Evaluation of an Application for Mobile Telephones (e-12HR) to Increase Adherence to the Mediterranean Diet in University Students: A Controlled, Randomized and Multicentric Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
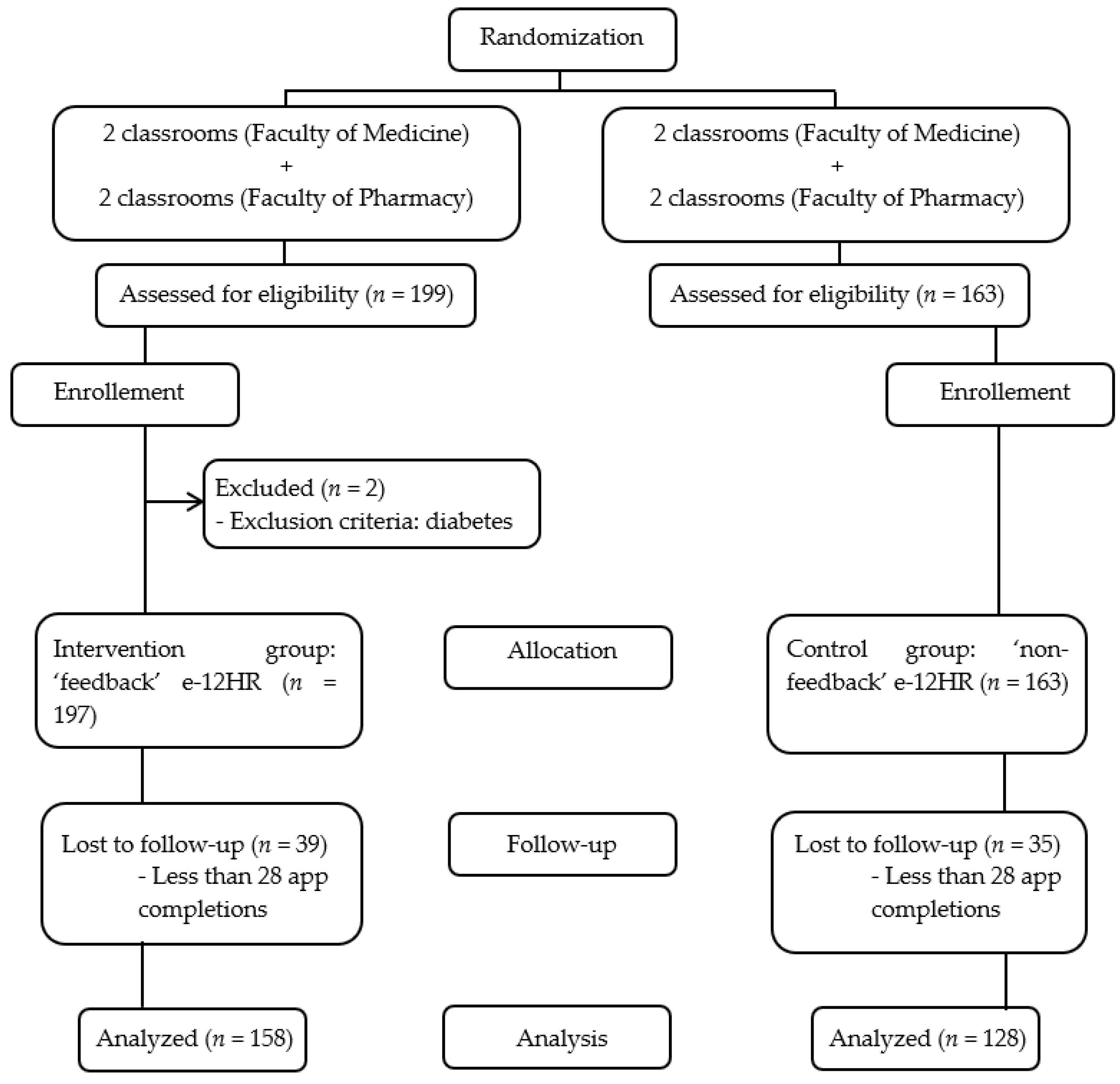
2.3. Randomization and Masking
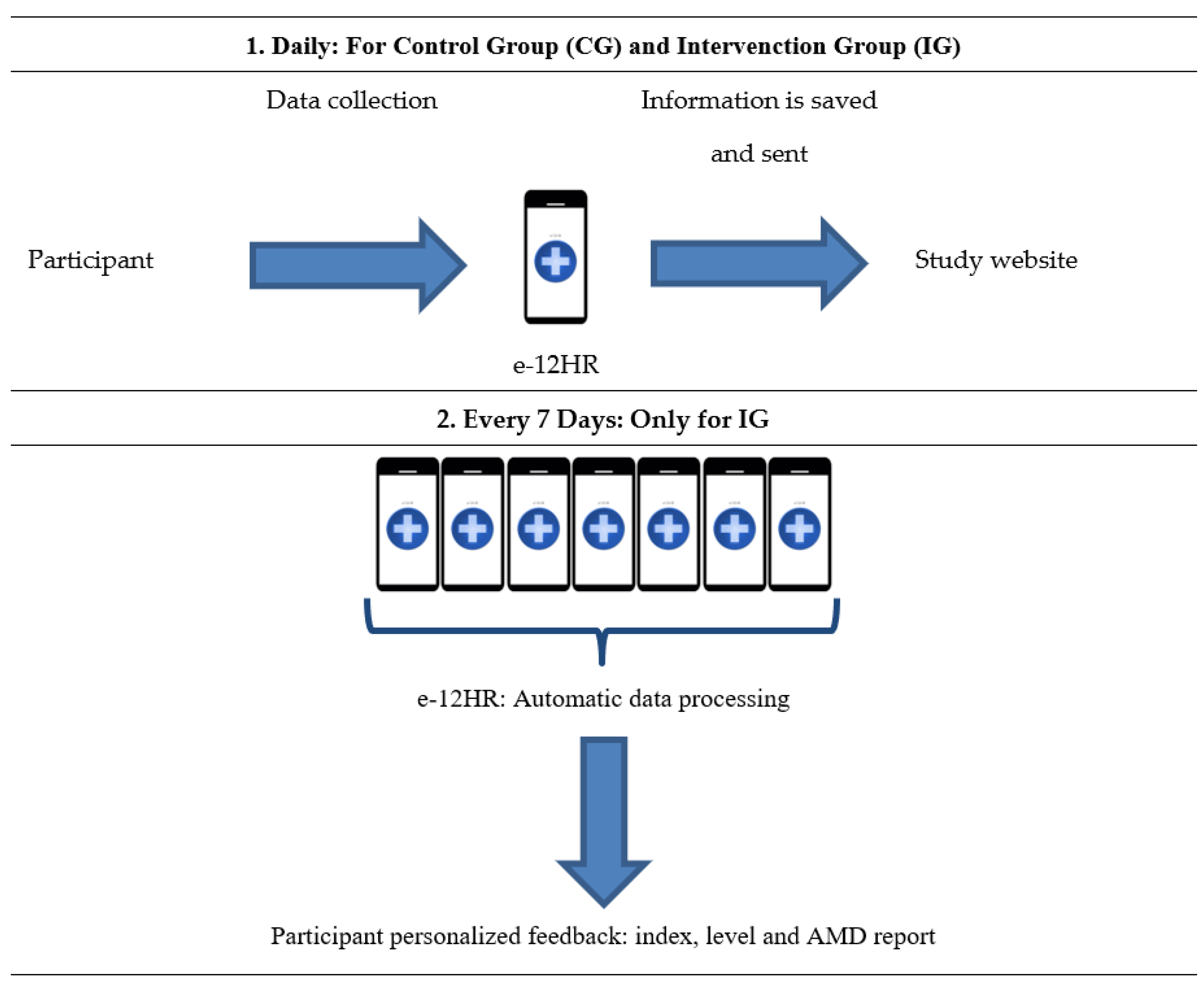
2.4. Intervention
- e-12HR App
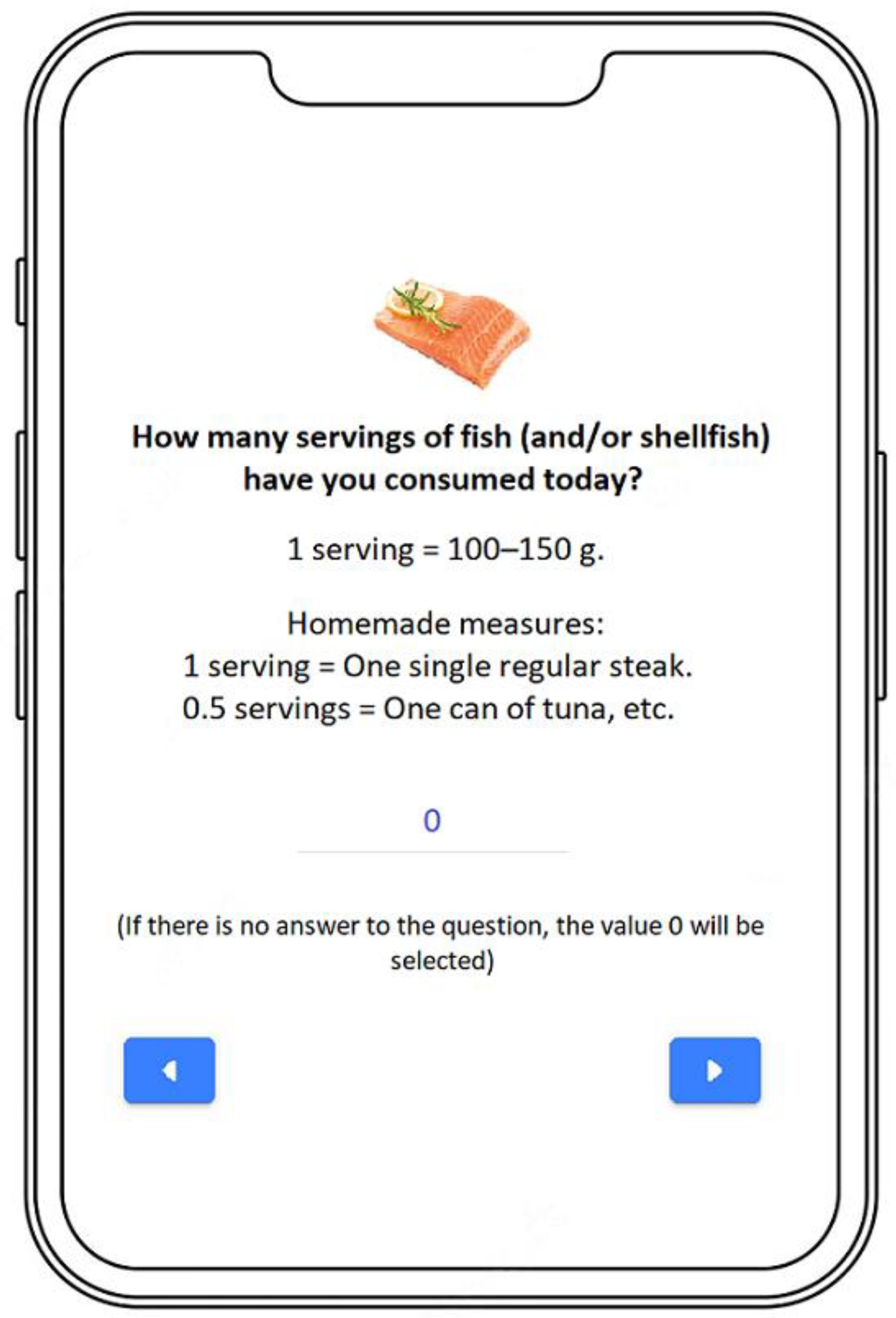
- Data Collection on Food Group Intake
- 2.
- Feedback from e-12HR
- AMD Assessment
- The specific food groups (compatible with the MD);
- Consumption recommendations for frequency of standard servings (per meal, daily or weekly);
- A numerical score assigned to each item (Appendix A, Table A2).
- AMD Report
2.5. Follow-Up and Outcome Measures
Adherence to the Mediterranean Diet
2.6. Sample Size Calculation
2.7. Usability Rating Questionnaire for e-12HR
2.8. Ethical Considerations
2.9. Statistical Analysis
3. Results
3.1. Sample and Adherence to the Study
3.2. Personal Information of the Participants
3.3. Effect of the Intervention
3.4. Usability Rating Questionnaire for e-12HR
4. Discussion
Future Research Related to the Current Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Questions about Food Groups: |
|---|
| 1. How many servings of fruits (orange, apple, pear, peach, strawberry, watermelon, etc. -including fresh juice-) have you consumed today? 1 serving = 150–200 g. Homemade measures: 1 serving = A medium-sized piece of apple, pear or orange, a cup of cherries or strawberries, two slices of melon, a glass of natural juice. |
| 2. How many servings of vegetables (tomato, carrot, bell pepper, lettuce, zucchini, etc.) have you consumed today? 1 serving = 150–250 g. Homemade measures: 1 serving = One normal single plate of salad, one normal single plate of cooked vegetables, one large tomato, two carrots. |
| 3. How many servings of breakfast cereals have you consumed today? 1 serving = 20–30 g. Homemade measures: 1 serving = One individual normal bowl. |
| 4. How many servings of pasta have you consumed today? 1 serving = 50–70 g. Homemade measures: 1 serving = One normal individual plate. |
| 5. How many servings of rice have you consumed today? 1 serving = 50–70 g. Homemade measures: 1 serving = One normal individual plate. |
| 6. How many servings of bread have you consumed today? 1 serving = 30–60 g. Homemade measures: 1 serving = Three or four slices of bread or a muffin. |
| 7. How many servings of olive oil have you consumed today (used for salad, to add to bread, or for cooking)? 1 serving = 15 mL. Homemade measures: 1 serving = One tablespoon. |
| 8. How many servings of milk and dairy products (yogurt, fresh cheese, aged cheese) have you consumed today? 1 serving of milk = 200–250 mL. 1 serving of yogurt = 125 g. 1 serving of fresh cheese = 60–80 g. 1 serving of aged cheese = 30–40 g. Homemade measures: 1 serving = A glass of milk, a yogurt, a tub or individual portion of fresh cheese, two or three slices of aged cheese. |
| 9. How many servings of nuts (almonds, walnuts, hazelnuts, etc.) and/or olives have you consumed today? 1 serving = 20–30 g. Homemade measures: 1 serving = A handful of olives (8–10 units), a handful of hazelnuts (18–20 units), three or four walnuts. |
| 10. How many servings of beer have you consumed today? 1 serving = 200 mL. Homemade measures: 1 serving = A beer or a bottle (200 mL). 1.5 servings = A bottle (large bottle), a can or a large beer glass (330 mL). |
| 11. How many servings of wine have you consumed today? 1 serving = 100 mL. Homemade measures: 1 serving = A glass of wine (100 mL). |
| 12. How many servings of potatoes have you consumed today (cooked, roasted, or fried)? 1 serving = 100–150 g. Homemade measures: 1 serving = One large potato or two small potatoes. |
| 13. How many servings of legumes (lentils, beans, chickpeas, peas, etc.) have you consumed today? 1 serving = 50–70 g. Homemade measures: 1 serving = One normal individual plate. |
| 14. How many servings of eggs have you consumed today? 1 serving = One medium egg (50–70 g). |
| 15. How many servings of fish (and/or shellfish) have you consumed today? 1 serving = 100–150 g. Homemade measures: 1 serving = One single regular steak. 0.5 servings = One can of tuna, etc. |
| 16. How many servings of white meat (poultry) have you consumed today? 1 serving = 100–125 g. Homemade measures: 1 serving = One regular single filet, or a chicken leg quarter. 0.5 servings = Two or three slices of chicken or turkey. |
| 17. How many servings of red meat (beef, pork, or lamb) have you consumed today? 1 serving = 100–125 g. Homemade measures: 1 serving = One single regular steak. |
| 18. How many servings of processed meats (hamburgers, sausages) and/or cold cuts (salami, chorizo, cooked ham) have you consumed today? 1 serving = 90–100 g. Homemade measures: 1 serving = a hamburger, a large sausage or two small sausages, eight or ten thin slices of cold cuts (salami, chorizo), four or five thin slices of cooked ham. |
| 19. How many sweets (sugar, candies, pastries, sweetened fruit juices and soft drinks) have you consumed today? Consider the number of units consumed regardless of the serving size. |
| Additional questions about physical activities |
| 20. For how many minutes have you practiced moderate physical activity today? Consider only those moderate physical activities that you practiced for at least 10 min at a time. According to the WHO, these activities require moderate effort, which perceptibly accelerates the heart rate. Examples: fast walking, cycling, dancing, housework, active participation in games and sports with children, etc. |
| 21. For how many minutes have you practiced intense physical activity today? Consider only those intense physical activities that you practiced for at least 10 min at a time. According to the WHO, these activities require a great deal of effort and cause rapid breathing and a substantial increase in heart rate. Examples: running, cycling fast, competitive sports and games, etc. |
| Food Group | Recommendation | Score |
|---|---|---|
| Fruit | 1–2 servings/main meal | 3 |
| Vegetables | ≥2 servings/main meal | 3 |
| Cereals | 1–2 servings/main meal | 3 |
| Olive Oil | 1 serving/main meal | 3 |
| Dairy products | 2 servings/day | 2 |
| Nuts | 1–2 servings/day | 2 |
| Fermented beverages | 1–2 glass/day | 1 |
| Potatoes | ≤3 servings/week | 1 |
| Legumes | ≥2 servings/week | 1 |
| Eggs | 2–4 servings/week | 1 |
| Fish | ≥2 servings/week | 1 |
| White meat | 2 servings/week | 1 |
| Red meat | <2 servings/week | 1 |
| Sweets | ≤2 servings/week | 1 |
| Total maximum score | 24 | |
| Levels of AMD | Score | Colour |
|---|---|---|
| Low | 0–8 | Red |
| Medium | 9–15 | Orange |
| High | 16–24 | Green |
| 1. I found e-12HR easy to complete: |
| Strongly agree. Agree. Neither agree nor disagree. Disagree. Strongly disagree. |
| 2. I found e-12HR interesting to complete: |
| Strongly agree. Agree. Neither agree nor disagree. Disagree. Strongly disagree. |
| 3. I found the questions of e-12HR understandable (for control group): 3. I found the questions/feedback from e-12HR understandable (for intervention group): |
| Strongly agree. Agree. Neither agree nor disagree. Disagree. Strongly disagree. |
| 4. In the future, I would be willing to complete e-12HR again: |
| Strongly agree. Agree. Neither agree nor disagree. Disagree. Strongly disagree. |
| 5. How much time was needed to complete the daily questionnaire on the app: |
| Less than 1 min per day. Approximately 1 min per day. Approximately 2 min per day. Approximately 3 min per day. Approximately 4 min per day. 5 min per day or more. |
References
- WHO. The-Top-10-Causes-of-Death. Available online: https://www.who.int/es/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 20 September 2022).
- GBD 2019 Risk Factors Collaborators. Global Burden of 87 Risk factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Vasiloglou, M.F.; Christodoulidis, S.; Reber, E.; Stathopoulou, T.; Lu, Y.; Stanga, Z.; Mougiakakou, S. What Healthcare Professionals Think of "Nutrition & Diet" Apps: An International Survey. Nutrients 2020, 12, 2214. [Google Scholar] [CrossRef]
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Available online: https://www.who.int/publications/i/item/9789241506236 (accessed on 20 September 2022).
- Aranceta Bartrina, J.; Arija Val, V.V.; Maíz Aldalur, E.; Martínez de Victoria Muñoz, E.; Ortega Anta, R.M.; Pérez Rodrigo, C.; Quiles Izquierdo, J.; Rodríguez Martín, A.; Román Viñas, B.; Salvador Castell, G.; et al. Dietary Guidelines for the Spanish population (SENC, diciembre 2016); the new graphic icon of healthy food. Nutr. Hosp. 2016, 33, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health benefits of the Mediterranean Diet: An update of research over the last 5 years. Angiology 2015, 66, 304–318. [Google Scholar] [CrossRef]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and its Benefits on Health and Mental Health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Lăcătușu, C.M.; Grigorescu, E.D.; Floria, M.; Onofriescu, A.; Mihai, B.M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef]
- Katz, D.L.; Meller, S. Can we say what diet is best for health? Annu. Rev. Public Health 2014, 35, 83–103. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.M. Sustainable Food Systems and the Mediterranean Diet. Nutrients 2019, 11, 2229. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M. Mediterranean Diet: From a Healthy Diet to a Sustainable Dietary Pattern. Front. Nutr. 2015, 7, 15. [Google Scholar] [CrossRef]
- Berry, E.M.; Dernini, S.; Burlingame, B.; Meybeck, A.; Conforti, P. Food security and sustainability: Can one exist without the other? Public Health Nutr. 2015, 18, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Burlingame, B.; Dernini, S. Sustainable Diets and Biodiversity; FAO: Rome, Italy, 2012; p. 309. [Google Scholar]
- Lacirignola, C.; Capone, R. Mediterranean Food Consumption Patterns Diet, Environment, Society, Economy and Health; FAO: Rome, Italy, 2015; p. 77. [Google Scholar]
- Burlingame, B.; Dernini, S. Sustainable diets: The Mediterranean diet as an example. Public Health Nutr. 2011, 14, 2285–2287. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Cabañero-Martínez, M.J.; Hurtado-Sánchez, J.A.; Laguna-Pérez, A.; Ferrer-Cascales, R. Evaluation of Mediterranean diet adherence scores: A systematic review. BMJ Open 2018, 8, e019033. [Google Scholar] [CrossRef]
- Gil, Á.; Martinez de Victoria, E.; Olza, J. Indicators for the evaluation of diet quality. Nutr. Hosp. 2015, 31, 128–144. [Google Scholar] [CrossRef]
- Vasiloglou, M.F.; Lu, Y.; Stathopoulou, T.; Papathanail, I.; Fäh, D.; Ghosh, A.; Baumann, M.; Mougiakakou, S. Assessing Mediterranean Diet Adherence with the Smartphone: The Medipiatto Project. Nutrients 2020, 12, 3763. [Google Scholar] [CrossRef]
- Béjar, L.M.; Sharp, B.N.; García-Perea, M.D. The e-EPIDEMIOLOGY Mobile Phone App for Dietary Intake Assessment: Comparison with a Food Frequency Questionnaire. JMIR Res. Protoc. 2016, 5, e208. [Google Scholar] [CrossRef] [PubMed]
- Béjar, L.M.; Vázquez-Limón, E. Is there any alternative to traditional food frequency questionnaire for evaluating habitual dietary intake? Nutr. Hosp. 2017, 34, 880–888. [Google Scholar] [CrossRef]
- Béjar, L.M. First evaluation steps of a new method for dietary intake estimation regarding a list of key food groups in adults and in different sociodemographic and health-related behaviour strata. Public Health Nutr. 2017, 20, 2660–2669. [Google Scholar] [CrossRef]
- Béjar, L.M.; Reyes, Ó.A.; García-Perea, M.D. Electronic 12-Hour Dietary Recall (e-12HR): Comparison of a Mobile Phone App for Dietary Intake Assessment with a Food Frequency Questionnaire and Four Dietary Records. JMIR mHealth uHealth 2018, 6, e10409. [Google Scholar] [CrossRef] [PubMed]
- Béjar, L.M.; García-Perea, M.D.; Reyes, Ó.A.; Vázquez-Limón, E. Relative Validity of a Method Based on a Smartphone App (Electronic 12-Hour Dietary Recall) to Estimate Habitual Dietary Intake in Adults. JMIR mHealth uHealth 2019, 7, e11531. [Google Scholar] [CrossRef] [PubMed]
- Bejar, L.M. Weekend–Weekday Differences in Adherence to the Mediterranean Diet among Spanish University Students. Nutrients 2022, 14, 2811. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, V.K.; Hatch, E.E.; Cueto, H.; Tucker, K.L.; Wise, L.; Christensen, T.; Mikkelsen, E.M. Relative validity of a semi-quantitative, web-based FFQ used in the ‘Snart Forældre’ cohort—A Danish study of diet and fertility. Public Health Nutr. 2016, 19, 1027–1034. [Google Scholar] [CrossRef]
- Medin, A.C.; Astrup, H.; Kåsin, B.M.; Andersen, L.F. Evaluation of a web-based food record for children using direct unobtrusive lunch observations: A validation study. J. Med. Internet Res. 2015, 17, e273. [Google Scholar] [CrossRef]
- Rodríguez, I.T.; Ballart, J.F.; Pastor, G.C.; Jordà, E.B.; Val, V.A. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- Monteagudo, C.; Mariscal-Arcas, M.; Rivas, A.; Lorenzo-Tovar, M.L.; Tur, J.A.; Olea-Serrano, F. Proposal of a Mediterranean Diet Serving Score. PLoS ONE 2015, 10, e0128594. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013; p. 552. [Google Scholar]
- Forster, H.; Fallaize, R.; Gallagher, C.; O’Donovan, C.B.; Woolhead, C.; Walsh, M.C.; Macready, A.L.; Lovegrove, J.A.; Mathers, J.C.; Gibney, M.J.; et al. Online dietary intake estimation: The Food4Me food frequency questionnaire. J. Med. Internet Res. 2014, 16, e150. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Larsson, C. A mobile phone app for dietary intake assessment in adolescents: An evaluation study. JMIR mHealth uHealth 2015, 3, e93. [Google Scholar] [CrossRef] [PubMed]
- Hongu, N.; Pope, B.T.; Bilgiç, P.; Orr, B.J.; Suzuki, A.; Kim, A.S.; Merchant, N.C.; Roe, D.J. Usability of a smartphone food picture app for assisting 24-hour dietary recall: A pilot study. Nutr. Res. Pract. 2015, 9, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Universidades, Gobierno de España. Datos y Cifras del Sistema Universitario Español Publicación 2020–2021. Available online: https://www.universidades.gob.es/ (accessed on 20 September 2022).
- Duan, Y.P.; Wienert, J.; Hu, C.; Si, G.Y.; Lippke, S. Web-Based Intervention for Physical Activity and Fruit and Vegetable Intake Among Chinese University Students: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e106. [Google Scholar] [CrossRef]
- García-Meseguer, M.J.; Burriel, F.C.; García, C.V.; Serrano-Urrea, R. Adherence to Mediterranean diet in a Spanish university population. Appetite 2014, 78, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Solera, A.; Gamero, A. Healthy habits in university students of health sciences and other branches of knowledge: A comparative study. Rev. Esp. Nutr. Hum. Diet 2019, 23, 271–282. [Google Scholar]
- Cancela-Carral, J.M.; Ayán-Pérez, C. Prevalence and relationship between physical activity and abnormal eating attitudes in Spanish women university students in Health and Education Sciences. Rev. Esp. Salud. Publica 2011, 85, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Yahia, N.; Achkar, A.; Abdallah, A.; Rizk, S. Eating habits and obesity among Lebanese university students. Nutr. J. 2008, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Dhawan, T.; Metzger, K.; Marshall, L.; Akbar, A.; Jain, T.; Young, H.A.; Katz, R.J. Image-Based Mobile System for Dietary Management in an American Cardiology Population: Pilot Randomized Controlled Trial to Assess the Efficacy of Dietary Coaching Delivered via a Smartphone App Versus Traditional Counseling. JMIR mHealth uHealth 2019, 7, e10755. [Google Scholar] [CrossRef]
- Alonso-Domínguez, R.; García-Ortiz, L.; Patino-Alonso, M.C.; Sánchez-Aguadero, N.; Gómez-Marcos, M.A.; Recio-Rodríguez, J.I. Effectiveness of A Multifactorial Intervention in Increasing Adherence to the Mediterranean Diet among Patients with Diabetes Mellitus Type 2: A Controlled and Randomized Study (EMID Study). Nutrients 2019, 11, 162. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, M.; Sanchez-Carrera, R.; Cejudo-Lopez, A.; Lozano-Navarrete, M.; Salamero Sánchez-Gabriel, E.; Torres-Bengoa, M.A.; Segura-Balbuena, M.; Sanchez-Cordero, M.J.; Barroso-Vazquez, M.; Perez-Barba, F.J.; et al. Short-Term Pilot Study to Evaluate the Impact of Salbi Educa Nutrition App in Macronutrients Intake and Adherence to the Mediterranean Diet: Randomized Controlled Trial. Nutrients 2022, 14, 2061. [Google Scholar] [CrossRef]
- McAleese, D.; Linardakis, M.; Papadaki, A. Quality and Presence of Behaviour Change Techniques in Mobile Apps for the Mediterranean Diet: A Content Analysis of Android Google Play and Apple App Store Apps. Nutrients 2022, 14, 1290. [Google Scholar] [CrossRef]
- Rutishauser, I.H. Dietary intake measurements. Public Health Nutr. 2005, 8, 1100–1107. [Google Scholar] [CrossRef]
- Tucker, K.L.; Smith, C.E.; Lai, C.Q.; Ordovas, J.M. Quantifying diet for nutrigenomic studies. Annu. Rev. Nutr. 2013, 33, 349–371. [Google Scholar] [CrossRef]
- Gibson, R. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: New York, NY, USA, 2005; 908p. [Google Scholar]
- Martín-Moreno, J.M.; Gorgojo, L. Assessment of dietary intake at the population level through individual questionnaires: Methodological shadows and lights. Rev. Esp. Salud Publica 2007, 81, 507–518. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.; Speakman, J.R.; Jeansonne, M.; Allison, D.B. Energy Balance Measurement Working Group. Energy balance measurement: When something is not better than nothing. Int. J. Obes. 2015, 39, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Illner, A.K.; Freisling, H.; Boeing, H.; Huybrechts, I.; Crispim, S.P.; Slimani, N. Review and evaluation of innovative technologies for measuring diet in nutritional epidemiology. Int. J. Epidemiol. 2012, 41, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Steele, R. An overview of the state of the art of automated capture of dietary intake information. Crit. Rev. Food Sci. Nutr. 2015, 55, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Hassannejad, H.; Matrella, G.; Ciampolini, P.; De Munari, I.; Mordonini, M.; Cagnoni, S. Automatic diet monitoring: A review of computer vision and wearable sensor-based methods. Int. J. Food Sci. Nutr. 2017, 68, 656–670. [Google Scholar] [CrossRef]
- Thong, Y.J.; Nguyen, T.; Zhang, Q.; Karunanithi, M.; Yu, L. Predicting food nutrition facts using pocket-size near-infrared sensor. Annu. Int. Conf. IEEE Eng. Med. Bio.l Soc. 2017, 2017, 742–745. [Google Scholar] [CrossRef]
- Carroll, R.J.; Midthune, D.; Subar, A.F.; Shumakovich, M.; Freedman, L.S.; Thompson, F.E.; Kipnis, V. Taking advantage of the strengths of 2 different dietary assessment instruments to improve intake estimates for nutritional epidemiology. Am. J. Epidemiol. 2012, 175, 340–347. [Google Scholar] [CrossRef]

| Scoring of Food Group Calculated on a Daily Basis | ||
|---|---|---|
| Food Group | Servings per Day | Score (e-12HR) |
| Fruits | 3–6 servings | 3 |
| Vegetables | ≥6 servings | 3 |
| Cereals | 3–6 servings | 3 |
| Olive oil | 3–4 servings | 3 |
| Milk and dairy products | 2–3 servings | 2 |
| Nuts | 1–2 servings | 2 |
| Fermented beverages | 1–2 servings | 1 |
| Scoring of Food Group Calculated on a Weekly Basis | ||
| Food Group | Servings per Week | Score (e-12HR) |
| Potatoes | ≤3 servings | 1 |
| Legumes | ≥2 servings | 1 |
| Eggs | 2–4 servings | 1 |
| Fish | ≥2 servings | 1 |
| White meat | 2–3 servings | 1 |
| Red/processed meat | <2 servings | 1 |
| Sweets | ≤2 servings | 1 |
| Total maximum score | 24 | |
| Your Global Index of Adherence to the Mediterranean Diet is the Result of Fulfilling the Following Recommendations:  | However, to Improve your Global Index of Adherence to the Mediterranean Diet, you Should Make a Little Effort to Follow These Recommendations: |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| Characteristics | Control Group (CG) | Intervention Group (IG) | |||
|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | p-Value | |
| Participants who completed the study | 128 (100) | - * | 158 (100) | - | - |
| Age (years) | - | 20.6 (2.1) | - | 20.7 (3.6) | 0.453 a |
| Gender | |||||
| Females | 91 (71.1) | - | 121 (76.6) | - | 0.292 b |
| Males | 37 (28.9) | - | 37 (23.4) | - | |
| Schools | |||||
| Pharmacy | 59 (46.1) | - | 40 (25.3) | - | 0.000 b |
| Medicine | 69 (53.9) | - | 118 (74.7) | - | |
| BMI (kg/m2) | - | 22.1 (3.4) | - | 21.8 (3.2) | 0.432 a |
| Smoking status | |||||
| No | 115 (89.9) | - | 147 (93.0) | - | 0.333 b |
| Yes | 13 (10.2) | - | 11 (7.0) | - | |
| Physical activity status (minutes/week) | |||||
| ≥150 | 99 (77.3) | - | 117 (74.1) | - | 0.520 b |
| <150 | 29 (22.7) | - | 41 (25.9) | - | |
| AMD Index | |||
|---|---|---|---|
| Control Group (CG) | Intervention Group (IG) | ||
| Number of Week | Mean (SD) | Mean (SD) | p |
| Week 1 | 7.91 (2.57) | 8.08 (2.70) | 0.697 b |
| Week 2 | 7.91 (2.55) | 8.62 (2.73) | 0.039 b |
| Week 3 | 7.95 (2.49) | 9.51 (3.03) | 0.000 b |
| Week 4 | 8.20 (2.35) | 9.63 (3.09) | 0.000 b |
| p | 0.446 a | 0.000 a | |
| Control Group (CG) | Intervention Group (IG) | ||||
|---|---|---|---|---|---|
| AMD index | |||||
| Number of Week | p | Number of Week | p | ||
| Mean (SD) | Mean (SD) | ||||
| Week 1 7.91 (2.57) | Week 2 7.91 (2.55) | 0.915 | Week 1 8.08 (2.70) | Week 2 8.62 (2.73) | 0.006 |
| Week 1 7.91 (2.57) | Week 3 7.95 (2.49) | 0.902 | Week 1 8.08 (2.70) | Week 3 9.51 (3.03) | 0.000 |
| Week 1 7.91 (2.57) | Week 4 8.20 (2.35) | 0.172 | Week 1 8.08 (2.70) | Week 4 9.63 (3.09) | 0.000 |
| Questions | Easy to Complete | Interesting to Complete | Understandable Questions/Feedbacks | I Would be Willing to Complete Again | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Control Group, | Intervention Group, | Control Group, | Intervention Group, | Control Group, | Intervention Group, | Control Group, | Intervention Group, |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Options | ||||||||
| Strongly agree | 46 (74.2) | 59 (73.8) | 19 (30.6) | 33 (41.3) | 42 (67.7) | 44 (55.0) | 16 (25.8) | 29 (36.3) |
| Agree | 12 (19.3) | 19 (23.7) | 34 (54.8) | 39 (48.7) | 18 (29.0) | 28 (35.0) | 32 (51.6) | 37 (46.2) |
| Neither agree nor disagree | 2 (3.2) | 2 (2.5) | 8 (12.9) | 6 (7.5) | 2 (3.2) | 8 (10.0) | 11 (17.7) | 10 (12.5) |
| Disagree | 2 (3.2) | 0 (0.0) | 1 (1.6) | 2 (2.5) | 0 (0.0) | 0 (0.0) | 3 (4.8) | 4 (5.0) |
| Strongly disagree | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Questions | Time to Complete | |||||||
| Control Group, | Intervention Group, | |||||||
| n (%) | n (%) | |||||||
| Options | ||||||||
| <1 min/day | 0 (0.0) | 6 (7.5) | ||||||
| Approximately 1 min/day | 8 (12.9) | 10 (12.5) | ||||||
| Approximately 2 min/day | 23 (37.1) | 36 (45.0) | ||||||
| Approximately 3 min/day | 21 (33.9) | 22 (27.5) | ||||||
| Approximately 4 min/day | 10 (16.1) | 4 (5.0) | ||||||
| 5 min/day or more | 0 (0.0) | 2 (2.5) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béjar, L.M.; García-Perea, M.D.; Mesa-Rodríguez, P. Evaluation of an Application for Mobile Telephones (e-12HR) to Increase Adherence to the Mediterranean Diet in University Students: A Controlled, Randomized and Multicentric Study. Nutrients 2022, 14, 4196. https://doi.org/10.3390/nu14194196
Béjar LM, García-Perea MD, Mesa-Rodríguez P. Evaluation of an Application for Mobile Telephones (e-12HR) to Increase Adherence to the Mediterranean Diet in University Students: A Controlled, Randomized and Multicentric Study. Nutrients. 2022; 14(19):4196. https://doi.org/10.3390/nu14194196
Chicago/Turabian StyleBéjar, Luis M., María Dolores García-Perea, and Pedro Mesa-Rodríguez. 2022. "Evaluation of an Application for Mobile Telephones (e-12HR) to Increase Adherence to the Mediterranean Diet in University Students: A Controlled, Randomized and Multicentric Study" Nutrients 14, no. 19: 4196. https://doi.org/10.3390/nu14194196
APA StyleBéjar, L. M., García-Perea, M. D., & Mesa-Rodríguez, P. (2022). Evaluation of an Application for Mobile Telephones (e-12HR) to Increase Adherence to the Mediterranean Diet in University Students: A Controlled, Randomized and Multicentric Study. Nutrients, 14(19), 4196. https://doi.org/10.3390/nu14194196






