The Relationship between Dietary Patterns and High Blood Glucose among Adults Based on Structural Equation Modelling
Abstract
1. Introduction
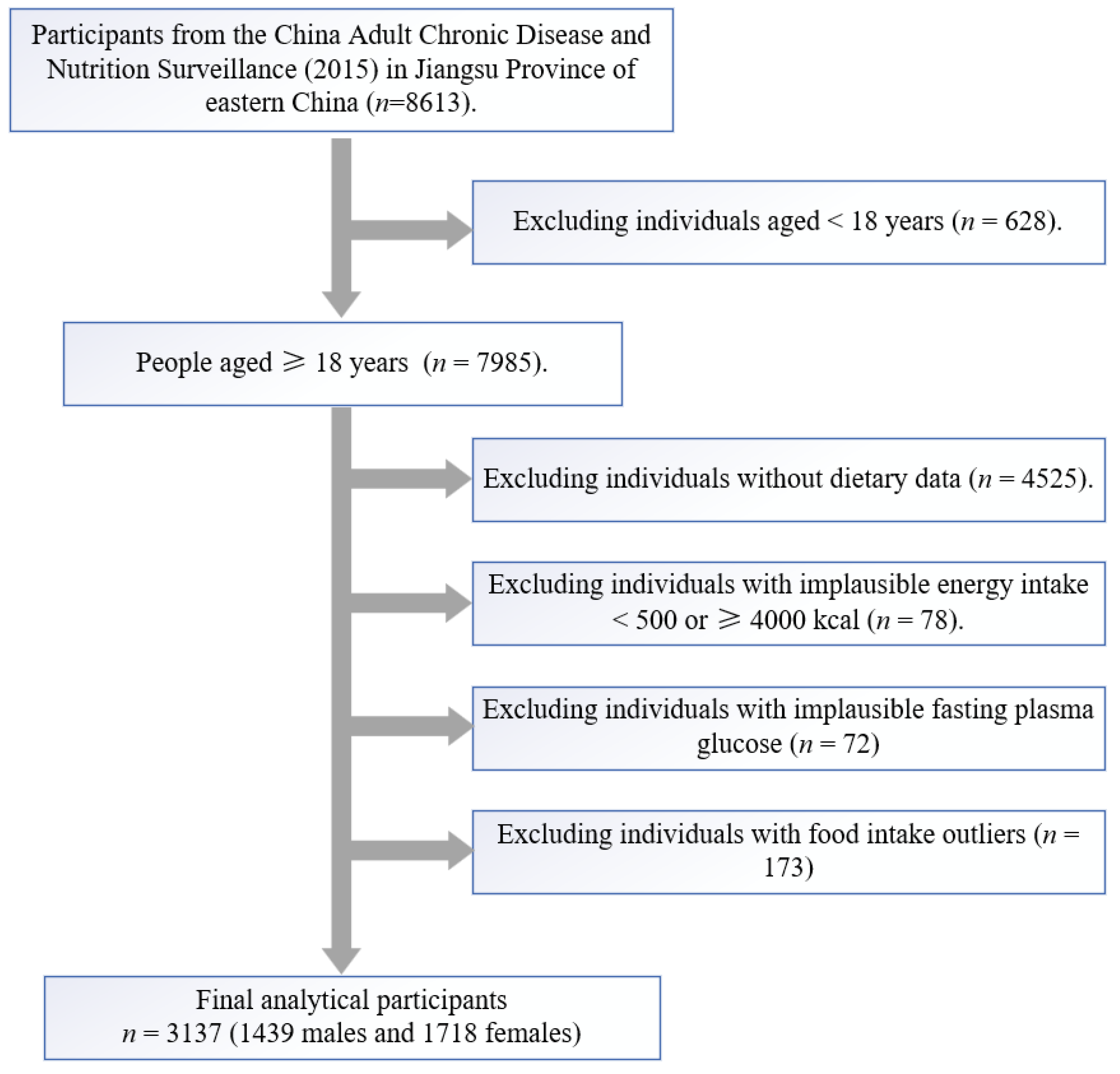
2. Participants and Methods
2.1. Study Population
2.2. Anthropometric Measurement
2.3. Biochemical Indicator
2.4. Dietary Assessment
2.5. Dietary Pattern Analysis
2.6. Structural Equation Modelling
2.7. Statistical Analysis
3. Results
3.1. Basic Information of Participants
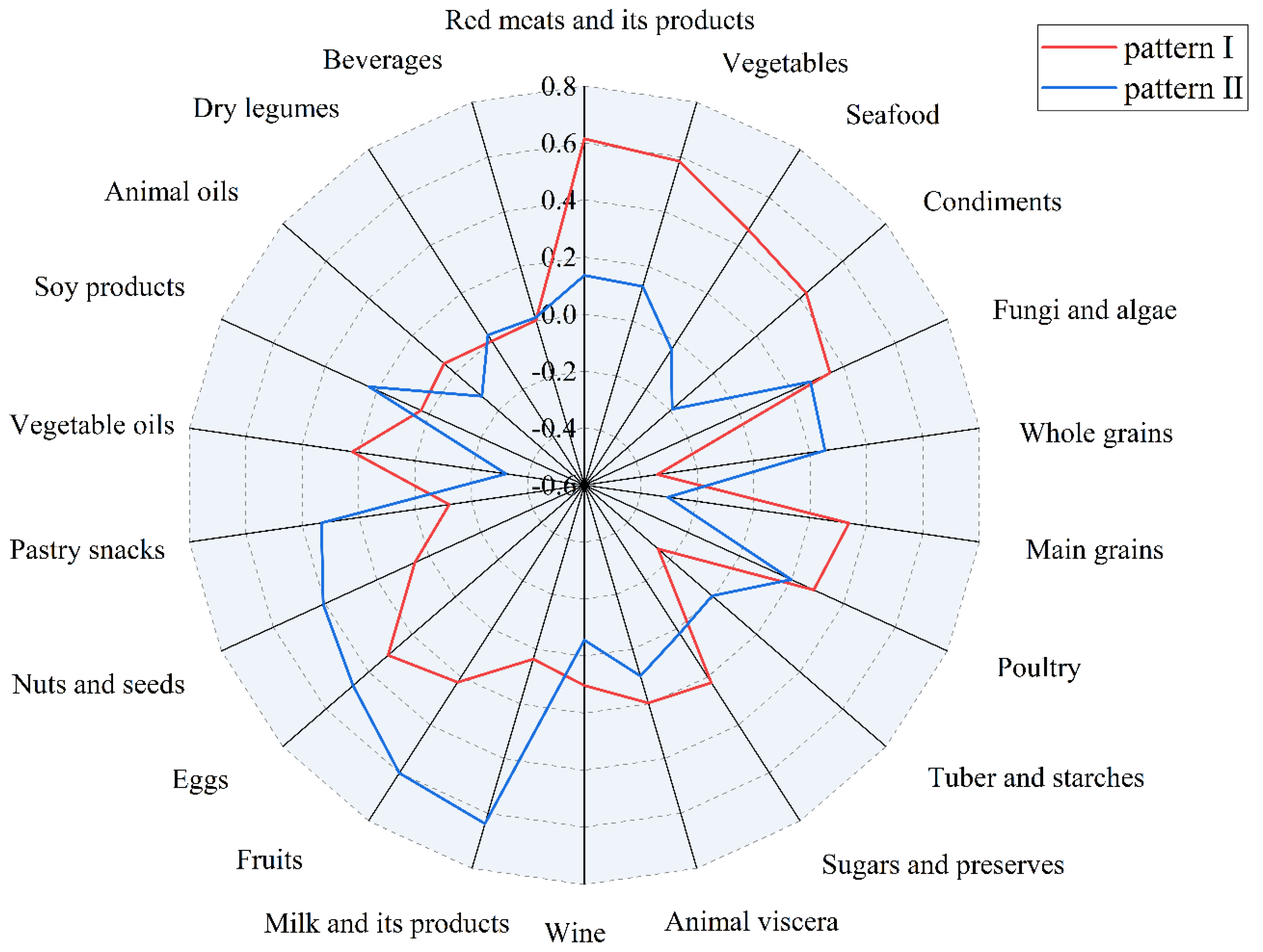
3.2. Determination of Dietary Patterns
3.3. Analysis of the Relationship between Dietary Patterns and High Blood Glucose by Multivariate Logistic Regression
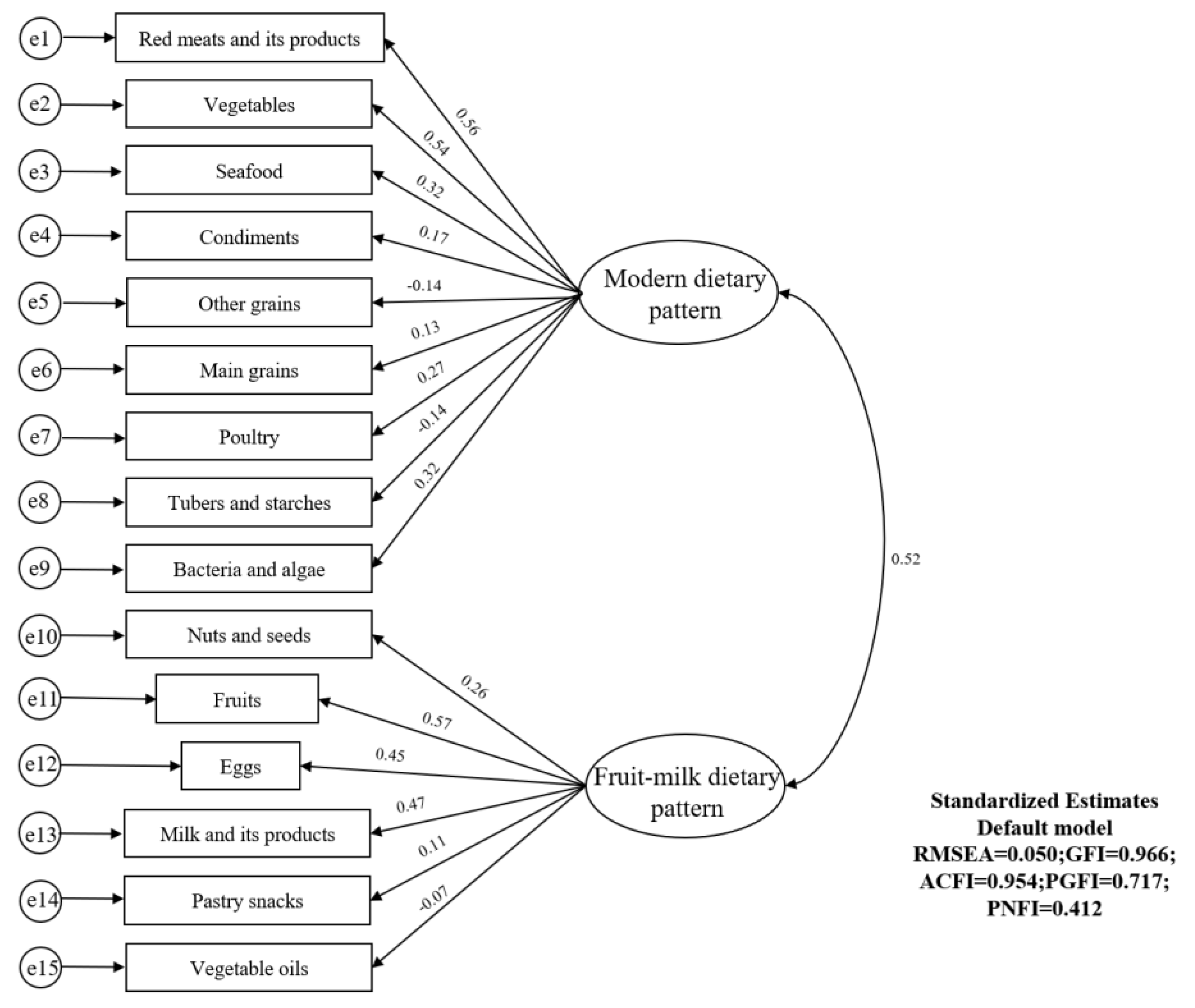
3.4. Structural Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.V.; McDonnell, M.E. Pathogenesis of Cardiovascular Disease in Diabetes. Endocrinol. Metab. Clin. N. Am. 2018, 47, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.S.; Janson, B.J.; Skeie, J.M.; Ling, J.J.; Greiner, M.A. The effects of diabetes mellitus on the corneal endothelium: A review. Surv. Ophthalmol. 2020, 65, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. Christian Bommer Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- China. Report on the Status of Nutrition and Chronic Diseases Among Chinese Residents 2020; People’s Publishing House: Beijing, China, 2020. [Google Scholar]
- Zaroudi, M.; Charati, J.Y.; Mehrabi, S.; Ghorbani, E.; Norouzkhani, J.; Shirashiani, H.; Nikzad, B.; Seiedpour, M.; Izadi, M.; Mirzaei, M.; et al. Dietary Patterns Are Associated with Risk of Diabetes Type 2: A Population-Based Case-Control Study. Arch. Iran. Med. 2016, 19, 166–172. [Google Scholar]
- Ojo, O. Dietary Intake and Type 2 Diabetes. Nutrients 2019, 11, 2177. [Google Scholar] [CrossRef]
- Ozcariz, S.G.; Bernardo Cde, O.; Cembranel, F.; Peres, M.A.; González-Chica, D.A. Dietary practices among individuals with diabetes and hypertension are similar to those of healthy people: A population-based study. BMC Public Health 2015, 15, 479. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef]
- Sprake, E.F.; Russell, J.M.; Cecil, J.E.; Cooper, R.J.; Grabowski, P.; Pourshahidi, L.K.; Barker, M.E. Dietary patterns of university students in the UK: A cross-sectional study. Nutr. J. 2018, 17, 90. [Google Scholar] [CrossRef]
- O’Hara, C.; Gibney, E.R. Meal Pattern Analysis in Nutritional Science: Recent Methods and Findings. Adv. Nutr. 2021, 12, 1365–1378. [Google Scholar] [CrossRef]
- Iqbal, K.; Schwingshackl, L.; Floegel, A.; Schwedhelm, C.; Stelmach-Mardas, M.; Wittenbecher, C.; Galbete, C.; Knüppel, S.; Schulze, M.B.; Boeing, H. Graphical models identified food intake networks and risk of type 2 diabetes, CVD, and cancer in the EPIC-Potsdam study. Eur. J. Nutr. 2019, 58, 1673–1686. [Google Scholar] [CrossRef]
- Archundia Herrera, M.C.; Subhan, F.B.; Chan, C.B. Dietary Patterns and Cardiovascular Disease Risk in People with Type 2 Diabetes. Curr. Obes. Rep. 2017, 6, 405–413. [Google Scholar] [CrossRef]
- Schulz, C.A.; Oluwagbemigun, K.; Nöthlings, U. Advances in dietary pattern analysis in nutritional epidemiology. Eur. J. Nutr. 2021, 60, 4115–4130. [Google Scholar] [CrossRef]
- Castro, M.A.; Baltar, V.T.; Marchioni, D.M.; Fisberg, R.M. Examining associations between dietary patterns and metabolic CVD risk factors: A novel use of structural equation modelling. Br. J. Nutr. 2016, 115, 1586–1597. [Google Scholar] [CrossRef]
- Tod, D.; Edwards, C.; Hall, G. Drive for leanness and health-related behavior within a social/cultural perspective. Body Image 2013, 10, 640–643. [Google Scholar] [CrossRef]
- Jöreskog, K.G. Structural analysis of covariance and correlation matrices. Psychometrika 1978, 43, 443–477. [Google Scholar] [CrossRef]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef]
- Yue, J.; Mao, X.; Xu, K.; Lü, L.; Liu, S.; Chen, F.; Wang, J. Awareness, Treatment and Control of Diabetes Mellitus in a Chinese Population. PLoS ONE 2016, 11, e0153791. [Google Scholar] [CrossRef]
- Papamichou, D.; Panagiotakos, D.B.; Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 531–543. [Google Scholar] [CrossRef]
- Zhen, S.; Ma, Y.; Zhao, Z.; Yang, X.; Wen, D. Dietary pattern is associated with obesity in Chinese children and adolescents: Data from China Health and Nutrition Survey (CHNS). Nutr. J. 2018, 17, 68. [Google Scholar] [CrossRef]
- Xu, X.; Hall, J.; Byles, J.; Shi, Z. Dietary Pattern Is Associated with Obesity in Older People in China: Data from China Health and Nutrition Survey (CHNS). Nutrients 2015, 7, 8170–8188. [Google Scholar] [CrossRef]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.S.; Mookadam, F.; Krasuski, R.A.; Ahmed, H. Association Between Obesity and Cardiovascular Outcomes: A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA Netw. Open. 2018, 1, e183788. [Google Scholar] [CrossRef]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef]
- Talaei, M.; Wang, Y.L.; Yuan, J.M.; Pan, A.; Koh, W.P. Meat, Dietary Heme Iron, and Risk of Type 2 Diabetes Mellitus: The Singapore Chinese Health Study. Am. J. Epidemiol. 2017, 186, 824–833. [Google Scholar] [CrossRef]
- Maghsoudi, Z.; Ghiasvand, R.; Salehi-Abargouei, A. Empirically derived dietary patterns and incident type 2 diabetes mellitus: A systematic review and meta-analysis on prospective observational studies. Public Health Nutr. 2016, 19, 230–241. [Google Scholar] [CrossRef]
- Jiang, R.; Ma, J.; Ascherio, A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: A prospective cohort study. Am. J. Clin. Nutr. 2004, 79, 70–75. [Google Scholar] [CrossRef] [PubMed]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [PubMed]
- Wang, Y.-Y.; Zhang, J.-X.; Tian, T.; Gao, M.-Y.; Zhu, Q.-R.; Xie, W.; Fu, L.-M.; Wang, S.-K.; Dai, Y. Dietary patterns in association with the risk of elevated blood pressure, lipid profile and fasting plasma glucose among adults in Jiangsu Province of China. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Åberg, S.; Mann, J.; Neumann, S.; Ross, A.B.; Reynolds, A.N. Whole-Grain Processing and Glycemic Control in Type 2 Diabetes: A Randomized Crossover Trial. Diabetes Care 2020, 43, 1717–1723. [Google Scholar] [CrossRef]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Haus, J.M.; Filion, J.; Pagadala, M.R.; Jean-Philippe, G.; Kochhar, S.; Ross, A.B.; Kirwan, J.P. A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: A randomized-controlled trial. Metabolism 2018, 82, 111–117. [Google Scholar] [CrossRef]
- Stephen, A.M.; Cummings, J.H. Mechanism of action of dietary fibre in the human colon. Nature 1980, 284, 283–284. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of whole grain foods and risk of type 2 diabetes: Results from three prospective cohort studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, T.-Y.; He, Y.; Gou, W.; Zuo, L.-S.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 371. [Google Scholar] [CrossRef]
- Feng, Q.; Kim, J.H.; Omiyale, W.; Bešević, J.; Conroy, M.; May, M.; Yang, Z.; Wong, S.Y.; Tsoi, K.K.; Allen, N.; et al. Raw and Cooked Vegetable Consumption and Risk of Cardiovascular Disease: A Study of 400,000 Adults in UK Biobank. Front. Nutr. 2022, 9, 831470. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Fan, M.; Cui, S.; Li, L. Meat and fish intake and type 2 diabetes: Dose-response meta-analysis of prospective cohort studies. Diabetes Metab. 2020, 46, 345–352. [Google Scholar] [CrossRef]
- Cruz, N.G.; Sousa, L.P.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 85–92. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; Ohashi, Y.; et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: Analysis of the Japan Diabetes Complications Study (JDCS). J. Clin. Endocrinol. Metab. 2014, 99, 3635–3643. [Google Scholar] [CrossRef]
- Talaei, M.; Pan, A.; Yuan, J.M.; Koh, W.P. Dairy intake and risk of type 2 diabetes. Clin. Nutr. 2018, 37, 712–718. [Google Scholar] [CrossRef]
- Gao, D.; Ning, N.; Wang, C.; Wang, Y.; Li, Q.; Meng, Z.; Liu, Y.; Li, Q. Dairy products consumption and risk of type 2 diabetes: Systematic review and dose-response meta-analysis. PLoS ONE 2013, 8, e73965. [Google Scholar] [CrossRef]
- Yu, Z.; Malik, V.S.; Keum, N.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Bao, Y. Associations between nut consumption and inflammatory biomarkers. Am. J. Clin. Nutr. 2016, 104, 722–728. [Google Scholar] [CrossRef]
- Muraki, I.; Imamura, F.; Manson, E.J.; Hu, F.B.; Willett, W.C.; van Dam, R.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef]

| Groups | Male | Female | p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 56.5 | 14.5 | 54.3 | 14.6 | <0.001 |
| Blood glucose (mmol/L) | 5.5 | 1.4 | 5.4 | 1.3 | 0.031 |
| BMI (kg/m2) | 24.9 | 3.3 | 24.8 | 3.6 | 0.091 |
| Energy intake (kcal/d) | 1847.5 | 540.4 | 1540.8 | 440.9 | <0.001 |
| n | % | n | % | p-value | |
| Age group (years) | <0.001 | ||||
| 18~34 | 143 | 10.0 | 214 | 12.5 | |
| 35~49 | 269 | 18.8 | 375 | 22.0 | |
| 50~64 | 542 | 37.9 | 671 | 39.3 | |
| 65~ | 475 | 33.2 | 448 | 26.2 | |
| BMI level | <0.001 | ||||
| Thinness | 22 | 1.5 | 38 | 2.2 | |
| Normal | 528 | 36.9 | 703 | 41.2 | |
| Overweight | 635 | 44.4 | 667 | 39.1 | |
| Obesity | 244 | 17.1 | 300 | 17.6 | |
| Central obesity | 0.574 | ||||
| No | 865 | 60.5 | 1017 | 59.5 | |
| Yes | 564 | 39.5 | 691 | 40.5 | |
| Smoking behaviour | <0.001 | ||||
| No | 776 | 54.3 | 1684 | 98.6 | |
| Yes | 653 | 45.7 | 24 | 1.4 | |
| Diabetes | 0.223 | ||||
| No | 1296 | 90.7 | 1570 | 91.9 | |
| Yes | 133 | 9.3 | 138 | 8.1 |
| Groups | OR | 95% CI | p-Value | p for Trend |
|---|---|---|---|---|
| Modern Dietary Pattern | ||||
| Q1 | 1.000 | 0.021 | ||
| Q2 | 1.441 | (0.992~2.094) | 0.055 | |
| Q3 | 1.566 | (1.063~2.308) | 0.023 | |
| Q4 | 1.561 | (1.025~2.379) | 0.038 | |
| Fruit–milk dietary pattern | ||||
| Q1 | 1.000 | 0.232 | ||
| Q2 | 0.998 | (0.687~1.451) | 0.992 | |
| Q3 | 1.060 | (0.734~1.531) | 0.755 | |
| Q4 | 1.269 | (0.887~1.814) | 0.192 |
| Path Analysis | Non-Standardised Coefficient | Standardised Coefficients | S.E. | C.R. | p-Value |
|---|---|---|---|---|---|
| Modern dietary pattern →diabetes | 0.001 | 0.127 | 0.000 | 3.417 | <0.001 |
| Fruit-milk dietary pattern→diabetes | −0.003 | −0.032 | 0.003 | −0.903 | 0.366 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, W.; Tian, T.; Zhang, J.; Zhu, Q.; Pan, D.; Xu, D.; Lu, Y.; Sun, G.; Dai, Y. The Relationship between Dietary Patterns and High Blood Glucose among Adults Based on Structural Equation Modelling. Nutrients 2022, 14, 4111. https://doi.org/10.3390/nu14194111
Wang Y, Xie W, Tian T, Zhang J, Zhu Q, Pan D, Xu D, Lu Y, Sun G, Dai Y. The Relationship between Dietary Patterns and High Blood Glucose among Adults Based on Structural Equation Modelling. Nutrients. 2022; 14(19):4111. https://doi.org/10.3390/nu14194111
Chicago/Turabian StyleWang, Yuanyuan, Wei Xie, Ting Tian, Jingxian Zhang, Qianrang Zhu, Da Pan, Dengfeng Xu, Yifei Lu, Guiju Sun, and Yue Dai. 2022. "The Relationship between Dietary Patterns and High Blood Glucose among Adults Based on Structural Equation Modelling" Nutrients 14, no. 19: 4111. https://doi.org/10.3390/nu14194111
APA StyleWang, Y., Xie, W., Tian, T., Zhang, J., Zhu, Q., Pan, D., Xu, D., Lu, Y., Sun, G., & Dai, Y. (2022). The Relationship between Dietary Patterns and High Blood Glucose among Adults Based on Structural Equation Modelling. Nutrients, 14(19), 4111. https://doi.org/10.3390/nu14194111








