Age-Related Sex Differences in Glucose Tolerance by 75 g Oral Glucose Tolerance Test in Japanese
Abstract
1. Introduction
2. Materials and Methods
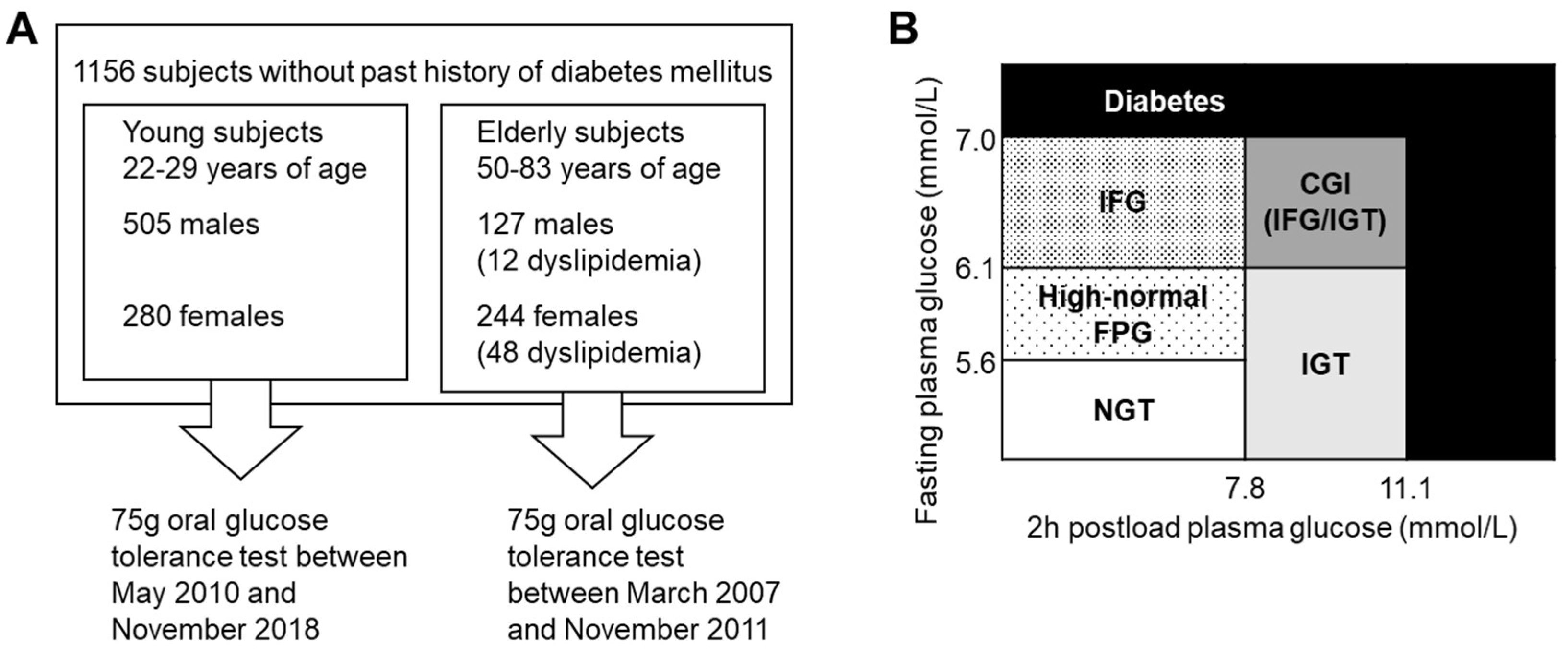
2.1. Participants
2.2. Study Design
2.3. Laboratory Assays
2.4. Statistical Methods
3. Results
3.1. Comparison of Young Males and Middle-Aged Males
3.2. Comparison of Young Females and Middle-Aged Females
3.3. Comparison of Young Males and Young Females
3.4. Comparison of Middle-Aged Males and Middle-Aged Females
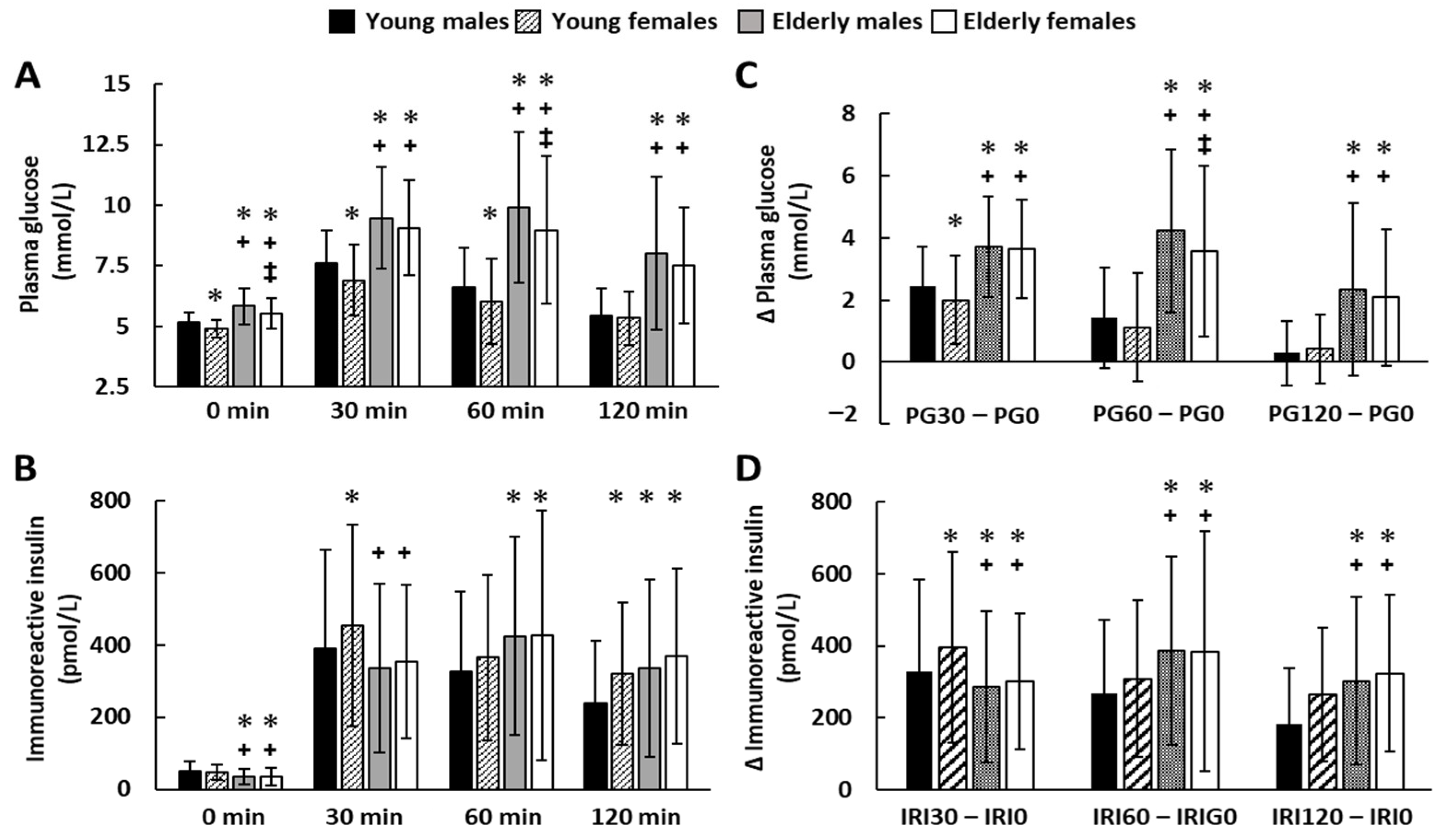
3.5. Sequential Changes in the Concentrations of Plasma Glucose and Insulin during the 75 gOGTT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF Diabetes Atlas. Available online: https://diabetesatlas.org/ (accessed on 14 November 2022).
- Khan, S.; Ijaz, A.; Bokhari, S.R.; Hanif, M.; Azam, N. Frequency of impaired glucose tolerance and diabetes mellitus in subjects with fasting blood glucose below 6.1 mmol/L (110 mg/dL). East. Mediterr. Health J. 2013, 19, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, R.; Fukushima, M.; Taniguchi, A.; Nakai, Y.; Aoyama, S.; Takahashi, Y.; Tsuji, H.; Yabe, D.; Yasuda, K.; Kurose, T.; et al. Insulin secretory capacity and insulin sensitivity in impaired fasting glucose in Japanese. J. Diabetes Investig. 2012, 3, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Kato, M.; Takahashi, Y.; Matsushita, Y.; Mizoue, T.; Inoue, M.; Tsugane, S.; Kadowaki, T. Fasting plasma glucose and 5-year incidence of diabetes in the JPHC diabetes study—Suggestion for the threshold for impaired fasting glucose among Japanese. Endocr. J. 2010, 57, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.; Polonsky, K.S.; Beltz, W.F.; Wallace, P.; Brechtel, G.; Fink, R.I. Effects of aging on insulin secretion. Diabetes 1989, 38, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Lee, N.P.; Cohan, P.; Chuang, L.-M. Beta cell function declines with age in glucose tolerant Caucasians. Clin. Endocrinol. 2000, 53, 569–575. [Google Scholar] [CrossRef]
- Toyoda, K.; Fukushima, M.; Mitsui, R.; Harada, N.; Suzuki, H.; Takeda, T.; Taniguchi, A.; Nakai, Y.; Kawakita, T.; Yamada, Y.; et al. Factors responsible for age-related elevation in fasting plasma glucose: A cross-sectional study in Japanese men. Metabolism 2008, 57, 299–303. [Google Scholar] [CrossRef]
- Fukushima, M.; Suzuki, H.; Seino, Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res. Clin. Prac. 2004, 66, S37–S43. [Google Scholar] [CrossRef]
- Kosaka, K.; Kuzuya, T.; Yoshinaga, H.; Hagura, R. A prospective study of health check examinees for the development of non-insulin-dependent diabetes mellitus: Relationship of the incidence of diabetes with the initial insulinogenic index and degree of obesity. Diabet. Med. 1996, 13, S120–S126. [Google Scholar] [CrossRef]
- The National Health and Nutrition Survey (NHNS) Japan. Results of Basic Items. Part II. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/download_files/eiyouchousa/2019.pdf (accessed on 14 November 2022).
- Tura, A.; Pacini, G.; Moro, E.; Vrbíková, J.; Bendlová, B.; Kautzky-Willer, A. Sex- and age-related differences of metabolic parameters in impaired glucose metabolism and type 2 diabetes compared to normal glucose tolerance. Diabetes Res. Clin. Pract. 2018, 146, 67–75. [Google Scholar] [CrossRef]
- Kim, S.H.; Reaven, G. Sex differences in insulin resistance and cardiovascular disease risk. J. Clin. Endocrinol. Metab. 2013, 98, E1716–E1721. [Google Scholar] [CrossRef]
- Ishigaki, H.; Yoshida, A.; Araki, O.; Kimura, T.; Tsunekawa, K.; Shoho, Y.; Nara, M.; Aoki, T.; Ogiwara, T.; Murakami, M. Prolonged plasma glucose elevation on oral glucose tolerance test in young healthy Japanese individuals. Endocrinol. Diabetes Metab. 2020, 3, e00098. [Google Scholar] [CrossRef] [PubMed]
- Martha, L.; Kimura, T.; Yoshida, A.; Tsunekawa, K.; Aoki, T.; Araki, O.; Nara, M.; Ogiwara, T.; Sumino, H.; Murakami, M. Association between insulin resistance and cardinal rheological parameters in young healthy Japanese individuals during 75 g oral glucose tolerance test. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.-F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in Diabetes. Diabetes Care 2010, 33, S11–S61. [Google Scholar] [CrossRef]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Weiss, R.; Taksali, S.E.; Tamborlane, W.V.; Burgert, T.S.; Savoye, M.; Caprio, S. Predictors of Changes in Glucose Tolerance Status in Obese Youth. Diabetes Care 2005, 28, 902–909. [Google Scholar] [CrossRef]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct Homeostasis Model Assessment (HOMA) Evaluation Uses the Computer Program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef]
- Ahren, B.; Pratley, R.; Soubt, M.; Dunning, B.; Foley, J. Clinical Measures of Islet Function: Usefulness to Characterize Defects in Diabetes. Curr. Diabetes Rev. 2008, 4, 129–145. [Google Scholar] [CrossRef]
- Lundsgaard, A.-M.; Kiens, B. Gender Differences in Skeletal Muscle Substrate Metabolism—Molecular Mechanisms and Insulin Sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-C.; Chuang, H.-H.; Li, W.-C.; Tzeng, I.-S.; Chen, J.-Y. Gender difference in the association between lower muscle mass and metabolic syndrome independent of insulin resistance in a middle-aged and elderly Taiwanese population. Arch. Gerontol. Geriatr. 2017, 72, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-S. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr. J. 2014, 61, 61–70. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Glucose Intolerance and Aging: Evidence for Tissue Insensitivity to Insulin. Diabetes 1979, 28, 1095–1101. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Mendes, I.; Ong, B.; Prado, C.M.; Mocciaro, G.; Mazidi, M.; Lubrano, C.; Lenzi, A.; Donini, L.M.; Siervo, M. Sarcopenic obesity and insulin resistance: Application of novel body composition models. Nutrition 2020, 75–76, 110765. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, W.; Shen, Y.; Tang, J.; Wang, Y.; Ma, X.; Bao, Y. The relationship between sex hormones and glycated hemoglobin in a non-diabetic middle-aged and elderly population. BMC Endocr. Disord. 2022, 22, 91. [Google Scholar] [CrossRef]
- Kernohan, A.F.B.; Sattar, N.; Hilditch, T.; Cleland, S.J.; Small, M.; Lumsden, M.A.; Connell, J.M.C.; Petrie, J.R. Effects of low-dose continuous combined hormone replacement therapy on glucose homeostasis and markers of cardiovascular risk in women with type 2 diabetes. Clin. Endocrinol. 2007, 66, 27–34. [Google Scholar] [CrossRef]
- Praveen, E.P.; Khurana, M.L.; Kulshreshtha, B.; Prabhakaran, D.; Khadgawat, R.; Dwivedi, S.N.; Gupta, N.; Kumar, G.; Ammini, A.C. Plasma testosterone in adult normoglycaemic men: Impact of hyperinsulinaemia. Andrologia 2012, 44, 293–298. [Google Scholar] [CrossRef]
- Corbould, A. Effects of androgens on insulin action in women: Is androgen excess a component of femen metabolic syndrome? Diabetes Metab. Res. Rev. 2008, 24, 520–532. [Google Scholar] [CrossRef]
- Lutz, S.Z.; Wagner, R.; Fritsche, L.; Peter, A.; Rettig, I.; Willmann, C.; Fehlert, E.; Martus, P.; Todenhöfer, T.; Stefan, N.; et al. Sex-Specific Associations of Testosterone with Metabolic Traits. Front. Endocrinol. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Locatelli, V. Testosterone a key factor in women related metabolic syndrome. Obes. Rev. 2018, 19, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kimura, T.; Aoki, T.; Tsunekawa, K.; Araki, O.; Shoho, Y.; Nara, M.; Sumino, H.; Murakami, M. Fasting serum insulin levels and insulin resistance are associated with blood rheology in Japanese young adults without diabetes. J. Int. Med. Res. 2016, 44, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Razzak, R.A.; Alshaiji, A.F.; Qareeballa, A.A.; Mohamed, M.W.; Bagust, J.; Docherty, S. High-normal blood glucose levels may be associated with decreased spatial perception in young healthy adults. PLoS ONE 2018, 13, e0199051. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, T.; Harada, T.; Hashimoto, Y.; Takaeko, Y.; Kajikawa, M.; Kihara, Y.; Hida, E.; Chayama, K.; Goto, C.; Han, Y.; et al. Pre-impaired fasting glucose state is a risk factor for endothelial dysfunction: Flow-mediated Dilation Japan (FMD-J) study. BMJ Open Diabetes Res. Care 2020, 8, e001610. [Google Scholar] [CrossRef]
- Pervanidou, P.; Chrousos, G.P. Stress and obesity/metabolic syndrome in childhood and adolescence. Int. J. Pediatr. Obes. 2011, 6, 21–28. [Google Scholar] [CrossRef]
- Pantoja-Torres, B.; Toro-Huamanchumo, C.J.; Urrunaga-Pastor, D.; Guarnizo-Poma, M.; Lazaro-Alcantara, H.; Paico-Palacios, S.; Ranilla-Seguin, V.D.C.; Benites-Zapata, V.A. High triglycerides to HDL-cholesterol ratio is associated with insulin resistance in normal-weight healthy adults. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 13, 382–388. [Google Scholar] [CrossRef]
- Krawczyk, M.; Rumińska, M.; Sędek, E.W.; Majcher, A.; Pyrżak, B. Usefulness of the Triglycerides to High-Density Lipoprotein Cholesterol ratio (TG/HDL-C) in prediction of metabolic syndrome in Polish obese children and adolescents. Acta Biochim. Pol. 2018, 65, 605–611. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z.; Min, R.; Dong, Y.; Sun, Q.; Li, Y. Log (TG)/HDL-C ratio as a predictor of decreased islet beta cell function in patients with type 2 diabetes: 6-year cohort study. J. Diabetes 2015, 7, 689–698. [Google Scholar] [CrossRef]
- Messier, V.; Karelis, A.D.; Prud’Homme, D.; Primeau, V.; Brochu, M.; Rabasa-Lhoret, R. Identifying Metabolically Healthy but Obese Individuals in Sedentary Postmenopausal Women. Obesity 2010, 18, 911–917. [Google Scholar] [CrossRef]
- Cassader, M.; Gambino, R.; Ruiu, G.; Marena, S.; Bodoni, P.; Pagano, G. Postprandial triglyceride-rich lipoprotein changes in elderly and young subjects. Aging Milan Italy 1996, 8, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, M.A.; Rizk, N.M.; Fadel, A.S.; Kerkadi, A. Prevalence and Predictors of Insulin Resistance in Non-Obese Healthy Young Females in Qatar. Int. J. Environ. Res. Public Health 2020, 17, 5088. [Google Scholar] [CrossRef] [PubMed]
| Young Males (n = 505) | Young Females (n = 280) | Middle-Aged Males (n = 127) | Middle-Aged Females (n = 244) | |
|---|---|---|---|---|
| Age (year) | 23.7 ± 1.7 | 23.3 ± 1.4 | 68.3 ± 7.5 * | 65.1 ± 6.4 +,‡ |
| Height (cm) | 172.4 ± 5.7 | 159.7 ± 5.7 * | 163.3 ± 5.8 * | 152.2 ± 5.9 +,‡ |
| Weight (kg) | 65.2 ± 8.9 | 50.8 ± 5.8 * | 62.8 ± 7.9 * | 52.5 ± 7.2 +,‡ |
| BMI (kg/m2) | 21.9 ± 2.7 | 19.9 ± 1.8 * | 23.5 ± 2.4 * | 22.6 ± 2.8 +,‡ |
| PG0 (mmol/L) | 5.2 ± 0.4 | 4.9 ± 0.3 * | 5.9 ± 0.8 * | 5.6 ± 0.6 +,‡ |
| AUCg (mmol/L·min) | 768 ± 130 | 713 ± 137 * | 1078 ± 275 * | 1000 ± 247 +,‡ |
| IRI0 (pmol/L) | 49 ± 29 | 47 ± 22 | 35 ± 21 * | 35 ± 23 + |
| AUCi (pmol/L·min) | 35,588 ± 20,485 | 41,019 ± 20,112 * | 41,730 ± 24,395 * | 42,447 ± 26,009 |
| HbA1c (%) | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.5 ± 0.6 * | 5.4 ± 0.4 + |
| HOMA-IR | 1.6 ± 1.1 | 1.4 ± 0.7 * | 1.3 ± 0.8 * | 1.2 ± 0.9 + |
| HOMA-β | 81.0 ± 41.5 | 92.7 ± 39.4 * | 44.5 ± 29.9 * | 48.7 ± 30.9 + |
| Matsuda index | 7.7 ± 3.9 | 7.2 ± 3.2 | 6.9 ± 3.8 * | 7.1 ± 3.6 |
| Insulinogenic index | 1.4 ± 2.7 | 1.9 ± 6.3 | 0.8 ± 0.8 * | 1.0 ± 2.2 + |
| Disposition index | 9.4 ± 20.4 | 12.0 ± 39.8 | 5.1 ± 6.9 * | 7.8 ± 23.2 + |
| TG (mmol/L) | 2.1 ± 1.1 | 1.6 ± 0.7 * | 3.1 ± 1.7 * | 2.8 ± 1.2 + |
| HDL-C (mmol/L) | 1.5 ± 0.3 | 1.8 ± 0.3 * | 1.5 ± 0.8 | 1.8 ± 0.6 ‡ |
| LDL-C (mmol/L) | 2.6 ± 0.7 | 2.5 ± 0.6 | 3.2 ± 0.9 * | 3.2 ± 1.0 + |
| TG/HDL-C | 1.49 ± 1.02 | 0.95 ± 0.45 * | 2.46 ± 1.88 * | 1.74 ± 1.03 +,‡ |
| LDL-C/HDL-C | 1.79 ± 0.7 | 1.45 ± 0.45 * | 2.42 ± 0.98 * | 1.95 ± 0.86 +,‡ |
| Young Males Number (%) | Young Females Number (%) | Middle-Aged Males Number (%) | Middle-Aged Females Number (%) | |
|---|---|---|---|---|
| NGT | 414 (82.0%) | 264 (94.3%) * | 32 (25.2%) * | 97 (39.8%) +,‡ |
| High-normal FPG | 77 (15.2%) | 11 (3.9) * | 27 (21.3%) * | 47 (19.3%) + |
| IFG | 2 (0.4%) | 0 (0%) | 14 (11.0%) * | 16 (6.6%) + |
| IGT | 10 (2.0%) | 5 (0.2%) | 25 (19.7%) * | 51 (20.9%) + |
| CGI | 1 (0.2%) | 0 (0%) | 13 (10.2%) * | 8 (3.3%) +,‡ |
| Diabetes | 1 (0.2%) | 0 (0%) | 16 (12.6%) * | 25 (10.2%) + |
| Total | 505 (100%) | 280 (100%) | 127 (100%) | 244 (100%) |
| Young Males | ||||||||
| Insulinogenic Index | HOMA-β | HOMA-IR | Matsuda Index | |||||
| Parameters | β | P | β | P | β | P | β | P |
| BMI | 0.346 | 0.000 | 0.375 | 0.000 | −0.099 | 0.035 | ||
| HbA1c | 0.196 | 0.000 | ||||||
| TG | −0.227 | 0.034 | −0.144 | 0.003 | ||||
| HDL-C | 0.115 | 0.034 | ||||||
| TG/HDL-C | 0.425 | 0.000 | ||||||
| Young Females | ||||||||
| Insulinogenic Index | HOMA-β | HOMA-IR | Matsuda Index | |||||
| Parameters | β | P | β | P | β | P | β | P |
| BMI | ||||||||
| HbA1c | −0.126 | 0.037 | ||||||
| TG | 0.212 | 0.000 | ||||||
| HDL-C | 0.130 | 0.032 | ||||||
| TG/HDL-C | ||||||||
| Middle-Aged Males | ||||||||
| Insulinogenic Index | HOMA-β | HOMA-IR | Matsuda Index | |||||
| Parameters | β | P | β | P | b | P | β | P |
| BMI | 0.229 | 0.006 | 0.308 | 0.000 | −0.369 | 0.000 | ||
| HbA1c | −0.253 | 0.001 | −0.280 | 0.001 | ||||
| TG | ||||||||
| HDL-C | 0.197 | 0.018 | ||||||
| TG/HDL-C | 0.505 | 0.000 | 0.229 | 0.008 | 0.231 | 0.006 | ||
| Middle-Aged Females | ||||||||
| Insulinogenic Index | HOMA-β | HOMA-IR | Matsuda Index | |||||
| Parameters | β | P | β | P | b | P | β | P |
| BMI | 0.412 | 0.000 | 0.458 | 0.000 | −0.408 | 0.000 | ||
| HbA1c | −0.229 | 0.000 | −0.171 | 0.002 | ||||
| TG | ||||||||
| HDL-C | 0.132 | 0.043 | ||||||
| TG/HDL-C | 0.291 | 0.000 | 0.180 | 0.001 | −0.194 | 0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, A.; Kimura, T.; Tsunekawa, K.; Shoho, Y.; Yanagawa, Y.; Araki, O.; Aoki, T.; Ogiwara, T.; Murakami, M. Age-Related Sex Differences in Glucose Tolerance by 75 g Oral Glucose Tolerance Test in Japanese. Nutrients 2022, 14, 4868. https://doi.org/10.3390/nu14224868
Yoshida A, Kimura T, Tsunekawa K, Shoho Y, Yanagawa Y, Araki O, Aoki T, Ogiwara T, Murakami M. Age-Related Sex Differences in Glucose Tolerance by 75 g Oral Glucose Tolerance Test in Japanese. Nutrients. 2022; 14(22):4868. https://doi.org/10.3390/nu14224868
Chicago/Turabian StyleYoshida, Akihiro, Takao Kimura, Katsuhiko Tsunekawa, Yoshifumi Shoho, Yoshimaro Yanagawa, Osamu Araki, Tomoyuki Aoki, Takayuki Ogiwara, and Masami Murakami. 2022. "Age-Related Sex Differences in Glucose Tolerance by 75 g Oral Glucose Tolerance Test in Japanese" Nutrients 14, no. 22: 4868. https://doi.org/10.3390/nu14224868
APA StyleYoshida, A., Kimura, T., Tsunekawa, K., Shoho, Y., Yanagawa, Y., Araki, O., Aoki, T., Ogiwara, T., & Murakami, M. (2022). Age-Related Sex Differences in Glucose Tolerance by 75 g Oral Glucose Tolerance Test in Japanese. Nutrients, 14(22), 4868. https://doi.org/10.3390/nu14224868






