Effects of Ambient Particulate Matter (PM2.5) Exposure on Calorie Intake and Appetite of Outdoor Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. PM2.5 Measurements
2.2. Study Population
2.3. Dietary Intakes
2.4. Statistical Analysis
3. Results
3.1. PM2.5 Data
3.2. Characteristics of Participant
3.3. Simplified Nutrition Appetite Questionnaire (SNAQ)
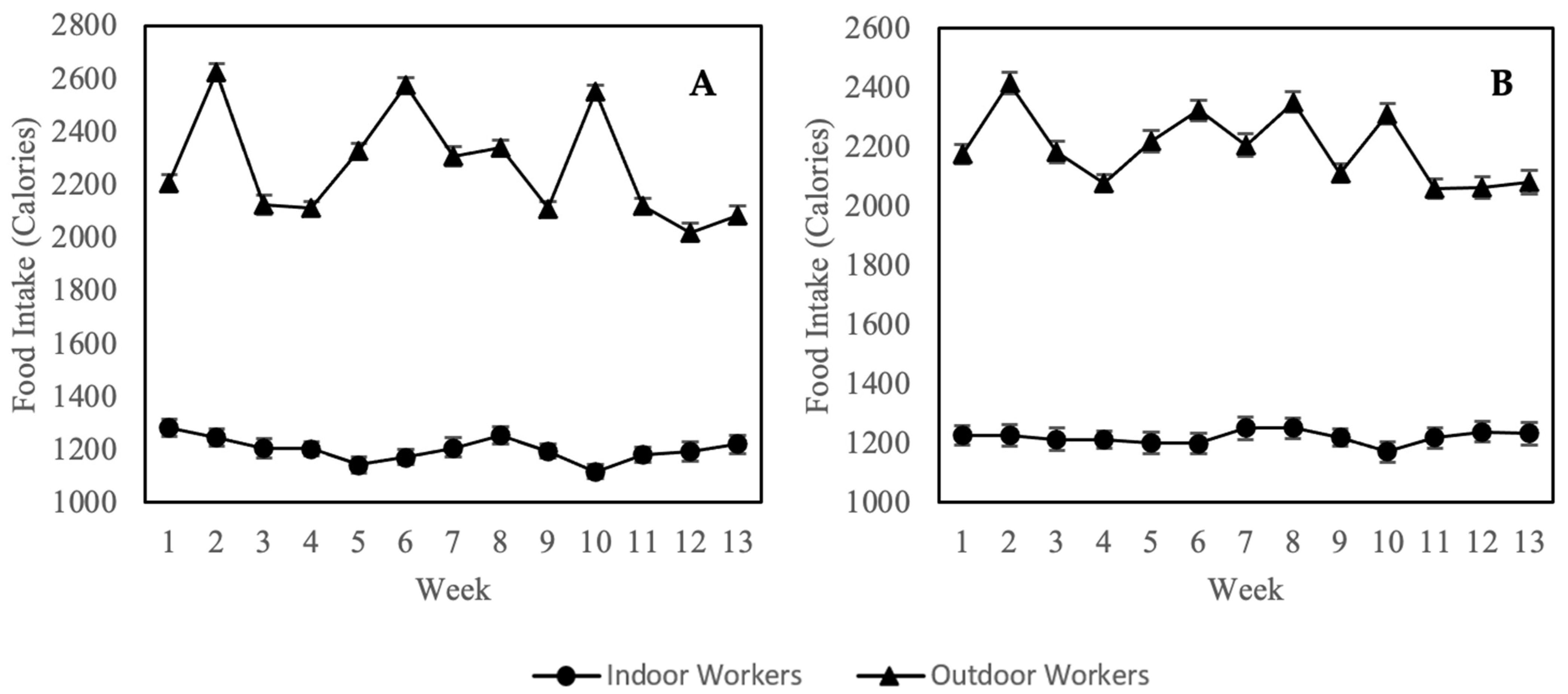
3.4. Calorie Intake (24-Hour Diet Recall)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, R.; Kan, H. Air pollution, disease burden, and health economic loss in China. Ambient. Air Pollut. Health Impact China 2017, 1017, 233–242. [Google Scholar]
- GBD MAPS Working Group. Burden of Disease Attributable to Major Air Pollution Sources in India; Special Report 21; Health Effects Institute: Boston, MA, USA, 2018. [Google Scholar]
- Kitamori, K.; Manders, T.; Dellink, R.; Tabeau, A. OECD Environmental Outlook to 2050: The Consequences of Inaction; Report No.: 9264122168; OECD: Paris, France, 2012. [Google Scholar]
- Ostro, B.; Spadaro, J.V.; Gumy, S.; Mudu, P.; Awe, Y.; Forastiere, F.; Peters, A. Assessing the recent estimates of the global burden of disease for ambient air pollution: Methodological changes and implications for low-and middle-income countries. Environ. Res. 2018, 166, 713–725. [Google Scholar] [CrossRef]
- Kaur, R.; Pandey, P. Air pollution, climate change, and human health in Indian cities: A Brief review. Front. Sustain. Cities 2021, 3, 705131. [Google Scholar] [CrossRef]
- Sundram, T.K.M.; Tan, E.S.S.; Cheah, S.C.; Lim, H.S.; Seghayat, M.S.; Bustami, N.A.; Tan, C.K. Impacts of particulate matter (PM2.5) on the health status of outdoor workers: Observational evidence from Malaysia. Environ. Sci. Pollut. Res. 2022, 29, 71064–71074. [Google Scholar] [CrossRef]
- Van, D.-A.; Vu, T.V.; Nguyen, T.-H.T.; Vo, L.-H.T.; Le, N.H.; Nguyen, P.H.T.; Pongkiatkul, P.; Ly, B.T. A Review of Characteristics, Causes, and Formation Mechanisms of Haze in Southeast Asia. Curr. Pollut. Rep. 2022, 8, 201–220. [Google Scholar] [CrossRef]
- Aguilera, R.; Corringham, T.; Gershunov, A.; Benmarhnia, T. Wildfire smoke impacts respiratory health more than fine particles from other sources: Observational evidence from Southern California. Nat. Commun. 2021, 12, 1493. [Google Scholar] [CrossRef]
- Sulong, N.A.; Latif, M.T.; Khan, M.F.; Amil, N.; Ashfold, M.J.; Wahab, M.I.A.; Chan, K.M.; Sahani, M. Source apportionment and health risk assessment among specific age groups during haze and non-haze episodes in Kuala Lumpur, Malaysia. Sci. Total Environ. 2017, 601, 556–570. [Google Scholar] [CrossRef]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69. [Google Scholar]
- Zeng, X.; Liu, D.; Wu, W. PM2. 5 exposure and pediatric health in e-waste dismantling areas. Environ. Toxicol. Pharmacol. 2022, 89, 103774. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Qi, W.; Zhao, T.; Zhang, L.; Zhou, L.; Ye, L. Adverse effects of PM2.5 on cardiovascular diseases. Rev. Environ. Health 2022, 37, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Warren, S.H.; Krantz, Q.T.; King, C.; Jaskot, R.; Preston, W.T.; George, B.J.; Hays, M.D.; Landis, M.S.; Higuchi, M. Mutagenicity and lung toxicity of smoldering vs. flaming emissions from various biomass fuels: Implications for health effects from wildland fires. Environ. Health Perspect. 2018, 126, 017011. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.; Rochereau, T.; Maesano, C.N.; Com-Ruelle, L.; Annesi-Maesano, I. Long-Term Effect of Outdoor Air Pollution on Mortality and Morbidity: A 12-Year Follow-Up Study for Metropolitan France. Int. J. Environ. Res. Public Health 2018, 15, 2487. [Google Scholar] [CrossRef]
- Mahmud, N.; Noor Ani, A.; Noraida, M.; Lim, K.; Mohd Azza, A. Prevalence and Associated Factor of Walking Disability among Adults: Finding from National Health & Morbidity Survey 2015 (NHMS 2015). Prim. Care Epidemiol. Glob. Health 2018, 1, 13–18. [Google Scholar]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Mohd-Sidik, S.; Lekhraj, R.; Foo, C.N. Prevalence, Associated Factors and Psychological Determinants of Obesity among Adults in Selangor, Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef]
- Collaboration NRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Ma, S.; Xi, B.; Yang, L.; Sun, J.; Zhao, M.; Bovet, P. Trends in the prevalence of overweight, obesity, and abdominal obesity among Chinese adults between 1993 and 2015. Int. J. Obes. 2021, 45, 427–437. [Google Scholar] [CrossRef]
- Singh, P.; Rai, S.N. Factors affecting obesity and its treatment. Obes. Med. 2019, 16, 100140. [Google Scholar] [CrossRef]
- Crovesy, L.; Rosado, E.L. Interaction between genes involved in energy intake regulation and diet in obesity. Nutrition 2019, 67, 110547. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, O.; Wang, H.; Wang, S.; Zhang, P. The effect of air pollution on body weight and obesity: Evidence from China. J. Dev. Econ. 2020, 145, 102461. [Google Scholar] [CrossRef]
- An, R.; Ji, M.; Yan, H.; Guan, C. Impact of ambient air pollution on obesity: A systematic review. Int. J. Obes. 2018, 42, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, X.; Zhong, M.; Hotchkiss, I.P.; Lewandowski, R.P.; Wagner, J.G.; Bramble, L.A.; Yang, Y.; Wang, A.; Harkema, J.R. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part. Fibre Toxicol. 2011, 8, 20. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Bai, Y.; Wang, T.-Y.; Rao, X.; Wang, A.; Sun, L.; Ying, Z.; Gushchina, L.; Maiseyeu, A. Air pollution–mediated susceptibility to inflammation and insulin resistance: Influence of CCR2 pathways in mice. Environ. Health Perspect. 2014, 122, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Corral, C.M.; Alderete, T.L.; Habre, R.; Berhane, K.; Lurmann, F.W.; Weigensberg, M.J.; Goran, M.; Gilliland, F. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr. Obes. 2018, 13, 54–62. [Google Scholar] [CrossRef]
- Chen, Z.; Herting, M.M.; Chatzi, L.; Belcher, B.R.; Alderete, T.; McConnell, R.; Gilliland, F.D. Regional and traffic-related air pollutants are associated with higher consumption of fast food and trans fat among adolescents. Am. J. Clin. Nutr. 2019, 109, 99–108. [Google Scholar] [CrossRef]
- Bolton, J.L.; Smith, S.H.; Huff, N.C.; Gilmour, M.I.; Foster, W.M.; Auten, R.L.; Bilbo, S.D. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012, 26, 4743–4754. [Google Scholar] [CrossRef]
- McConnell, R.; Gilliland, F.; Goran, M.; Allayee, H.; Hricko, A.; Mittelman, S. Does near-roadway air pollution contribute to childhood obesity? Pediatr. Obes. 2016, 11, 1. [Google Scholar] [CrossRef]
- Ustulin, M.; Park, S.Y.; Chin, S.O.; Chon, S.; Woo, J.-T.; Rhee, S.Y. Air pollution has a significant negative impact on intentional efforts to lose weight: A global scale analysis. Diabetes Metab. J. 2018, 42, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, S.; Dales, R.; Leech, J.; Liu, L. The influence of air pollution on cardiovascular and pulmonary function and exercise capacity: Canadian Health Measures Survey (CHMS). Environ. Res. 2011, 111, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Voss, J.D.; Knight, B. The association of ambient air pollution and physical inactivity in the United States. PLoS ONE 2014, 9, e90143. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Zhang, S.; Ji, M.; Guan, C. Impact of ambient air pollution on physical activity among adults: A systematic review and meta-analysis. Perspect. Public Health 2018, 138, 111–121. [Google Scholar] [CrossRef]
- Shabani, D.; Islami, P.; Ibraimi, Z.; Islami, H. Manifestation of Bronchial Reactivity in Mining Workers who are Exposed to different Pollutants. Toxicol. Int. 2020, 26, 137–146. [Google Scholar]
- Ali, M.U.; Rashid, A.; Yousaf, B.; Kamal, A. Health outcomes of road-traffic pollution among exposed roadside workers in Rawalpindi City, Pakistan. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1330–1339. [Google Scholar] [CrossRef]
- Brucker, N.; do Nascimento, S.N.; Bernardini, L.; Charão, M.F.; Garcia, S.C. Biomarkers of exposure, effect, and susceptibility in occupational exposure to traffic-related air pollution: A review. J. Appl. Toxicol. 2020, 40, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.-M.; Zhao, L.-Y.; Mu, X.; Li, Y.-J.; Mao, X.-X.; Huang, T.; Ma, J.-M.; Gao, H. Pollution Characteristics and Occupational Exposure Risk of Heavy Metals in Indoor and Outdoor Ambient Particles at a Scaled Electronic Waste Dismantling Plant, Northwest China. Huan Jing Ke Xue Huanjing Kexue 2019, 40, 1101–1110. [Google Scholar] [PubMed]
- Dana, S.H.F.; Esha, I.; Yunus, F.; Adrianison, A.; Saad, A.; Restilla, R. Risk Factors Affecting Respiratory Symptoms and Impaired Lung Function of Palm Oil Mill Workers in the District of Kandis. J. Respirologi Indones. 2021, 41, 180–186. [Google Scholar] [CrossRef]
- Ji, W.; Liu, C.; Liu, Z.; Wang, C.; Li, X. Concentration, composition, and exposure contributions of fine particulate matter on subway concourses in China. Environ. Pollut. 2021, 275, 116627. [Google Scholar] [CrossRef]
- Mohammad, W.H.B.W. Exposure of Inhalable Dust and Respiratory Symptoms among Workers in Construction Industry. Bachelor’s Thesis, University of Malaysia Pahang, Pahang, Malaysia, 2019. [Google Scholar]
- Putri Anis Syahira, M.; Karmegam, K.; Nur Athirah Diyana, M.; Irniza, R.; Shamsul Bahri, M.; Vivien, H.; Nurul Maizura, H.; Sivasankar, S. Impacts of PM2.5 on respiratory system among traffic policemen. Work 2020, 66, 25–29. [Google Scholar] [CrossRef]
- Jayaratne, R.; Liu, X.; Ahn, K.H.; Asumadu-Sakyi, A.; Fisher, G.; Gao, J.; Mabon, A.; Mazaheri, M.; Mullins, B.; Nyaku, M. Low-cost PM2.5 sensors: An assessment of their suitability for various applications. Aerosol Air Qual. Res. 2020, 20, 520–532. [Google Scholar] [CrossRef]
- Gao, M.; Cao, J.; Seto, E. A distributed network of low-cost continuous reading sensors to measure spatiotemporal variations of PM2.5 in Xi’an, China. Environ. Pollut. 2015, 199, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.K.; Sreekanth, V.; Salmon, M.; Tonne, C.; Marshall, J.D. Use of spatiotemporal characteristics of ambient PM2.5 in rural South India to infer local versus regional contributions. Environ. Pollut. 2018, 239, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.T.; Othman, M.; Idris, N.; Juneng, L.; Abdullah, A.M.; Hamzah, W.P.; Khan, M.F.; Sulaiman, N.M.N.; Jewaratnam, J.; Aghamohammadi, N. Impact of regional haze towards air quality in Malaysia: A review. Atmos. Environ. 2018, 177, 28–44. [Google Scholar] [CrossRef]
- Mathey, M.; de Jong, N.; de Groot, L.C.; de Graaf, C.; van Staveren, W.A. Assessing appetite in Dutch elderly with the Appetite, Hunger and Sensory Perception (AHSP) questionnaire. J. Nutr. Health Aging 2001, 5, 22–28. [Google Scholar]
- Hanisah, R.; Shahar, S.; Lee, F. Validation of screening tools to assess appetite among geriatric patients. J. Nutr. Health Aging 2012, 16, 660–665. [Google Scholar] [CrossRef]
- Lau, S.; Pek, K.; Chew, J.; Lim, J.P.; Ismail, N.H.; Ding, Y.Y.; Cesari, M.; Lim, W.S. The Simplified Nutritional Appetite Questionnaire (SNAQ) as a Screening Tool for Risk of Malnutrition: Optimal Cutoff, Factor Structure, and Validation in Healthy Community-Dwelling Older Adults. Nutrients 2020, 12, 2885. [Google Scholar] [CrossRef]
- Gibson, R.S.; Ferguson, E.L. An Interactive 24-Hour Recall for Assessing the Adequacy of Iron and Zinc Intakes in Developing Countries; ILSI Press: Washington, DC, USA, 1999. [Google Scholar]
- Zalilah, M., Jr.; Mirnalini, K.; Safiah, M.; Tahir, A.; Haslinda, S.; MY, K.Z.; Hasyami, S.; Normah, H.; Fatimah, A. Daily Energy Intake from Meals and Afternoon Snacks: Findings from the Malaysian Adults Nutrition Survey (MANS). Malays. J. Nutr. 2008, 14, 41–55. [Google Scholar]
- Gautam, S.; Yadav, A.; Tsai, C.-J.; Kumar, P. A review on recent progress in observations, sources, classification and regulations of PM2.5 in Asian environments. Environ. Sci. Pollut. Res. 2016, 23, 21165–21175. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global update. World Health Organization, Geneva. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/78638E.2006;90038 (accessed on 8 September 2022).
- Huijnen, V.; Wooster, M.; Kaiser, J.W.; Gaveau, D.L.A.; Flemming, J.; Parrington, M.; Inness, A.; Murdiyarso, D.; Main, B.; van Weele, M. Fire carbon emissions over maritime southeast Asia in 2015 largest since 1997. Sci. Rep. 2016, 6, 26886. [Google Scholar] [CrossRef]
- Fujii, Y.; Tohno, S.; Amil, N.; Latif, M.T. Quantitative assessment of source contributions to PM2. 5 on the west coast of Peninsular Malaysia to determine the burden of Indonesian peatland fire. Atmos. Environ. 2017, 171, 111–117. [Google Scholar] [CrossRef]
- McCarter, C.; Wilkinson, S.; Moore, P.; Waddington, J. Ecohydrological trade-offs from multiple peatland disturbances: The interactive effects of drainage, harvesting, restoration and wildfire in a southern Ontario bog. J. Hydrol. 2021, 601, 126793. [Google Scholar] [CrossRef]
- Dahari, N.; Latif, M.T.; Muda, K.; Hussein, N. Influence of meteorological variables on suburban atmospheric PM2.5 in the southern region of peninsular Malaysia. Aerosol Air Qual. Res. 2020, 20, 14–25. [Google Scholar] [CrossRef]
- Ruiz Estrada, M.A.; Swee Kheng, K.; Ating, R. The Evaluation of Obesity in Malaysia. 2019. Available online: https://ssrn.com/abstract=3455108 (accessed on 13 September 2022).
- Lim, Z.-M.; Chie, Q.-T.; Teh, L.-K. Influence of dopamine receptor gene on eating behaviour and obesity in Malaysia. Meta Gene 2020, 25, 100736. [Google Scholar] [CrossRef]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Wang, Z.; Usyk, M.; Sotres-Alvarez, D.; Daviglus, M.L.; Schneiderman, N.; Talavera, G.A.; Gellman, M.D.; Thyagarajan, B.; Moon, J.-Y. Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biol. 2019, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, R.T.; Kwong, J.C.; Villeneuve, P.J.; Goldberg, M.S.; Brook, R.D.; van Donkelaar, A.; Jerrett, M.; Martin, R.V.; Brook, J.R. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ. Health Perspect. 2013, 121, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Eze, I.; Hemkens, L.; Bucher, H.C.; Hoffmann, B.; Schindler, C.; Künzli, N.; Schikowski, T.; Probst-Hensch, N.M. Association between ambient air pollution and diabetes mellitus in Europe and North America: A systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 381–389. [Google Scholar] [CrossRef]
- Jiang, S.; Bo, L.; Gong, C.; Du, X.; Kan, H.; Xie, Y.; Song, M.; Zhao, J. Traffic-related air pollution is associated with cardio-metabolic biomarkers in general residents. Int. Arch. Occup. Environ. Health 2016, 89, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Paciência, I.; Rufo, J.C.; Silva, D.; Martins, C.; Mendes, F.; Farraia, M.; Delgado, L.; de Oliveira Fernandes, E.; Padrão, P.; Moreira, P. Exposure to indoor endocrine-disrupting chemicals and childhood asthma and obesity. Allergy 2019, 74, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental endocrine-disrupting chemical exposure: Role in non-communicable diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef] [PubMed]
- Morin, V.; Hozer, F.; Costemale-Lacoste, J.-F. The effects of ghrelin on sleep, appetite, and memory, and its possible role in depression: A review of the literature. L’encephale 2018, 44, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.; Bowers, C.Y.; Broglio, C.Y. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Keith, S.W.; Redden, D.T.; Katzmarzyk, P.T.; Boggiano, M.M.; Hanlon, E.C.; Benca, R.M.; Ruden, D.; Pietrobelli, A.; Barger, J.L.; Fontaine, K. Putative contributors to the secular increase in obesity: Exploring the roads less traveled. Int. J. Obes. 2006, 30, 1585–1594. [Google Scholar] [CrossRef]
- Thomson, E.M. Air pollution, stress, and allostatic load: Linking systemic and central nervous system impacts. J. Alzheimer’s Dis. 2019, 69, 597–614. [Google Scholar] [CrossRef]
- Miller, J.G.; Gillette, J.S.; Manczak, E.M.; Kircanski, K.; Gotlib, I.H. Fine particle air pollution and physiological reactivity to social stress in adolescence: The moderating role of anxiety and depression. Psychosom. Med. 2019, 81, 641. [Google Scholar] [CrossRef]
- Schellekens, H.; Finger, B.C.; Dinan, T.G.; Cryan, J.F. Ghrelin signalling and obesity: At the interface of stress, mood and food reward. Pharmacol. Ther. 2012, 135, 316–326. [Google Scholar] [CrossRef]
- Blundell, J.E.; Gibbons, C.; Beaulieu, K.; Casanova, N.; Duarte, C.; Finlayson, G.; Stubbs, R.J.; Hopkins, M. The drive to eat in homo sapiens: Energy expenditure drives energy intake. Physiol. Behav. 2020, 219, 112846. [Google Scholar] [CrossRef]
- Mandic, I.; Ahmed, M.; Rhind, S.; Goodman, L.; L’Abbe, M.; Jacobs, I. The effects of exercise and ambient temperature on dietary intake, appetite sensation, and appetite regulating hormone concentrations. Nutr. Metab. 2019, 16, 29. [Google Scholar] [CrossRef]
- Chen, S.; Oliva, P.; Zhang, P. Air Pollution and Mental Health: Evidence from China; National Bureau of Economic Research: Cambridge, MA, USA, 2018. [Google Scholar]
- Zhao, J.; Zuo, L.; Sun, J.; Su, C.; Wang, H. Trends and Urban-Rural Disparities of Energy Intake and Macronutrient Composition among Chinese Children: Findings from the China Health and Nutrition Survey (1991 to 2015). Nutrients 2021, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Bekki, K.; Ito, T.; Yoshida, Y.; He, C.; Arashidani, K.; He, M.; Sun, G.; Zeng, Y.; Sone, H.; Kunugita, N.; et al. PM2.5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways. Environ. Toxicol. Pharmacol. 2016, 45, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Nääv, Å.; Erlandsson, L.; Isaxon, C.; Åsander Frostner, E.; Ehinger, J.; Sporre, M.K.; Krais, A.M.; Strandberg, B.; Lundh, T.; Elmér, E. Urban PM2.5 induces cellular toxicity, hormone dysregulation, oxidative damage, inflammation, and mitochondrial interference in the HRT8 trophoblast cell line. Front. Endocrinol. 2020, 11, 75. [Google Scholar] [CrossRef]
- Sun, J.; Yu, J.; Shen, Z.; Niu, X.; Wang, D.; Wang, X.; Xu, H.; Chuang, H.-C.; Cao, J.; Ho, K.-F. Oxidative stress–inducing effects of various urban PM2.5 road dust on human lung epithelial cells among 10 Chinese megacities. Ecotoxicol. Environ. Saf. 2021, 224, 112680. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Campolim, C.M.; Weissmann, L.; Ferreira, C.K.d.O.; Zordão, O.P.; Dornellas, A.P.S.; de Castro, G.; Zanotto, T.M.; Boico, V.F.; Quaresma, P.G.F.; Lima, R.P.A.; et al. Short-term exposure to air pollution (PM2.5) induces hypothalamic inflammation, and long-term leads to leptin resistance and obesity via Tlr4/Ikbke in mice. Sci. Rep. 2020, 10, 10160. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, Z.; Kim, H.; Yang, Z.; Zhang, G.; Shi, X.; Sun, F.; Peng, C.; Ding, Y.; Wang, A.; et al. Inhalation Exposure to PM2.5 Counteracts Hepatic Steatosis in Mice Fed High-fat Diet by Stimulating Hepatic Autophagy. Sci. Rep. 2017, 7, 16286. [Google Scholar] [CrossRef]
- Du, Z.; Lin, L.; Li, Y.; Sun, M.; Liang, Q.; Sun, Z.; Duan, J. Combined exposure to PM2.5 and high-fat diet facilitate hepatic lipid metabolism disorders via ROS/miR-155/PPARγ pathway. Free. Radic. Biol. Med. 2022, 190, 16–27. [Google Scholar] [CrossRef]
- Costa Beber, L.C.; da Silva, M.O.A.F.; Dos Santos, A.B.; Mai, A.S.; Goettems-Fiorin, P.B.; Frizzo, M.N.; Hirsch, G.E.; Ludwig, M.S.; Heck, T.G. The association of subchronic exposure to low concentration of PM2.5 and high-fat diet potentiates glucose intolerance development, by impairing adipose tissue antioxidant defense and eHSP72 levels. Environ. Sci. Pollut. Res. 2020, 27, 32006–32016. [Google Scholar] [CrossRef]
- Long, M.-h.; Zhang, C.; Xu, D.-q.; Fu, W.-l.; Gan, X.-d.; Li, F.; Wang, Q.; Xia, W.; Xu, D.-g. PM2.5 aggravates diabetes via the systemically activated IL-6-mediated STAT3/SOCS3 pathway in rats’ liver. Environ. Pollut. 2020, 256, 113342. [Google Scholar] [CrossRef]
- Liu, J.; Su, X.; Lu, J.; Ning, J.; Lin, M.; Zhou, H. PM2.5 induces intestinal damage by affecting gut microbiota and metabolites of rats fed a high-carbohydrate diet. Environ. Pollut. 2021, 279, 116849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gan, X.; Li, F.; Chen, Y.; Fu, W.; Zhu, X.; Xu, D.; Long, M.; Xu, D. PM2.5 Exposure Induces More Serious Apoptosis of Cardiomyocytes Mediated by Caspase3 through JNK/ P53 Pathway in Hyperlipidemic Rats. Int. J. Biol. Sci. 2019, 15, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Jia, R.; Wei, M.; Meng, X.; Zhang, X.; Du, R.; Sun, W.; Wang, L.; Song, L. Oxidative stress activates Ryr2-Ca2+ and apoptosis to promote PM2.5-induced heart injury of hyperlipidemia mice. Ecotoxicol. Environ. Saf. 2022, 232, 113228. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, H.; Ge, P.; Hu, T.; Zhang, Y.; Zhang, X.; Xu, B.; Wang, B.; Xie, J. PM2.5 promotes plaque vulnerability at different stages of atherosclerosis and the formation of foam cells via TLR4/MyD88/NFκB pathway. Ecotoxicol. Environ. Saf. 2019, 176, 76–84. [Google Scholar] [CrossRef]
- Soleimanifar, N.; Nicknam, M.H.; Bidad, K.; Jamshidi, A.R.; Mahmoudi, M.; Mostafaei, S.; Hosseini-Khah, Z.; Nikbin, B. Effect of food intake and ambient air pollution exposure on ankylosing spondylitis disease activity. Adv. Rheumatol. 2019, 59, 9. [Google Scholar] [CrossRef]
- Pon, L.W.; Kandiah, M.; Taib, M.N.M. Body image perception, dietary practices and physical activity of overweight and normal weight Malaysian female adolescents. Malays. J. Nutr. 2004, 10, 131–147. [Google Scholar] [PubMed]
- Badrin, S.; Daud, N.; Ismail, S.B. Body weight perception and weight loss practices among private college students in Kelantan State, Malaysia. Korean J. Fam. Med. 2018, 39, 355. [Google Scholar] [CrossRef]
- Lee, G.; Han, K.; Kim, H. Risk of mental health problems in adolescents skipping meals: The Korean National Health and Nutrition Examination Survey 2010 to 2012. Nurs. Outlook 2017, 65, 411–419. [Google Scholar] [CrossRef]


| Characteristic | Indoor Workers | Outdoor Workers | Overall |
|---|---|---|---|
| Gender (n/%) | |||
| Male | 75 (37.7) | 201 (95.7) | 276 (67.5) |
| Female | 124 (62.3) | 9 (4.3) | 133 (32.5) |
| Age (years) (n/%) | |||
| ≤30 | 106 (53.3) | 138 (65.7) | 244 (59.7) |
| 31–40 | 62 (31.2) | 49 (23.3) | 111 (27.1) |
| 41–50 | 13 (6.5) | 22 (10.5) | 35 (8.6) |
| >50 | 18 (9.0) | 1 (0.5) | 19 (4.6) |
| Smoking Habit (n/%) | |||
| Yes | 5 (2.5) | 47 (22.4) | 52 (12.7) |
| No | 194 (97.5) | 163 (77.6) | 357 (87.3) |
| Variables | PM2.5 Concentration | ||
|---|---|---|---|
| Cycle 1 | Cycle 2 | ||
| Appetite | Indoor Workers | 0.134 | 0.093 |
| Outdoor Workers | 0.541 * | 0.453 * | |
| Calorie Intake | Indoor Workers | 0.179 | 0.087 |
| Outdoor Workers | 0.493 * | 0.581 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundram, T.K.M.; Tan, E.S.S.; Lim, H.S.; Amini, F.; Bustami, N.A.; Tan, P.Y.; Rehman, N.; Ho, Y.B.; Tan, C.K. Effects of Ambient Particulate Matter (PM2.5) Exposure on Calorie Intake and Appetite of Outdoor Workers. Nutrients 2022, 14, 4858. https://doi.org/10.3390/nu14224858
Sundram TKM, Tan ESS, Lim HS, Amini F, Bustami NA, Tan PY, Rehman N, Ho YB, Tan CK. Effects of Ambient Particulate Matter (PM2.5) Exposure on Calorie Intake and Appetite of Outdoor Workers. Nutrients. 2022; 14(22):4858. https://doi.org/10.3390/nu14224858
Chicago/Turabian StyleSundram, Thavin Kumar Mathana, Eugenie Sin Sing Tan, Hwee San Lim, Farahnaz Amini, Normina Ahmad Bustami, Pui Yee Tan, Navedur Rehman, Yu Bin Ho, and Chung Keat Tan. 2022. "Effects of Ambient Particulate Matter (PM2.5) Exposure on Calorie Intake and Appetite of Outdoor Workers" Nutrients 14, no. 22: 4858. https://doi.org/10.3390/nu14224858
APA StyleSundram, T. K. M., Tan, E. S. S., Lim, H. S., Amini, F., Bustami, N. A., Tan, P. Y., Rehman, N., Ho, Y. B., & Tan, C. K. (2022). Effects of Ambient Particulate Matter (PM2.5) Exposure on Calorie Intake and Appetite of Outdoor Workers. Nutrients, 14(22), 4858. https://doi.org/10.3390/nu14224858






