Biotin Enhances Testosterone Production in Mice and Their Testis-Derived Cells
Abstract
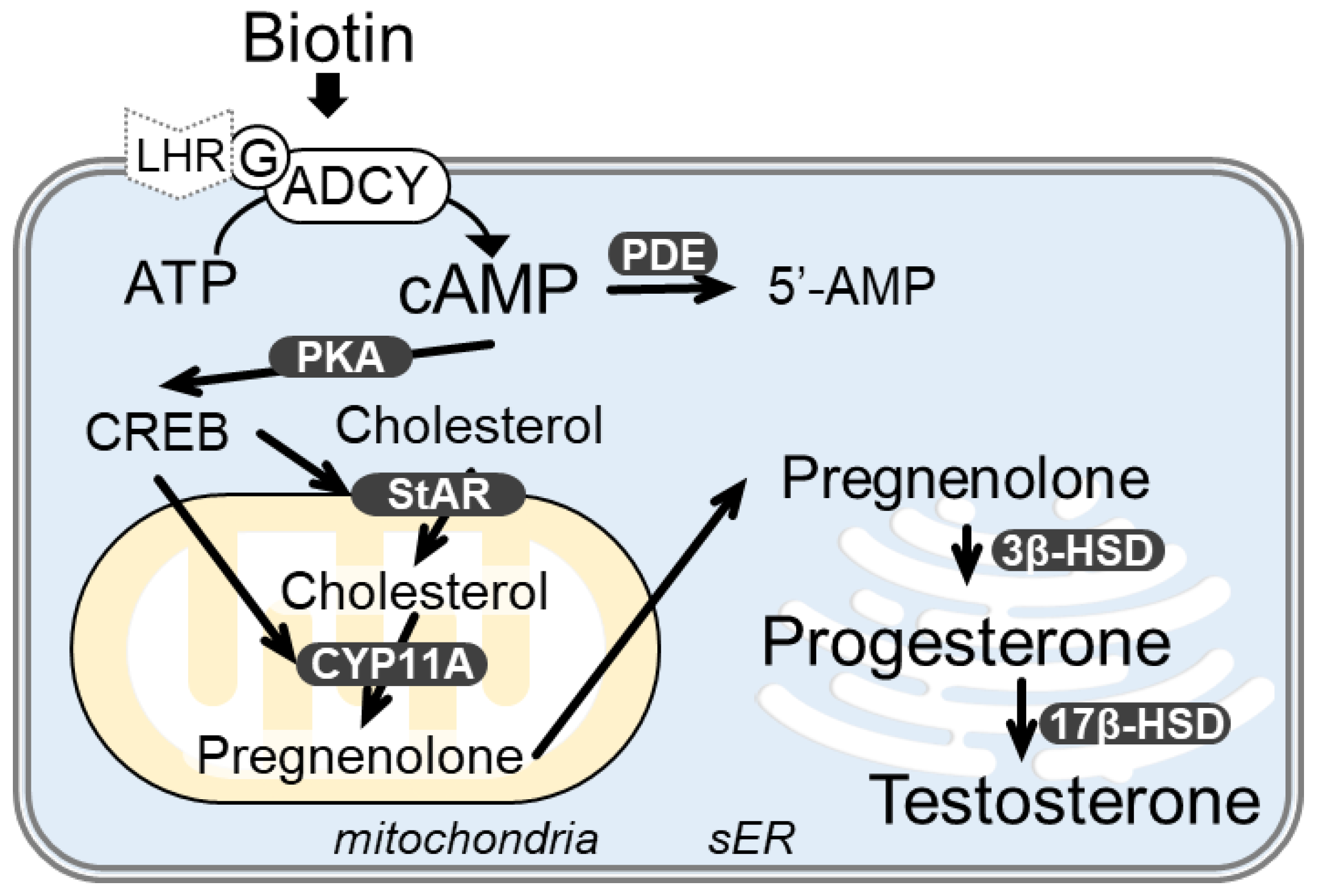
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Experiments
2.3. Cell Culture
2.4. Measurement of Testosterone, Progesterone, and Luteinizing Hormone Levels
2.5. Measurement of cAMP Levels
2.6. Reporter Gene and RNA Interference Assays
2.7. mRNA Quantification
2.8. Measurement of Biotin Levels
2.9. Statistical Analysis
3. Results
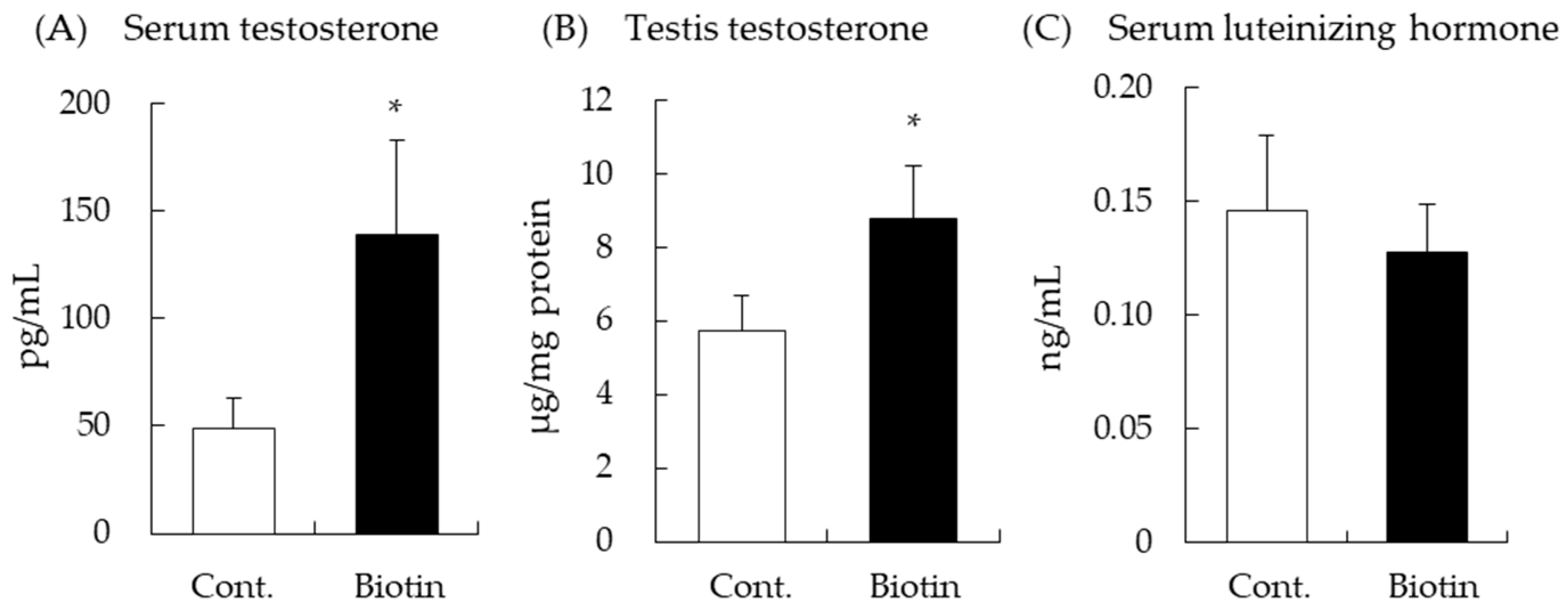
3.1. Effect of Biotin on Testes and Serum Testosterone Levels in Mice
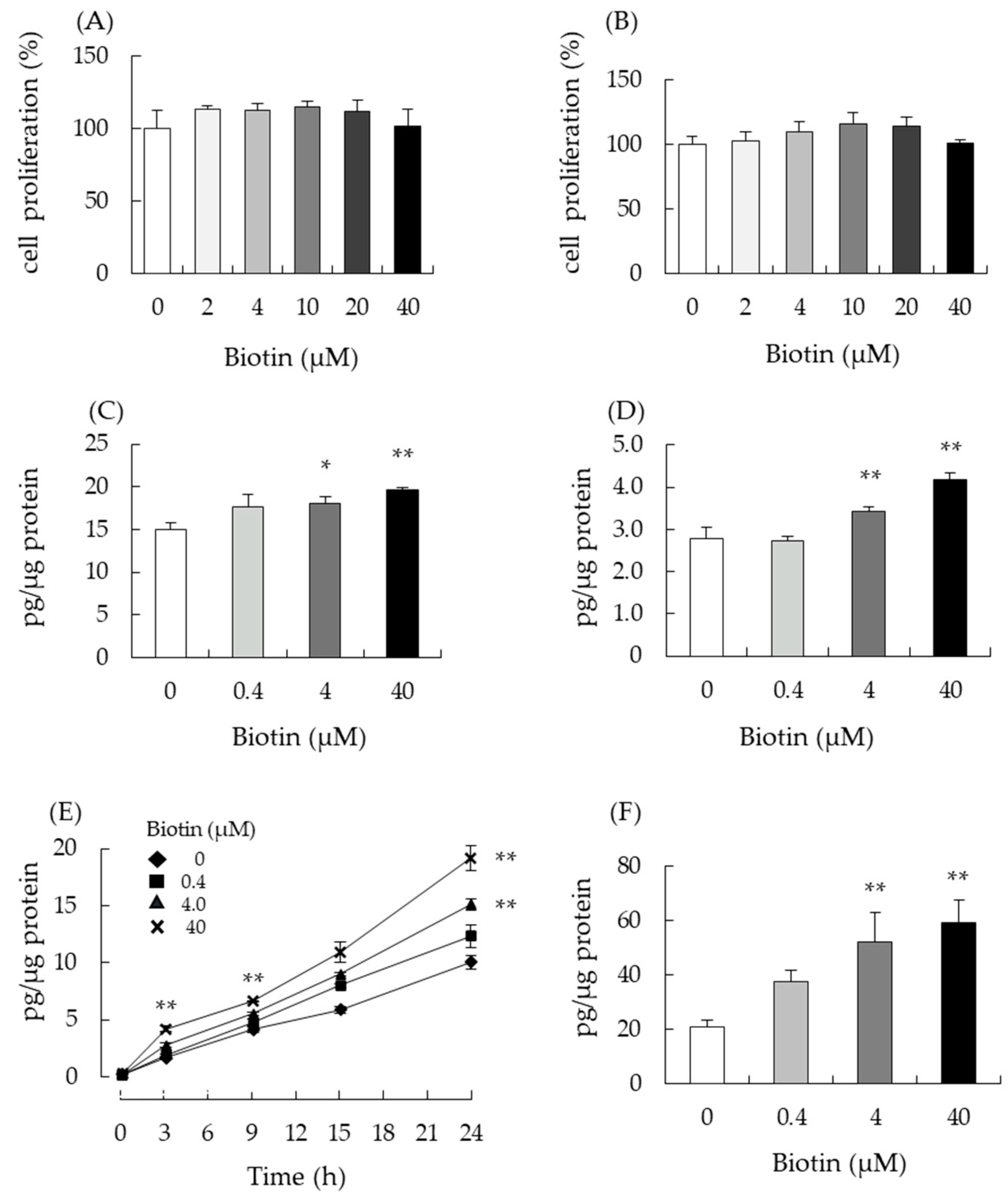
3.2. Biotin Stimulates Testosterone Production in Testis-Derived cells
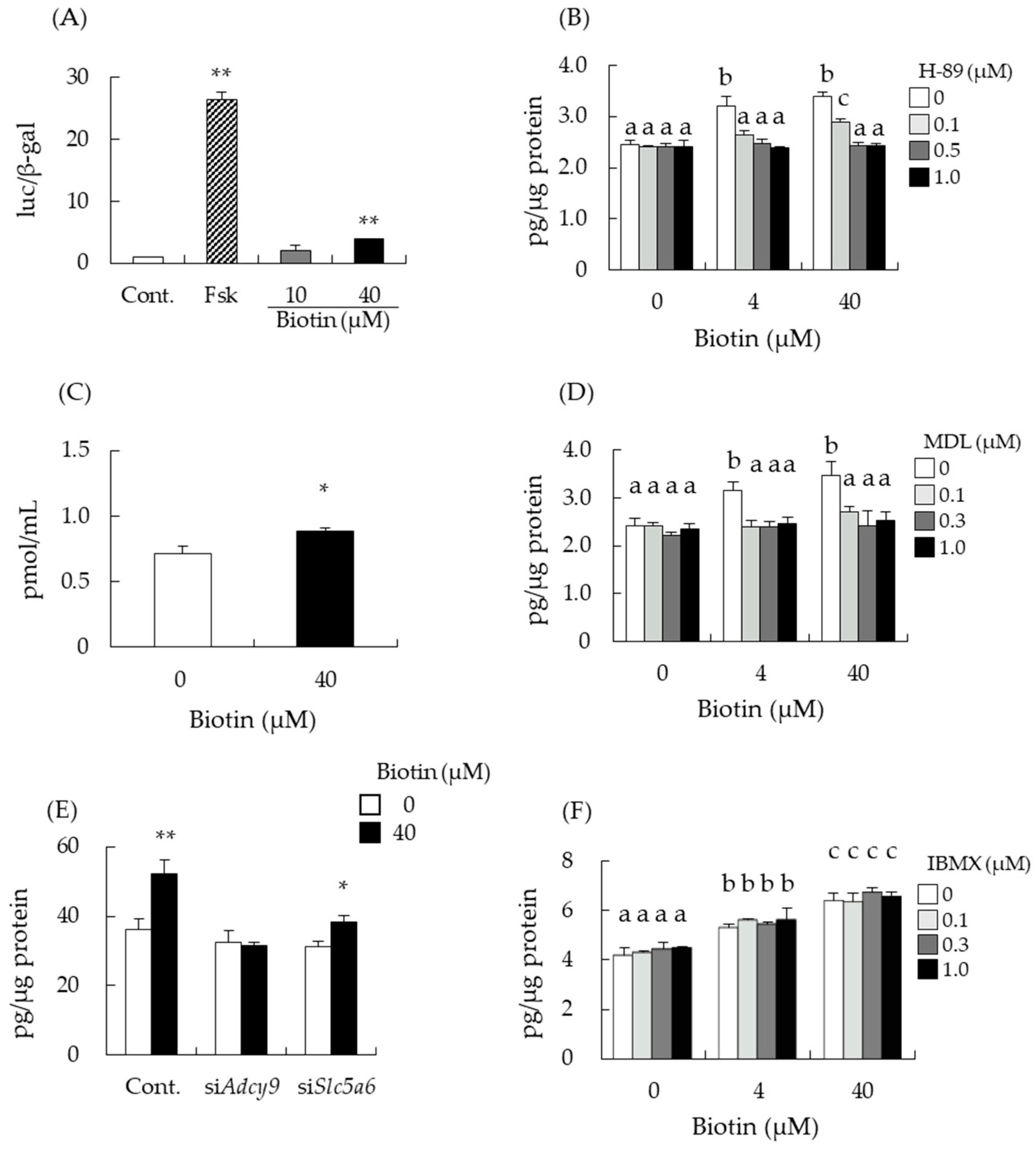
3.3. Biotin Stimulates the cAMP/PKA Pathway for Testosterone Synthesis
3.4. Intracellular Transport of Biotin Did Not Contribute to the Enhancement of Testosterone Levels
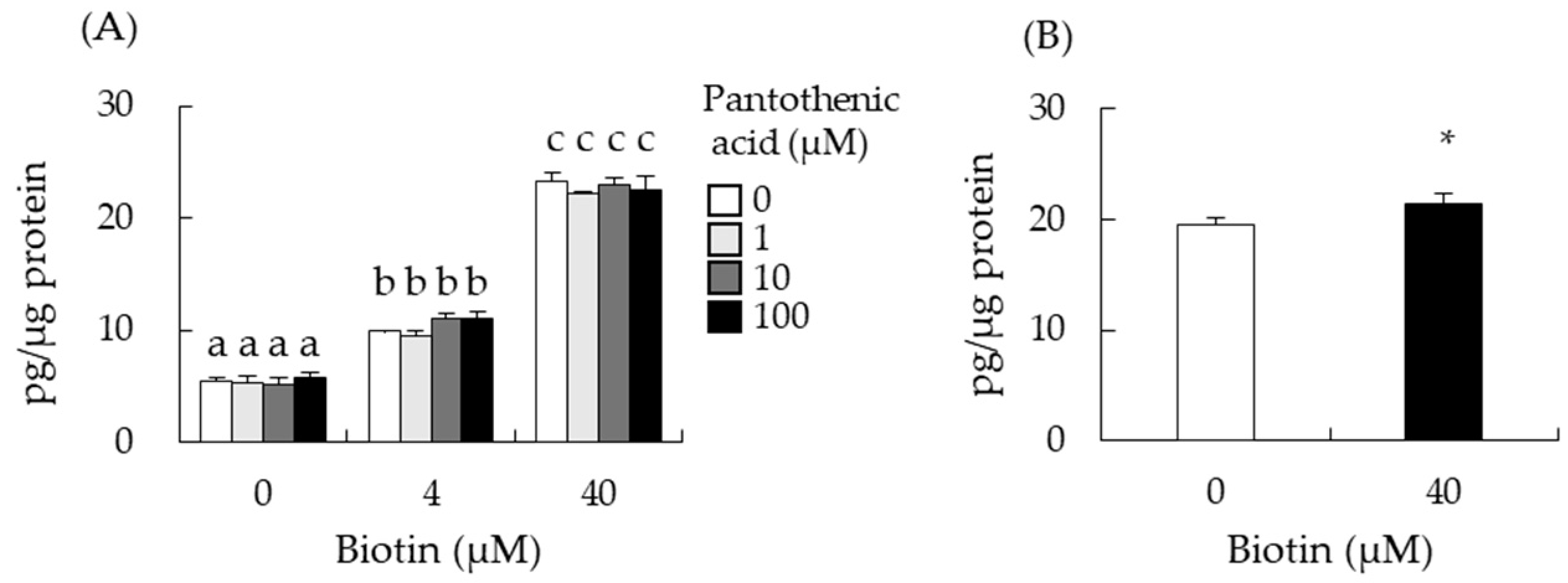
3.5. Effect of Biotin-Analogues on Testosterone Levels in I-10 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwamoto, T.; Yanase, T.; Koh, E.; Horie, H.; Baba, K.; Namiki, M.; Nawata, H. Reference ranges of total serum and free testosterone in Japanese male adults. Nihon Hinyokika Gakkai Zasshi 2004, 95, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.C.W.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef]
- Corona, G.; Goulis, D.G.; Huhtaniemi, I.; Zitzmann, M.; Toppari, J.; Forti, G.; Vanderschueren, D.; Wu, F.C. European Academy of Andrology (EAA) Guidelines on Investigation, Treatment and Monitoring of Functional Hypogonadism in Males: Endorsing Organization: European Society of Endocrinology. Andrology 2020, 8, 970–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocrinol. 2014, 35, 42–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.H.; Hardy, M.P. (Eds.) The Leydig Cell in Health and Disease; Humana Press: Totowa, NJ, USA, 2007; ISBN 9781588297549. [Google Scholar]
- Ito, A.; Shirakawa, H.; Takumi, N.; Minegishi, Y.; Ohashi, A.; Howlader, Z.H.; Ohsaki, Y.; Sato, T.; Goto, T.; Komai, M. Menaquinone-4 Enhances Testosterone Production in Rats and Testis-Derived Tumor Cells. Lipids Health Dis. 2011, 10, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, H.-J.; Shirakawa, H.; Yoshida, R.; Ito, A.; Maeda, M.; Goto, T.; Komai, M. Geranylgeraniol Enhances Testosterone Production via the cAMP/protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells. Biosci. Biotechnol. Biochem. 2016, 80, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Takumi, N.; Shirakawa, H.; Ohsaki, Y.; Ito, A.; Watanabe, T.; Giriwono, P.E.; Sato, T.; Komai, M. Dietary Vitamin K Alleviates the Reduction in Testosterone Production Induced by Lipopolysaccharide Administration in Rat Testis. Food Funct. 2011, 2, 406–411. [Google Scholar] [CrossRef]
- Shirakawa, H.; Ohsaki, Y.; Minegishi, Y.; Takumi, N.; Ohinata, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K Deficiency Reduces Testosterone Production in the Testis through down-Regulation of the Cyp11a a Cholesterol Side Chain Cleavage Enzyme in Rats. Biochim. Biophys. Acta 2006, 1760, 1482–1488. [Google Scholar] [CrossRef]
- Horigome, S.; Maeda, M.; Ho, H.-J.; Shirakawa, H.; Komai, M. Effect of Kaempferia parviflora Extract and Its Polymethoxyflavonoid Components on Testosterone Production in Mouse Testis-Derived Tumour Cells. J. Funct. Foods 2016, 26, 529–538. [Google Scholar] [CrossRef]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef]
- Nakayama, Y.; Ho, H.-J.; Yamagishi, M.; Ikemoto, H.; Komai, M.; Shirakawa, H. Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells. Molecules 2020, 25, 4694. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; Shiozawa, K.; Mukai, K.; Takayanagi, K.; Eguchi, K.; Sultana, H.; Ohsaki, Y.; Komai, M.; Shirakawa, H. S-Allyl Cysteine Enhances Testosterone Production in Mice and Mouse Testis-Derived I-10 Cells. Molecules 2021, 26, 1697. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Wijeratne, S.S.K.; Hassan, Y.I. Biotin. Biofactors 2009, 35, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Nagai, Y.; Taniguchi, A.; Ebara, S.; Kimura, S.; Fukui, T. Effects of Biotin Deficiency on Embryonic Development in Mice. Nutrition 2009, 25, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Báez-Saldaña, A.; Camacho-Arroyo, I.; Espinosa-Aguirre, J.J.; Neri-Gómez, T.; Rojas-Ochoa, A.; Guerra-Araiza, C.; Larrieta, E.; Vital, P.; Díaz, G.; Chavira, R.; et al. Biotin deficiency and biotin excess: Effects on the female reproductive system. Steroids 2009, 74, 863–869. [Google Scholar] [CrossRef]
- Tsuji, A.; Nakamura, T.; Shibata, K. Biotin-deficient diet induces chromosome misalignment and spindle defects in mouse oocytes. Biosci. Biotechnol. Biochem. 2015, 79, 292–299. [Google Scholar] [CrossRef]
- Zhang, H.; Osada, K.; Sone, H.; Furukawa, Y. Biotin administration improves the impaired glucose tolerance of streptozotocin-induced diabetic Wistar rats. J. Nutr. Sci. Vitaminol. 1997, 43, 271–280. [Google Scholar] [CrossRef]
- Sone, H.; Sasaki, Y.; Komai, M.; Toyomizu, M.; Kagawa, Y.; Furukawa, Y. Biotin Enhances ATP Synthesis in Pancreatic Islets of the Rat, Resulting in Reinforcement of Glucose-Induced Insulin Secretion. Biochem. Biophys. Res. Commun. 2004, 314, 824–829. [Google Scholar] [CrossRef]
- Sugita, Y.; Shirakawa, H.; Sugimoto, R.; Furukawa, Y.; Komai, M. Effect of Biotin Treatment on Hepatic Gene Expression in Streptozotocin-Induced Diabetic Rats. Biosci. Biotechnol. Biochem. 2008, 72, 1290–1298. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Sone, H.; Kamiyama, S.; Shimizu, M.; Shirakawa, H.; Kagawa, Y.; Komai, M.; Furukawa, Y. Administration of biotin prevents the development of insulin resistance in the skeletal muscles of Otsuka long-Evans Tokushima fatty rats. Food Funct. 2012, 3, 414–419. [Google Scholar] [CrossRef]
- Watanabe-Kamiyama, M.; Kamiyama, S.; Horiuchi, K.; Ohinata, K.; Shirakawa, H.; Furukawa, Y.; Komai, M. Antihypertensive Effect of Biotin in Stroke-Prone Spontaneously Hypertensive Rats. Br. J. Nutr. 2008, 99, 756–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riverón-Negrete, L.; Sicilia-Argumedo, G.; Álvarez-Delgado, C.; Coballase-Urrutia, E.; Alcántar-Fernández, J.; Fernandez-Mejia, C. Dietary Biotin Supplementation Modifies Hepatic Morphology without Changes in Liver Toxicity Markers. Biomed Res. Int. 2016, 2016, 7276463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastén-Hidalgo, K.; Riverón-Negrete, L.; Sicilia-Argumedo, G.; Canul-Medina, G.; Salazar-Anzures, T.; Tapia-Rodríguez, M.; Hernández-González, E.O.; Roa-Espitia, A.L.; Cedillo-Peláez, C.; Fernandez-Mejia, C. Dietary Biotin Supplementation Impairs Testis Morphology and Sperm Quality. J. Med. Food 2020, 23, 535–544. [Google Scholar] [CrossRef]
- Salazar-Anzures, T.; Pastén-Hidalgo, K.; Sicilia-Argumedo, G.; Riverón-Negrete, L.; de Jesús Hernández-Vázquez, A.; Fernanadez-Mejia, C. Dietary Biotin Supplementation Increases Proliferation Pathways in Mice Testes without Affecting Serum Follicle-Stimulating Hormone Levels and Stem Cell Factor Expression. Toxicol. Appl. Pharmacol. 2021, 433, 115774. [Google Scholar] [CrossRef]
- Sawamura, H.; Ikeda, C.; Shimada, R.; Yoshii, Y.; Watanabe, T. Dietary Intake of High-Dose Biotin Inhibits Spermatogenesis in Young Rats. Congenit. Anom. 2015, 55, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suemori, H.; Kadodawa, Y.; Nakatsuji, N.; Goto, K.; Araki, I.; Kondoh, H. A Mouse Embryonic Stem Cell Line Showing Pluripotency of Differentiation in Early Embryos and Ubiquitous β-Galactosidase Expression. Cell Differ. Dev. 1990, 29, 181–186. [Google Scholar] [CrossRef]
- Sultana, H.; Kato, A.; Ohashi, A.; Takashima, R.; Katsurai, T.; Sato, S.; Monma, M.; Ohsaki, Y.; Goto, T.; Komai, M.; et al. Effect of Vitamin K-Mediated PXR Activation on Drug-Metabolizing Gene Expression in Human Intestinal Carcinoma LS180 Cell Line. Nutrients 2021, 13, 1709. [Google Scholar] [CrossRef]
- Sone, H.; Kamiyama, S.; Higuchi, M.; Fujino, K.; Kubo, S.; Miyazawa, M.; Shirato, S.; Hiroi, Y.; Shiozawa, K. Biotin Augments Acetyl CoA Carboxylase 2 Gene Expression in the Hypothalamus, Leading to the Suppression of Food Intake in Mice. Biochem. Biophys. Res. Commun. 2016, 476, 134–139. [Google Scholar] [CrossRef]
- Prasad, P.D.; Ramamoorthy, S.; Leibach, F.H.; Ganapathy, V. Characterization of a sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin and lipoate in human placental choriocarcinoma cells. Placenta 1997, 18, 527–533. [Google Scholar] [CrossRef]
- Banihani, S.A. Ginger and Testosterone. Biomolecules 2018, 8, 119. [Google Scholar] [CrossRef]
- Banihani, S.A. Testosterone in Males as Enhanced by Onion (Allium cepa L.). Biomolecules 2019, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Stork, P.J.S.; Schmitt, J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002, 12, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Steven, A.; Seliger, B. Control of CREB expression in tumors: From molecular mechanisms and signal transduction pathways to therapeutic target. Oncotarget 2016, 7, 35454–35465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Q.L.; Lin, Y.H.; Xu, Y.K.T.; Fernandes, M.G.F.; Rao, V.T.S.; Kennedy, T.E.; Antel, J. Effects of biotin on survival, ensheathment, and ATP production by oligodendrocyte lineage cells in vitro. PLoS ONE 2020, 15, e0233859. [Google Scholar] [CrossRef] [PubMed]
- Vilches-Flores, A.; Tovar, A.R.; Marin-Hernandez, A.; Rojas-Ochoa, A.; Fernandez-Mejia, C. Biotin increases glucokinase expression via soluble guanylate cyclase/protein kinase G, adenosine triphosphate production and autocrine action of insulin in pancreatic rat islets. J. Nutr. Biochem. 2010, 21, 606–612. [Google Scholar] [CrossRef]
- Boone-Villa, D.; Aguilera-Méndez, A.; Miranda-Cervantes, A.; Fernandez-Mejia, C. Effects of Biotin Supplementation in the Diet on Adipose Tissue cGMP Concentrations, AMPK Activation, Lipolysis, and Serum-Free Fatty Acid Levels. J. Med. Food 2015, 18, 1150–1156. [Google Scholar] [CrossRef]
- Pacheco-Alvarez, D.; Solórzano-Vargas, R.S.; González-Noriega, A.; Michalak, C.; Zempleni, J.; León-Del-Río, A. Biotin Availability Regulates Expression of the Sodium-Dependent Multivitamin Transporter and the Rate of Biotin Uptake in HepG2 Cells. Mol. Genet. Metab. 2005, 85, 301–307. [Google Scholar] [CrossRef]
- Peretz, J.; Flaws, J.A. Bisphenol A Down-regulates Rate-Limiting Cyp11a1 to Acutely Inhibit Steroidogenesis in Cultured Mouse Antral Follicles. Toxicol. Appl. Pharmacol. 2013, 271, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Rebourcet, D.; Mackay, R.; Darbey, A.; Curley, M.K.; Jørgensen, A.; Frederiksen, H.; Mitchell, R.T.; O’Shaughnessy, P.J.; Nef, S.; Smith, L.B. Ablation of the Canonical Testosterone Production Pathway via Knockout of the Steroidogenic Enzyme HSD17B3, Reveals a Novel Mechanism of Testicular Testosterone Production. FASEB J. 2020, 34, 10373–10386. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, Y.F.; Nie, N.; Feng, W.P.; Bao, J.F.; Meng, X.P.; Qiao, X.L. Protective Effects of L-Arginine against Testosterone Synthesis Decreased by T-2 Toxin in Mouse Leydig Cells. Theriogenology 2019, 134, 98–103. [Google Scholar] [CrossRef]

| Gene Name | siRNA Sequence | Accession No. |
|---|---|---|
| Adcy9 | 5′-CAUAGGAGUAGAAGAGGCCAGUGAA-3′ | NM_009624.3 |
| Slc5a6 | 5′-GAGUACCUAGAGCUCCGCUUCAAUA-3′ | NM_001177621.1 |
| Gene Name | Forward Primer Reverse Primer | Product Size (bp) | Accession No. |
|---|---|---|---|
| Adcy1 | 5′-GGTCCAGTGTTTTCCAGGGT-3′ | 100 | NM_009622.22 |
| 5′-CACCACACAGCCTTGAGCTA-3′ | |||
| Adcy2 | 5′-TCAACCCCAAGGGAGAAAGAC-3′ | 64 | NM_153534.2 |
| 5′-CCATCCAGAGTGTGTCGAGG-3′ | |||
| Adcy3 | 5′-GGAAAAGGACTCTCCTATGGTGG-3′ | 80 | NM_138305.3 |
| 5′-GCCTGCTGTCAGTGCCATT-3′ | |||
| Adcy4 | 5′-ATTGCTGCGTGTTGGGTTTC-3′ | 89 | NM_001361604.1 |
| 5′-CACCAGCCACAGCAGAAGTA-3′ | |||
| Adcy6 | 5′-TTCCTTTGGAAGCAGCTCGG-3′ | 56 | NM_001368413.2 |
| 5′-ATGGCATTGGTGCAGAGGAA-3′ | |||
| Adcy7 | 5′-CAGGGTATTAAGGTCCCAGCC-3′ | 91 | NM_001037723.3 |
| 5′-GACATCTTCTTCCCTGGCTCT-3′ | |||
| Adcy8 | 5′-TCATGATCGCCATCTACGCC-3′ | 99 | NM_009623.2 |
| 5′-TCCCCAGGAAATCTTCTCCAC-3′ | |||
| Adcy9 | 5′-CCTGTGTCAGGACAGTTCCATT-3′ | 51 | NM_009624.3 |
| 5′-TTCTGTGCTGAGTCCAAGGG-3′ | |||
| Adcy10 | 5′-AGAGCTCGACTCGTACCTGG-3′ | 86 | NM_173029.3 |
| 5′-CTCTGTGGTGGTCGAGGTTT-3′ | |||
| Slc5a6 | 5′-AGTGAATCAGGCTCAGGTGC-3′ | 71 | NM_001177621.1 |
| 5′-CATAGCAGGAGAGCACAGCA-3′ | |||
| Eef1α1 | 5′-GATGGCCCCAAATTCTTGAAG-3′ | 52 | NM_010106.2 |
| 5′-GGACCATGTCAACAATGGCAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiozawa, K.; Maeda, M.; Ho, H.-J.; Katsurai, T.; Howlader, M.Z.H.; Horiuchi, K.; Sugita, Y.; Ohsaki, Y.; Agista, A.Z.; Goto, T.; et al. Biotin Enhances Testosterone Production in Mice and Their Testis-Derived Cells. Nutrients 2022, 14, 4761. https://doi.org/10.3390/nu14224761
Shiozawa K, Maeda M, Ho H-J, Katsurai T, Howlader MZH, Horiuchi K, Sugita Y, Ohsaki Y, Agista AZ, Goto T, et al. Biotin Enhances Testosterone Production in Mice and Their Testis-Derived Cells. Nutrients. 2022; 14(22):4761. https://doi.org/10.3390/nu14224761
Chicago/Turabian StyleShiozawa, Kota, Misato Maeda, Hsin-Jung Ho, Tomoko Katsurai, Md. Zakir Hossain Howlader, Kimiko Horiuchi, Yumi Sugita, Yusuke Ohsaki, Afifah Zahra Agista, Tomoko Goto, and et al. 2022. "Biotin Enhances Testosterone Production in Mice and Their Testis-Derived Cells" Nutrients 14, no. 22: 4761. https://doi.org/10.3390/nu14224761
APA StyleShiozawa, K., Maeda, M., Ho, H.-J., Katsurai, T., Howlader, M. Z. H., Horiuchi, K., Sugita, Y., Ohsaki, Y., Agista, A. Z., Goto, T., Komai, M., & Shirakawa, H. (2022). Biotin Enhances Testosterone Production in Mice and Their Testis-Derived Cells. Nutrients, 14(22), 4761. https://doi.org/10.3390/nu14224761







