The Effect of Dextrose or Protein Ingestion on Circulating Growth Differentiation Factor 15 and Appetite in Older Compared to Younger Women
Abstract
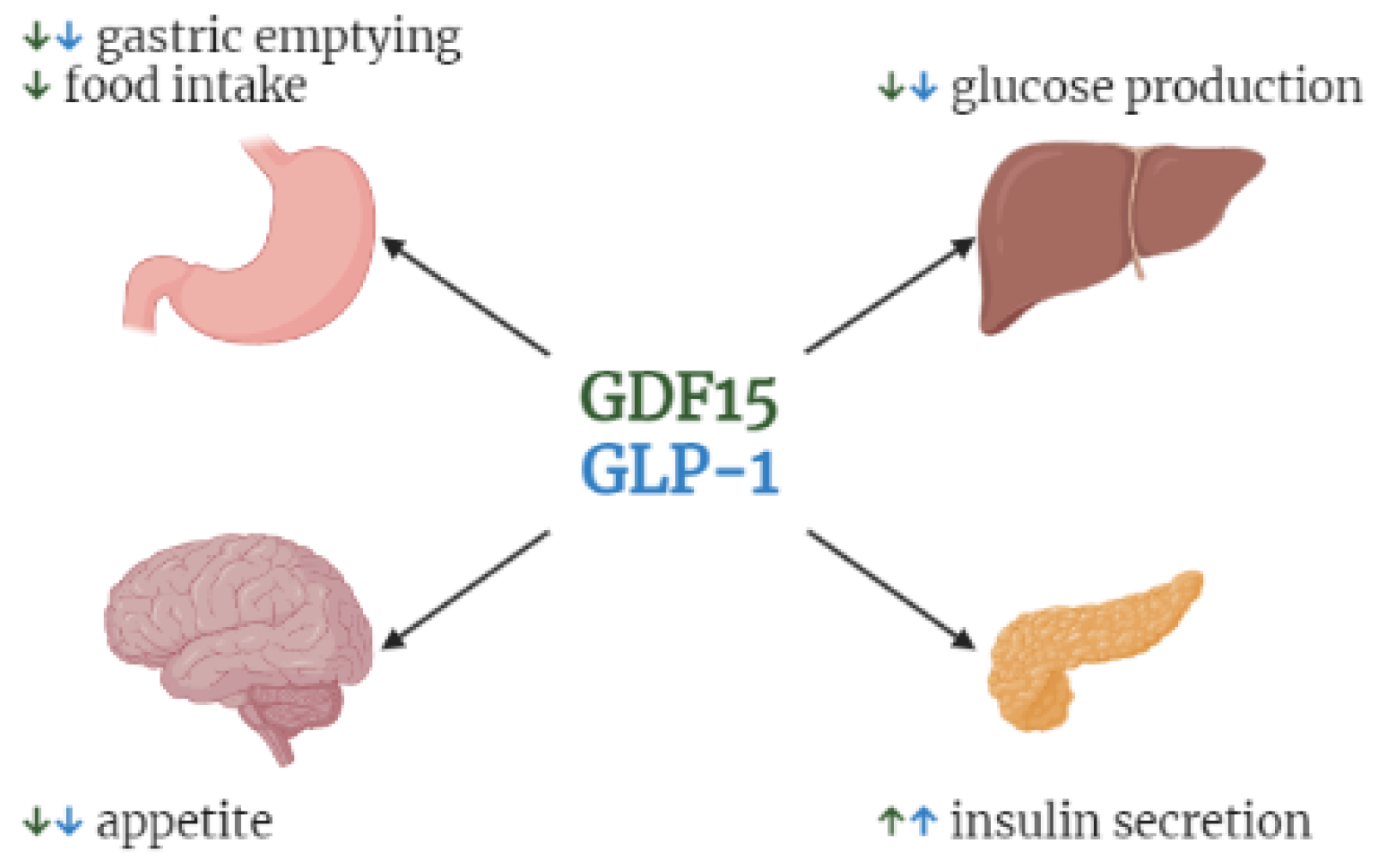
1. Introduction
2. Materials and Methods
3. Results
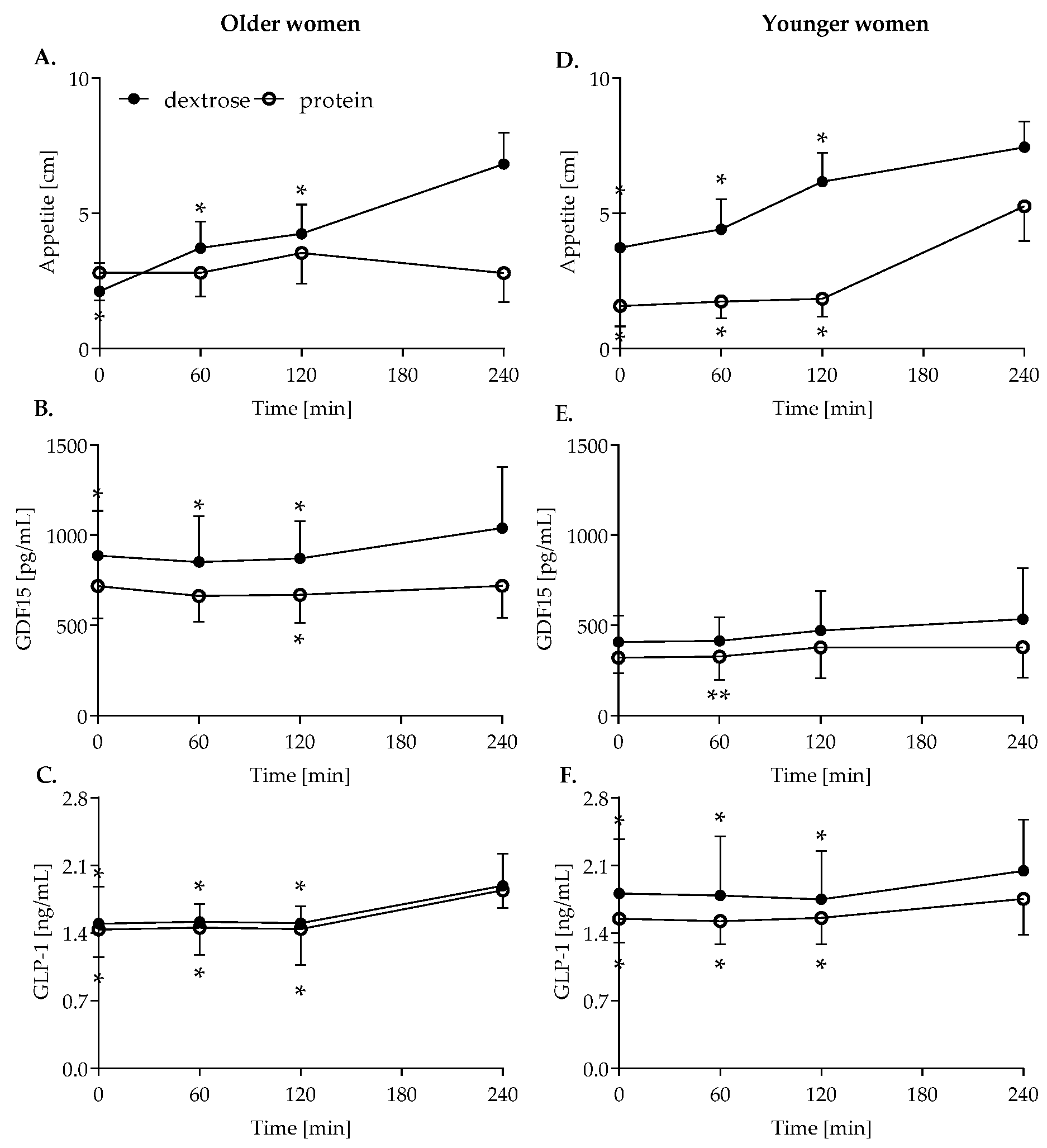
3.1. Appetite
3.2. GDF15
3.3. GLP-1
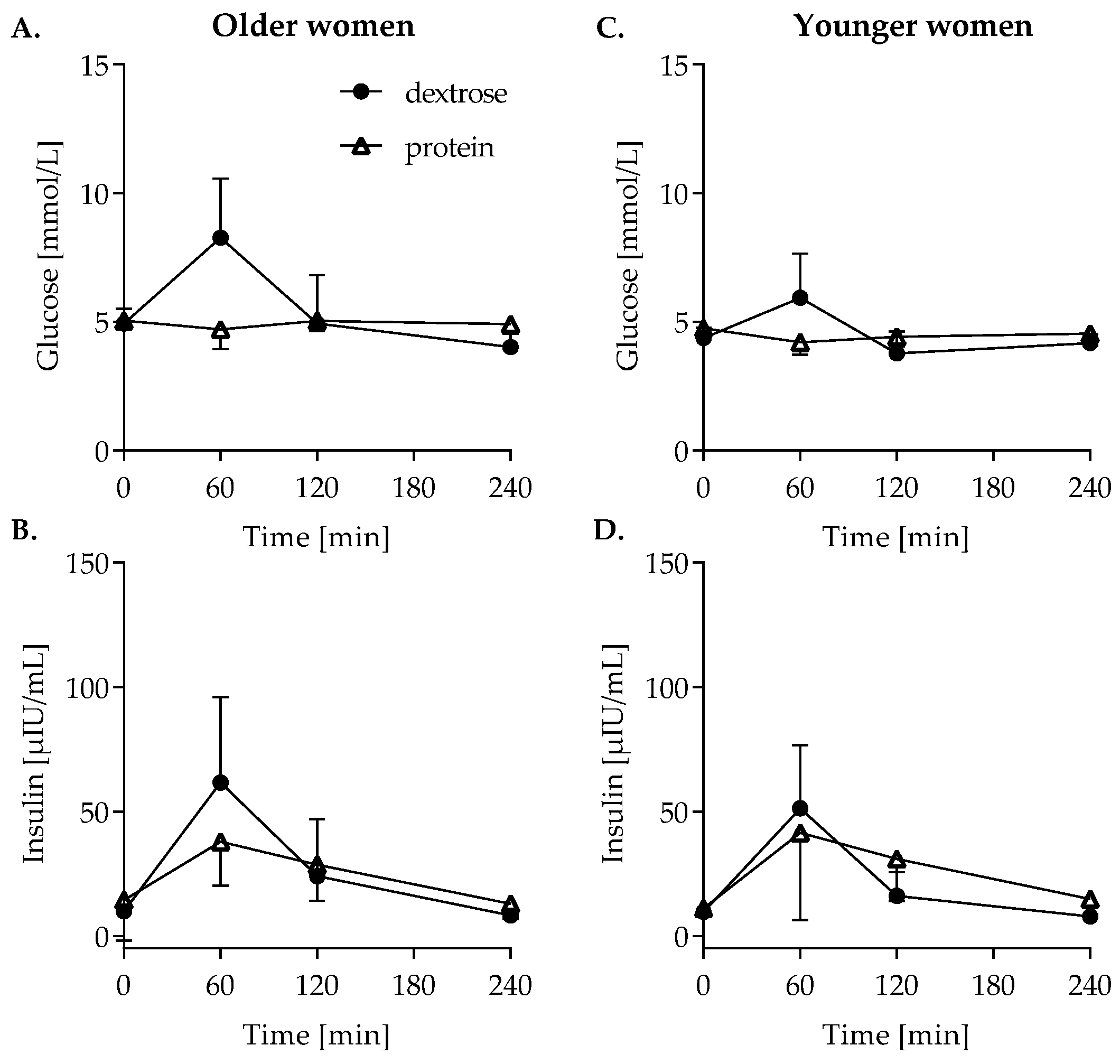
3.4. Glucose Metabolism
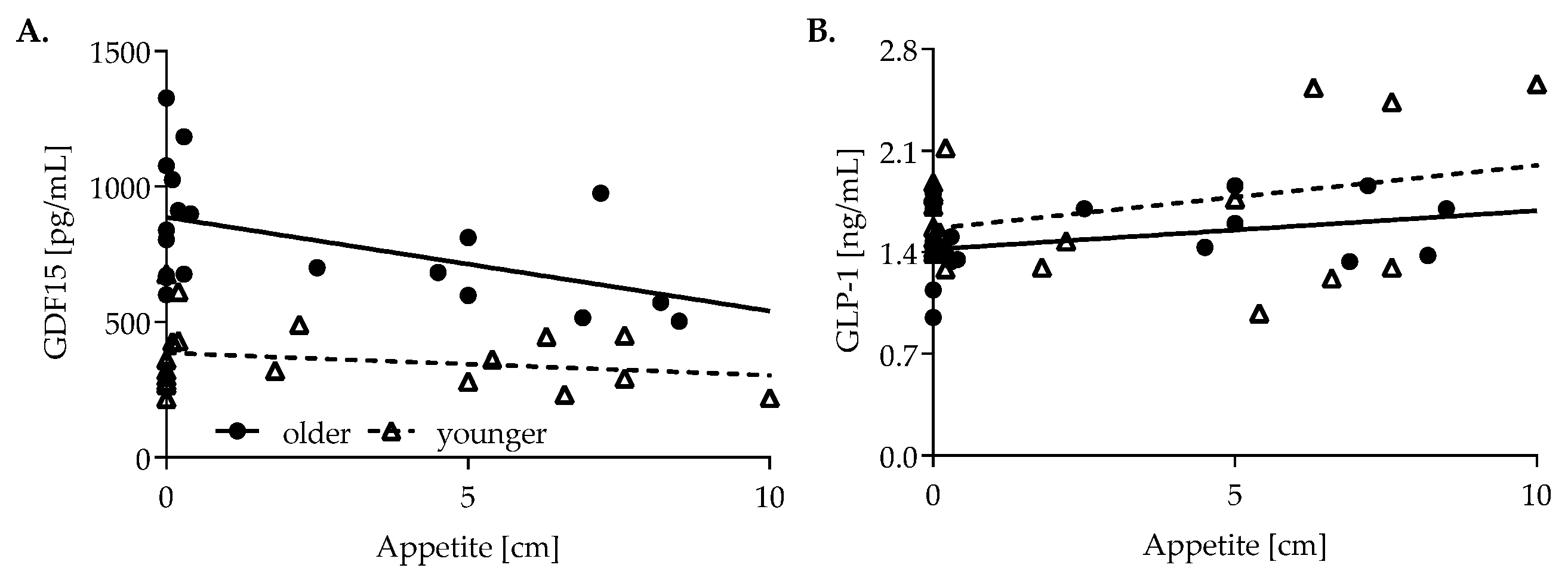
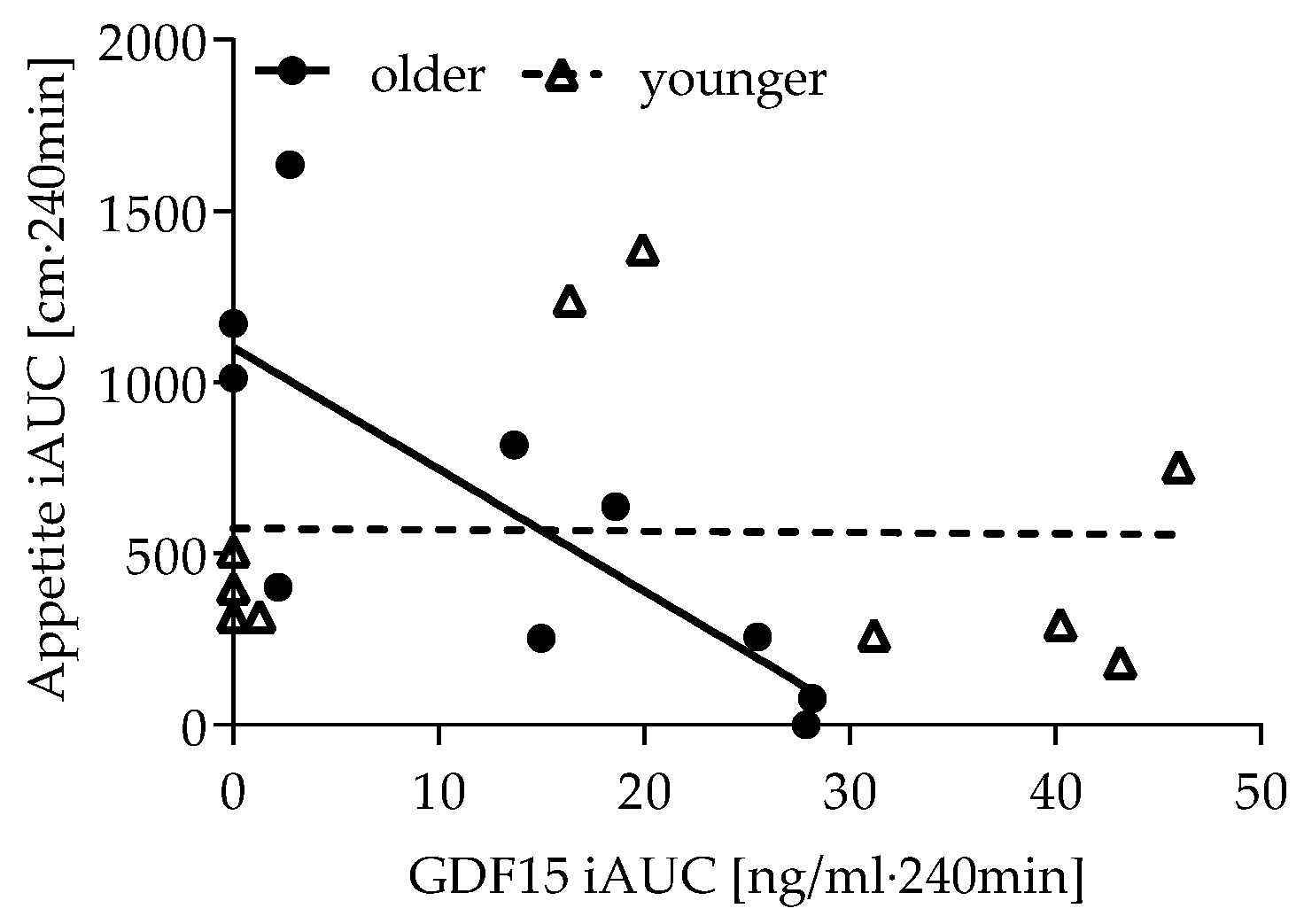
3.5. Association of Appetite, GDF15, GLP-1, Glucose and Insulin Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breit, S.N.; Brown, D.A.; Tsai, V.W.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Giuliani, C.; Chiariello, A.; Iannuzzi, V.; Franceschi, C.; Salvioli, S. GDF15, an emerging key player in human aging. Ageing Res. Rev. 2022, 75, 101569. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Alvarez-Guaita, A.; Melvin, A.; Rimmington, D.; Dattilo, A.; Miedzybrodzka, E.L.; Cimino, I.; Maurin, A.C.; Roberts, G.P.; Meek, C.L.; et al. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 2019, 29, 707–718.e8. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 550, 255–259. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Macia, L.; Johnen, H.; Kuffner, T.; Manadhar, R.; Jørgensen, S.B.; Lee-Ng, K.K.M.; Zhang, H.P.; Wu, L.; Marquis, C.P.; et al. TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS ONE 2013, 8, e55174. [Google Scholar] [CrossRef]
- Borner, T.; Wald, H.S.; Ghidewon, M.Y.; Zhang, B.; Wu, Z.; De Jonghe, B.C.; Breen, D.; Grill, H.J. GDF15 Induces an Aversive Visceral Malaise State that Drives Anorexia and Weight Loss. Cell Rep. 2020, 31, 107543. [Google Scholar] [CrossRef]
- Xiong, Y.; Walker, K.; Min, X.; Hale, C.; Tran, T.; Komorowski, R.; Yang, J.; Davda, J.; Nuanmanee, N.; Kemp, D.; et al. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci. Transl. Med. 2017, 9, eaan8732. [Google Scholar] [CrossRef]
- Hays, N.P.; Roberts, S.B. The anorexia of aging in humans. Physiol. Behav. 2006, 88, 257–266. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Di Francesco, V.; Zamboni, M.; Dioli, A.; Zoico, E.; Mazzali, G.; Omizzolo, F.; Bissoli, L.; Solerte, S.B.; Benini, L.; Bosello, O. Delayed postprandial gastric emptying and impaired gallbladder contraction together with elevated cholecystokinin and peptide YY serum levels sustain satiety and inhibit hunger in healthy elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1581–1585. [Google Scholar] [CrossRef]
- Kleinert, M.; Bojsen-Møller, K.N.; Jørgensen, N.B.; Svane, M.S.; Martinussen, C.; Kiens, B.; Wojtaszewski, J.F.; Madsbad, S.; Richter, E.A.; Clemmensen, C. Effect of bariatric surgery on plasma GDF15 in humans. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E615–E621. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, B.; Agazzi, C.; Pierpont, B.; Chick, J.; Li, Z.; Caprio, S.; Santoro, N. Growth differentiation factor 15 (GDF15) is associated with non-alcoholic fatty liver disease (NAFLD) in youth with overweight or obesity. Nutr. Diabetes 2022, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner-Reiter, M.H.; Kasses, D.; Tugendsam, C.; Riedl, M.; Peric, S.; Prager, G.; Krebs, M.; Promintzer-Schifferl, M.; Clodi, M.; Luger, A.; et al. Growth differentiation factor 15 increases following oral glucose ingestion: Effect of meal composition and obesity. Eur. J. Endocrinol. 2016, 175, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.W.; Macia, L.; Feinle-Bisset, C.; Manandhar, R.; Astrup, A.; Raben, A.; Lorenzen, J.K.; Schmidt, P.T.; Wiklund, F.; Pedersen, N.L.; et al. Serum Levels of Human MIC-1/GDF15 Vary in a Diurnal Pattern, Do Not Display a Profile Suggestive of a Satiety Factor and Are Related to BMI. PLoS ONE 2015, 10, e0133362. [Google Scholar] [CrossRef]
- Herpich, C.; Haß, U.; Kochlik, B.; Franz, K.; Laeger, T.; Klaus, S.; Bosy-Westphal, A.; Norman, K. Postprandial dynamics and response of fibroblast growth factor 21 in older adults. Clin. Nutr. 2021, 40, 3765–3771. [Google Scholar] [CrossRef]
- Herpich, C.; Franz, K.; Ost, M.; Otten, L.; Coleman, V.; Klaus, S.; Müller-Werdan, U.; Norman, K. Associations Between Serum GDF15 Concentrations, Muscle Mass, and Strength Show Sex-Specific Differences in Older Hospital Patients. Rejuvenation Res. 2021, 24, 14–19. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Slosman, D.O.; Pichard, C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition 2001, 17, 534–541. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Baek, S.J.; Eling, T. Growth differentiation factor 15 (GDF15): A survival protein with therapeutic potential in metabolic diseases. Pharmacol. Ther. 2019, 198, 46–58. [Google Scholar] [CrossRef]
- Borner, T.; Shaulson, E.D.; Ghidewon, M.Y.; Barnett, A.B.; Horn, C.C.; Doyle, R.P.; Grill, H.J.; Hayes, M.R.; De Jonghe, B.C. GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 2020, 31, 351–362.e5. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.B.; Nicolaisen, T.S.; Ørtenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T.; et al. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat. Commun. 2021, 12, 1041. [Google Scholar] [CrossRef] [PubMed]
- Aaboe, K.; Krarup, T.; Madsbad, S.; Holst, J.J. GLP-1: Physiological effects and potential therapeutic applications. Diabetes Obes. Metab. 2008, 10, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.A.; Ip, W.; Jin, T. The role of the Wnt signaling pathway in incretin hormone production and function. Front. Physiol. 2012, 3, 273. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, D.A. The non-regulation of food intake in humans: Hope for reversing the epidemic of obesity. Physiol. Behav. 2005, 86, 623–632. [Google Scholar] [CrossRef] [PubMed]
| Older Women n = 20 | Younger Women n = 20 | p-Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 73.7 ± 6.30 | 25.7 ± 4.39 | |
| BMI (kg/m2) | 23.9 ± 3.93 | 22.1 ± 2.33 | 0.090 |
| FMI (kg/m2) | 8.65 ± 2.78 | 7.04 ± 1.73 | 0.036 |
| Glucose (mmol/L) | 4.98 ± 0.44 | 4.54 ± 0.42 | 0.003 |
| Insulin (µUI/mL) | 12.2 ± 11.5 | 10.3 ± 2.49 | 0.488 |
| HOMA-IR | 2.70 ± 2.48 | 2.09 ± 0.58 | 0.309 |
| GDF15 (pg/mL) | 802 ± 227 | 364 ± 125 | <0.001 |
| GLP-1 (ng/mL) | 2.60 ± 1.37 | 3.62 ± 2.29 | 0.108 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herpich, C.; Lehmann, S.; Kochlik, B.; Kleinert, M.; Klaus, S.; Müller-Werdan, U.; Norman, K. The Effect of Dextrose or Protein Ingestion on Circulating Growth Differentiation Factor 15 and Appetite in Older Compared to Younger Women. Nutrients 2022, 14, 4066. https://doi.org/10.3390/nu14194066
Herpich C, Lehmann S, Kochlik B, Kleinert M, Klaus S, Müller-Werdan U, Norman K. The Effect of Dextrose or Protein Ingestion on Circulating Growth Differentiation Factor 15 and Appetite in Older Compared to Younger Women. Nutrients. 2022; 14(19):4066. https://doi.org/10.3390/nu14194066
Chicago/Turabian StyleHerpich, Catrin, Stephanie Lehmann, Bastian Kochlik, Maximilian Kleinert, Susanne Klaus, Ursula Müller-Werdan, and Kristina Norman. 2022. "The Effect of Dextrose or Protein Ingestion on Circulating Growth Differentiation Factor 15 and Appetite in Older Compared to Younger Women" Nutrients 14, no. 19: 4066. https://doi.org/10.3390/nu14194066
APA StyleHerpich, C., Lehmann, S., Kochlik, B., Kleinert, M., Klaus, S., Müller-Werdan, U., & Norman, K. (2022). The Effect of Dextrose or Protein Ingestion on Circulating Growth Differentiation Factor 15 and Appetite in Older Compared to Younger Women. Nutrients, 14(19), 4066. https://doi.org/10.3390/nu14194066






