 [14.0, 95.0] |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) |
| Body mass index (kg/m2) | 25.82 (5.02) | 25.56 (5.40) | 26.12 (4.53) |
| Missing | 23 (1.1%) | 18 (1.3%) | 5 (0.4%) |
| Weight status | |||
| Underweight (<18.5 kg/m2) | 49 (1.9%) | 37 (2.7%) | 12 (1.0%) |
| Normal weight (18.5–24.9 kg/m2) | 1173 (46.3%) | 684 (50.7%) | 489 (41.4%) |
| Overweight (25.0–29.9 kg/m2) | 924 (36.5%) | 393 (29.1%) | 531 (45.0%) |
| Obesity class I (30.0–34.9 kg/m2) | 239 (9.4%) | 144 (10.7%) | 95 (8.0%) |
| Obesity class II (35.0–39.9 kg/m2) | 73 (2.9%) | 44 (3.3%) | 29 (2.5%) |
| Obesity class III (≥40.0 kg/m2) | 50 (2.0%) | 30 (2.2%) | 20 (1.7%) |
| Missing | 23 (0.9%) | 18 (1.3%) | 5 (0.4%) |
| Household income (net, per month) | |||
| 500 –< 1000 EUR | 176 (7.0%) | 95 (7.0%) | 81 (6.9%) |
| 1000 –< 2000 EUR | 716 (28.3%) | 456 (33.8%) | 260 (22.0%) |
| 2000 –< 3500 EUR | 1042 (41.2%) | 491 (36.4%) | 551 (46.7%) |
| ≥3500 EUR | 544 (21.5%) | 276 (20.4%) | 268 (22.7%) |
| Missing | 53 (2.1%) | 32 (2.4%) | 21 (1.8%) |
| Marital status | |||
| Married/living together | 1101 (43.5%) | 553 (41.0%) | 548 (46.4%) |
| Married/separated | 70 (2.8%) | 43 (3.2%) | 27 (2.3%) |
| Single | 760 (30.0%) | 355 (26.3%) | 405 (34.3%) |
| Divorced | 368 (14.5%) | 226 (16.7%) | 142 (12.0%) |
| Widowed | 220 (8.7%) | 167 (12.4%) | 53 (4.5%) |
| Missing | 12 (0.5%) | 6 (0.4%) | 6 (0.5%) |
| Nationality | |||
| German | 2437 (96.3%) | 1299 (96.2%) | 1138 (96.4%) |
| Other | 90 (3.6%) | 47 (3.5%) | 43 (3.6%) |
| Missing | 4 (0.2%) | 4 (0.3%) | 0 (0%) |
| M | SD | rit | Pi | rsb | W | |
|---|---|---|---|---|---|---|
| Wanting | 1.38 | 0.88 | 34.50 | 0.75 | 0.95 | |
| Strong craving | 1.48 | 0.94 | 0.78 | 37.00 | 0.89 | |
| Always appetite | 1.29 | 1.02 | 0.75 | 32.25 | 0.88 | |
| Liking | 1.66 | 0.94 | 41.50 | 0.71 | 0.96 | |
| Greatest pleasure | 1.74 | 1.05 | 0.71 | 43.50 | 0.90 | |
| Greater enjoyment | 1.58 | 1.10 | 0.79 | 39.50 | 0.91 | |
| Dyscontrol | 0.62 | 0.76 | 15.50 | 0.70 | 0.79 | |
| Unable to stop | 0.80 | 0.91 | 0.71 | 20.00 | 0.79 | |
| Out of control | 0.44 | 0.79 | 0.61 | 11.00 | 0.59 | |
| HEDO–Q | 1.22 | 0.71 | 30.50 | 0.97 |
| χ2 | df | CFI | ΔCFI | RMSEA | ΔRMSEA | Measurement Invariance | |
|---|---|---|---|---|---|---|---|
| Sex (female, male) | |||||||
| 1 | 51.65 | 12 | 0.989 | 0.060 | |||
| 2 | 57.21 | 15 | 0.989 | 0.000 | 0.055 | −0.005 | √ |
| 3 | 59.52 | 17 | 0.989 | 0.000 | 0.051 | −0.004 | √ |
| 4 | 63.57 | 20 | 0.989 | 0.000 | 0.047 | −0.004 | √ |
| 5 | 68.10 | 22 | 0.989 | 0.000 | 0.045 | −0.002 | √ |
| 6 | 71.04 | 25 | 0.989 | 0.000 | 0.042 | −0.003 | √ |
| 7 | 69.91 | 31 | 0.990 | +0.001 | 0.036 | −0.006 | √ |
| Age (14–34 years, 35–54 years, 55–98 years) | |||||||
| 1 | 47.20 | 18 | 0.992 | 0.051 | |||
| 2 | 54.34 | 24 | 0.992 | 0.000 | 0.044 | −0.007 | √ |
| 3 | 63.08 | 28 | 0.991 | −0.001 | 0.044 | 0.000 | √ |
| 4 | 439.12 | 35 | 0.901 | −0.090 | 0.130 | +0.086 | x |
| 4 part 1 | 68.74 | 32 | 0.991 | 0.000 | 0.041 | −0.003 | √ |
| 5 | 73.39 | 36 | 0.991 | 0.000 | 0.039 | −0.002 | √ |
| 6 | 80.23 | 41 | 0.991 | 0.000 | 0.037 | −0.002 | √ |
| 7 | 115.46 | 53 | 0.984 | −0.007 | 0.043 | +0.006 | √ |
| Weight Status (nonobese, obese) | |||||||
| 1 | 50.13 | 12 | 0.989 | 0.059 | |||
| 2 | 75.79 | 15 | 0.983 | −0.006 | 0.065 | +0.006 | √ |
| 3 | 99.34 | 17 | 0.977 | −0.006 | 0.071 | +0.006 | √ |
| 4 | 130.44 | 20 | 0.971 | −0.006 | 0.074 | +0.003 | √ |
| 5 | 148.74 | 22 | 0.968 | −0.003 | 0.074 | +0.000 | √ |
| 6 | 176.41 | 25 | 0.963 | −0.005 | 0.075 | +0.001 | √ |
| 7 | 237.22 | 31 | 0.932 | −0.031 | 0.091 | +0.016 | x |
| 7 part 2 | 179.76 | 30 | 0.959 | −0.004 | 0.072 | −0.003 | √ |
| Total | Sex | Age | Weight Status | |||||
|---|---|---|---|---|---|---|---|---|
| HEDO–Q Score | Sample N = 2531 | Women N = 1350 | Men N = 1181 | 14–34 y N = 658 | 35–54 y N = 872 | 55–98 y N = 1001 | Nonobese N = 2144 | Obese N = 369 |
| 0.00 | 5 | 5 | 4 | 3 | 4 | 6 | 5 | 2 |
| 0.17 | 8 | 9 | 8 | 6 | 8 | 10 | 9 | 3 |
| 0.33 | 13 | 14 | 12 | 10 | 12 | 16 | 14 | 5 |
| 0.50 | 18 | 19 | 17 | 15 | 16 | 23 | 20 | 7 |
| 0.67 | 26 | 27 | 24 | 20 | 23 | 32 | 28 | 12 |
| 0.83 | 35 | 37 | 33 | 28 | 32 | 42 | 38 | 17 |
| 1.00 | 45 | 47 | 43 | 38 | 43 | 52 | 49 | 25 |
| 1.17 | 56 | 57 | 54 | 47 | 53 | 63 | 59 | 32 |
| 1.33 | 65 | 66 | 64 | 56 | 65 | 71 | 69 | 39 |
| 1.5 | 72 | 73 | 72 | 64 | 72 | 78 | 76 | 47 |
| 1.67 | 79 | 80 | 78 | 73 | 78 | 84 | 83 | 54 |
| 1.83 | 85 | 85 | 84 | 79 | 84 | 89 | 89 | 62 |
| 2.00 | 89 | 89 | 89 | 84 | 90 | 92 | 93 | 69 |
| 2.17 | 92 | 91 | 92 | 87 | 92 | 95 | 95 | 73 |
| 2.33 | 94 | 93 | 95 | 91 | 94 | 96 | 97 | 78 |
| 2.50 | 96 | 95 | 96 | 94 | 95 | 97 | 98 | 82 |
| 2.67 | 97 | 97 | 98 | 96 | 97 | 98 | 99 | 88 |
| 2.83 | 98 | 98 | 98 | 98 | 98 | 99 | 99 | 92 |
| 3.00 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 96 |
| 3.17 | 99 | 99 | 99 | 99 | 99 | >99 | 99 | 97 |
| 3.33 | 99 | 99 | 99 | 99 | 99 | >99 | >99 | 98 |
| 3.50 | >99 | 99 | >99 | 99 | 99 | >99 | >99 | 98 |
| 3.67 | >99 | >99 | >99 | 99 | >99 | >99 | >99 | 99 |
| 3.83 | >99 | >99 | >99 | >99 | >99 | >99 | >99 | 99 |
| 4.00 | >99 | >99 | >99 | >99 | >99 | >99 | >99 | >99 |
| Women (N = 1350) | Men (N = 1181) | Group Differences | |||||||
| M | SD | M | SD | t(2529) | p | d | 95% CI | ||
| Wanting | 1.35 | 0.88 | 1.41 | 0.87 | 1.69 | 0.092 | 0.07 | −0.01 | 0.15 |
| Strong craving | 1.44 | 0.94 | 1.51 | 0.95 | 1.81 | 0.071 | 0.07 | −0.01 | 0.15 |
| Always appetite | 1.27 | 1.03 | 1.31 | 1.00 | 1.23 | 0.220 | 0.05 | −0.03 | 0.13 |
| Liking | 1.63 | 0.96 | 1.69 | 0.93 | 1.49 | 0.137 | 0.06 | −0.02 | 0.14 |
| Greatest pleasure | 1.71 | 1.06 | 1.78 | 1.03 | 1.86 | 0.063 | 0.07 | 0.00 | 0.15 |
| Greater enjoyment | 1.56 | 1.11 | 1.60 | 1.08 | 0.79 | 0.428 | 0.03 | −0.05 | 0.11 |
| Dyscontrol | 0.62 | 0.77 | 0.61 | 0.74 | −0.27 | 0.786 | −0.01 | −0.09 | 0.07 |
| Unable to stop | 0.80 | 0.92 | 0.80 | 0.90 | 0.15 | 0.880 | 0.01 | −0.07 | 0.08 |
| Out of control | 0.45 | 0.83 | 0.42 | 0.79 | −0.67 | 0.502 | −0.03 | −0.10 | 0.05 |
| HEDO–Q | 1.2 | 0.72 | 1.24 | 0.69 | 1.26 | 0.209 | 0.05 | −0.03 | 0.13 |
| Nonobese (N = 2144) | Obese (N = 369) | Group Differences | |||||||
| M | SD | M | SD | t(2511) | p | d | 95% CI | ||
| Wanting | 1.29 | 0.83 | 1.92 | 0.96 | 13.23 | <0.001 | 0.75 | 0.86 | 0.63 |
| Strong craving | 1.38 | 0.90 | 2.05 | 1.00 | 12.99 | <0.001 | 0.73 | 0.84 | 0.62 |
| Always appetite | 1.20 | 0.98 | 1.80 | 1.12 | 10.59 | <0.001 | 0.60 | 0.71 | 0.49 |
| Liking | 1.60 | 0.94 | 2.03 | 0.92 | 8.17 | <0.001 | 0.46 | 0.57 | 0.35 |
| Greatest pleasure | 1.68 | 1.04 | 2.11 | 1.03 | 7.40 | <0.001 | 0.42 | 0.53 | 0.31 |
| Greater enjoyment | 1.52 | 1.08 | 1.94 | 1.13 | 6.98 | <0.001 | 0.39 | 0.50 | 0.28 |
| Dyscontrol | 0.52 | 0.65 | 1.18 | 1.03 | 16.20 | <0.001 | 0.91 | 1.03 | 0.80 |
| Unable to stop | 0.70 | 0.83 | 1.37 | 1.12 | 13.37 | <0.001 | 0.75 | 0.87 | 0.64 |
| Out of control | 0.34 | 0.69 | 0.99 | 1.17 | 14.82 | <0.001 | 0.84 | 0.95 | 0.72 |
| HEDO–Q | 1.14 | 0.65 | 1.71 | 0.83 | 14.91 | <0.001 | 0.84 | 0.95 | 0.73 |
| No Eating Disturbance (N = 1856) | Eating Disturbance (N = 675) | Group Differences | |||||||
| M | SD | M | SD | t(2511) | p | d | 95% CI | ||
| Wanting | 1.18 | 0.79 | 1.93 | 0.88 | 20.33 | <0.001 | 0.91 | 0.82 | 1.01 |
| Strong craving | 1.28 | 0.87 | 2.02 | 0.92 | 18.68 | <0.001 | 0.84 | 0.75 | 0.93 |
| Always appetite | 1.09 | 0.93 | 1.83 | 1.04 | 17.10 | <0.001 | 0.77 | 0.68 | 0.86 |
| Liking | 1.51 | 0.92 | 2.07 | 0.89 | 13.67 | <0.001 | 0.61 | 0.52 | 0.70 |
| Greatest pleasure | 1.60 | 1.01 | 2.13 | 1.05 | 11.48 | <0.001 | 0.52 | 0.43 | 0.61 |
| Greater enjoyment | 1.42 | 1.07 | 2.01 | 1.04 | 12.38 | <0.001 | 0.56 | 0.47 | 0.65 |
| Dyscontrol | 0.42 | 0.58 | 1.16 | 0.9 | 24.55 | <0.001 | 1.10 | 1.01 | 1.20 |
| Unable to stop | 0.57 | 0.76 | 1.41 | 1.00 | 22.3 | <0.001 | 1.00 | 0.91 | 1.09 |
| Out of control | 0.26 | 0.62 | 0.92 | 1.05 | 19.43 | <0.001 | 0.87 | 0.78 | 0.96 |
| HEDO–Q | 1.04 | 0.61 | 1.72 | 0.73 | 23.68 | <0.001 | 1.06 | 0.97 | 1.16 |
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. HEDO–Q | ||||||
| 2. Wanting | 0.88 ** | |||||
| [0.87, 0.89] | ||||||
| 3. Liking | 0.81 ** | 0.56 ** | ||||
| [0.80, 0.82] | [0.53, 0.59] | |||||
| 4. Dyscontrol | 0.78 ** | 0.63 ** | 0.38 ** | |||
| [0.77, 0.80] | [0.60, 0.65] | [0.35, 0.42] | ||||
| 5. Depressive Symptoms | 0.15 ** | 0.13 ** | 0.05 * | 0.21 ** | ||
| (PHQ–2) | [0.11, 0.19] | [0.09, 0.16] | [0.01, 0.09] | [0.17, 0.25] | ||
| 6. Generalized Anxiety | 0.16 ** | 0.15 ** | 0.04 * | 0.23 ** | 0.73 ** | |
| Disorder Symptoms (GAD–2) | [0.12, 0.20] | [0.11, 0.18] | [0.00, 0.08] | [0.19, 0.27] | [0.71, 0.75] | |
| 7. Global Eating Disorder | 0.41 ** | 0.37 ** | 0.21 ** | 0.46 ** | 0.24 ** | 0.26 ** |
| Psychopathology (EDE–Q8) | [0.37, 0.44] | [0.34, 0.40] | [0.17, 0.24] | [0.43, 0.49] | [0.20, 0.27] | [0.23, 0.30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilbert, A.; Witte, V.; Meule, A.; Braehler, E.; Kliem, S. Development of the Hedonic Overeating–Questionnaire (HEDO–Q). Nutrients 2022, 14, 1865. https://doi.org/10.3390/nu14091865
Hilbert A, Witte V, Meule A, Braehler E, Kliem S. Development of the Hedonic Overeating–Questionnaire (HEDO–Q). Nutrients. 2022; 14(9):1865. https://doi.org/10.3390/nu14091865
Chicago/Turabian StyleHilbert, Anja, Veronica Witte, Adrian Meule, Elmar Braehler, and Soeren Kliem. 2022. "Development of the Hedonic Overeating–Questionnaire (HEDO–Q)" Nutrients 14, no. 9: 1865. https://doi.org/10.3390/nu14091865
APA StyleHilbert, A., Witte, V., Meule, A., Braehler, E., & Kliem, S. (2022). Development of the Hedonic Overeating–Questionnaire (HEDO–Q). Nutrients, 14(9), 1865. https://doi.org/10.3390/nu14091865







