The Performances of SNAQ, GLIM, mNICE, and ASPEN for Identification of Neurocritically Ill Patients at High Risk of Developing Refeeding Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. RFS Risk Screening and Assessment
2.3. RFS Definition and Data Collection
2.4. Nutrition Management
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Differences between the RFS and Non-RFS Groups
3.3. Validation and Comparison of Four Screening Tools for Predicting RFS
3.4. Prediction Models of RFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doig, G.S.; Simpson, F.; Heighes, P.T.; Bellomo, R.; Chesher, D.; Caterson, I.D.; Reade, M.C.; Harrigan, P.W.J. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: A randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir. Med. 2015, 3, 943–952. [Google Scholar] [CrossRef]
- Mehanna, H.M.; Moledina, J.; Travis, J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ 2008, 336, 1495–1498. [Google Scholar] [CrossRef]
- Friedli, N.; Stanga, Z.; Sobotka, L.; Culkin, A.; Kondrup, J.; Laviano, A.; Mueller, B.; Schuetz, P. Revisiting the Refeeding Syndrome: Results of a Systematic Review. Nutrition 2016, 35, 151–160. [Google Scholar] [CrossRef]
- Vignaud, M.; Constantin, J.; Ruivard, M.; Villemeyre-Plane, M.; Futier, E.; Bazin, J.; Annane, D.; AZUREA, G.A.S.G. Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: An observational study. Crit. Care 2010, 14, R172. [Google Scholar] [CrossRef]
- Ponzo, V.; Pellegrini, M.; Cioffi, I.; Scaglione, L.; Bo, S. The Refeeding Syndrome: A neglected but potentially serious condition for inpatients. A narrative review. Intern. Emerg. Med. 2021, 16, 49–60. [Google Scholar] [CrossRef]
- Schnitker, M.A.; Mattman, P.E.; Bliss, T.L. A clinical study of malnutrition in Japanese prisoners of war. Ann. Intern. Med. 1951, 35, 69–96. [Google Scholar]
- Miller, S.J. Death Resulting From Overzealous Total Parenteral Nutrition: The Refeeding Syndrome Revisited. Nutr. Clin. Pr. 2008, 23, 166–171. [Google Scholar] [CrossRef]
- Yoshida, M.; Izawa, J.; Wakatake, H.; Saito, H.; Kawabata, C.; Matsushima, S.; Suzuki, A.; Nagatomi, A.; Yoshida, T.; Masui, Y.; et al. Mortality associated with new risk classification of developing refeeding syndrome in critically ill patients: A cohort study. Clin. Nutr. 2021, 40, 1207–1213. [Google Scholar] [CrossRef]
- Friedli, N.; Stanga, Z.; Culkin, A.; Crook, M.; Laviano, A.; Sobotka, L.; Kressig, R.W.; Kondrup, J.; Mueller, B.; Schuetz, P. Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition 2018, 47, 13–20. [Google Scholar] [CrossRef]
- National Collaborating Centre for Acute Care. Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition; National Collaborating Centre for Acute Care: London, UK, 2006. [Google Scholar]
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.W.; Wierdsma, N.J.; van Bokhorst De Van Der Schueren, M.A.E. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ©). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [CrossRef]
- Goyale, A.; Ashley, S.L.; Taylor, D.R.; Elnenaei, M.O.; Alaghband-Zadeh, J.; Sherwood, R.A.; le Roux, C.W.; Vincent, R.P. Predicting refeeding hypophosphataemia: Insulin growth factor 1 (IGF-1) as a diagnostic biochemical marker for clinical practice. Ann. Clin. Biochem. 2015, 52, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Zeki, S.; Culkin, A.; Gabe, S.M.; Nightingale, J.M. Refeeding hypophosphataemia is more common in enteral than parenteral feeding in adult in patients. Clin. Nutr. 2011, 30, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Kraaijenbrink, B.V.; Lambers, W.M.; Mathus-Vliegen, E.M.; Siegert, C.E. Incidence of refeeding syndrome in internal medicine patients. Neth. J. Med. 2016, 74, 116–121. [Google Scholar] [PubMed]
- Da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition–A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.M.; Livesay, S.L.; Abdelhak, T.; Bleck, T.P.; Human, T.; Karanjia, N.; Lamer-Rosen, A.; Medow, J.; Nyquist, P.A.; Rosengart, A.; et al. Standards for Neurologic Critical Care Units: A Statement for Healthcare Professionals from The Neurocritical Care Society. Neurocrit. Care 2018, 29, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.; Bretón, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L.; Gomes, F.; Jésus, P.; Leischker, A.; Muscaritoli, M.; et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar] [CrossRef]
- Xiong, R.; Huang, H.; Wu, Y.; Wang, S.; Wang, D.; Ji, Z.; Lin, Z.; Zang, N.; Pan, S.; Huang, K. Incidence and outcome of refeeding syndrome in neurocritically ill patients. Clin. Nutr. 2021, 40, 1071–1076. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef]
- Collins, P.F.; Elia, M.; Kurukulaaratchy, R.J.; Stratton, R.J. The influence of deprivation on malnutrition risk in outpatients with chronic obstructive pulmonary disease (COPD). Clin. Nutr. 2018, 37, 144–148. [Google Scholar] [CrossRef]
- Olthof, L.E.; Koekkoek, W.; van Setten, C.; Kars, J.; van Blokland, D.; van Zanten, A. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: A retrospective study. Clin. Nutr. 2018, 37, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Adika, E.; Jia, R.; Li, J.; Seres, D.; Freedberg, D.E. Evaluation of the ASPEN guidelines for refeeding syndrome among hospitalized patients receiving enteral nutrition: A retrospective cohort study. JPEN J. Parenter. Enter. Nutr. 2022. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, F.; Song, C.; Yin, R.; Chang, M.; Zhang, W.; Zhang, B.; Yu, L.; Jia, Y.; Ma, Y.; et al. Safety and efficacy of three enteral feeding strategies in patients with severe stroke in China (OPENS): A multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2022, 21, 319–328. [Google Scholar] [CrossRef]
- Brito, J.E.; Burgel, C.F.; Lima, J.; Chites, V.S.; Saragiotto, C.B.; Rabito, E.I.; Silva, F.M. GLIM criteria for malnutrition diagnosis of hospitalized patients presents satisfactory criterion validity: A prospective cohort study. Clin. Nutr. 2021, 40, 4366–4372. [Google Scholar] [CrossRef]
- Fung, A.T.; Rimmer, J. Hypophosphataemia secondary to oral refeeding syndrome in a patient with long-term alcohol misuse. Med. J. Aust. 2005, 183, 324–326. [Google Scholar] [CrossRef]
- Rio, A.; Whelan, K.; Goff, L.; Reidlinger, D.P.; Smeeton, N. Occurrence of refeeding syndrome in adults started on artificial nutrition support: Prospective cohort study. BMJ Open 2013, 3, e2173. [Google Scholar] [CrossRef]
- Brown, C.A.; Sabel, A.L.; Gaudiani, J.L.; Mehler, P.S. Predictors of hypophosphatemia during refeeding of patients with severe anorexia nervosa. Int. J. Eat Disord. 2015, 48, 898–904. [Google Scholar] [CrossRef]
- Kameoka, N.; Iga, J.; Tamaru, M.; Tominaga, T.; Kubo, H.; Watanabe, S.; Sumitani, S.; Tomotake, M.; Ohmori, T. Risk factors for refeeding hypophosphatemia in Japanese inpatients with anorexia nervosa. Int. J. Eat Disord. 2016, 49, 402–406. [Google Scholar] [CrossRef]
- Am, R.; Mbm, N. Refeeding hypophosphataemia after enteral nutrition in a Malaysian intensive care unit: Risk factors and outcome. Asia Pac. J. Clin. Nutr. 2018, 27, 329–335. [Google Scholar]
- González Avila, G.; Fajardo Rodríguez, A.; González Figueroa, E. The incidence of the refeeding syndrome in cancer patients who receive artificial nutritional treatment. Nutr. Hosp. 1996, 11, 98–101. [Google Scholar]
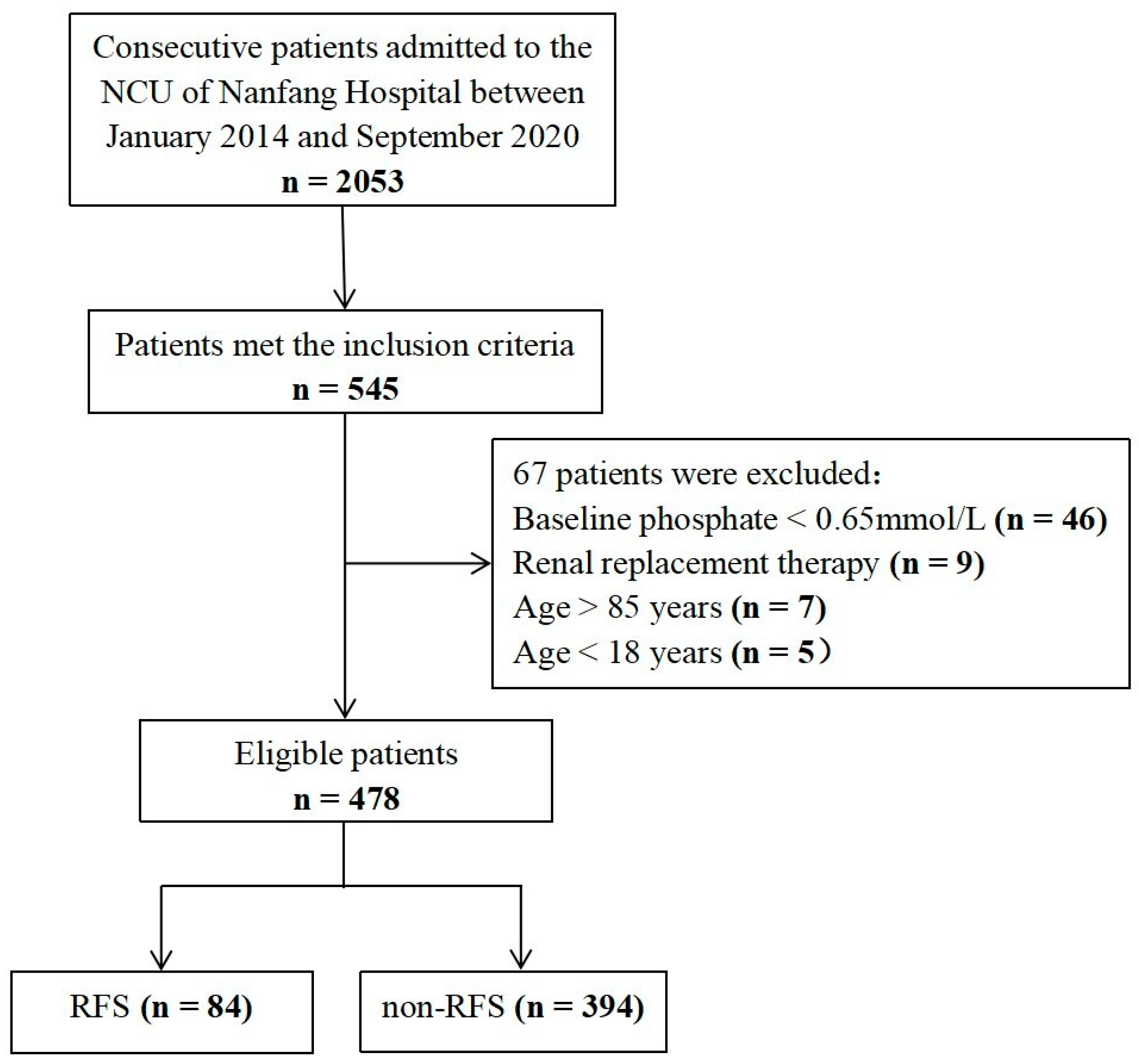
| Variables | RFS (n = 84) | Non-RFS (n = 394) | p |
|---|---|---|---|
| Age, years, median (IQR) | 65 (51–73) | 58 (47–67) | 0.003 |
| Female, n (%) | 19 (22.9%) | 119 (30.1%) | 0.164 |
| BMI, kg/m2, median (IQR) | 22.9 (19.6–25.5) | 23.2 (21.2–25.4) | 0.433 |
| Duration from the onset of diseases to our NCU, days, median (IQR) | 2 (0–7) | 3 (0–12) | 0.181 |
| History of alcoholism, n (%) | 8 (9.5%) | 59 (15.0%) | 0.191 |
| Hypertension, n (%) | 52 (62.7%) | 196 (49.6%) | 0.023 |
| Diabetes mellitus, n (%) | 19 (22.9%) | 77 (19.5%) | 0.523 |
| Heart disease, n (%) | 16 (19.3%) | 43 (10.9%) | 0.040 |
| Mechanical ventilation at admission, n (%) | 10 (12.0%) | 53 (13.4%) | 0.737 |
| APACHE II, median (IQR) | 17 (13–22) | 15 (11–19) | <0.001 |
| GCS, median (IQR) | 8 (6–11) | 10 (7–12) | 0.007 |
| SOFA, median (IQR) | 4 (3–7) | 4 (2–6) | 0.006 |
| NUTRIC, median (IQR) | 4 (3–5) | 3 (2–4) | 0.001 |
| MUST, median (IQR) | 2 (2–2) | 2 (2–2) | 0.107 |
| NRS 2002, median (IQR) | 4 (3–4) | 4(3–4) | 0.094 |
| SNAQ, median (IQR) | 1 (0–1) | 1 (0–1) | 0.631 |
| GLIM, median (IQR) | 1 (1–1) | 1 (1–1) | 0.1224 |
| mMICE, median (IQR) | 2 (1–3) | 2 (1–3) | 0.035 |
| ASPEN, median (IQR) | 1 (0–2) | 0 (0–1) | 0.001 |
| Baseline serum electrolytes | |||
| Phosphorous, mmol/L, median (IQR) | 0.98 (0.86–1.14) | 1.05 (0.92–1.21) | 0.015 |
| Potassium, mmol/L, median (IQR) | 3.91 (3.53–4.24) | 3.90 (3.63–4.20) | 0.953 |
| Sodium, mmol/L, median (IQR) | 140 (135–144) | 140 (137–143) | 0.490 |
| Magnesium, mmol/L, median (IQR) | 0.84 (0.79–0.89) | 0.86 (0.79–0.94) | 0.039 |
| Caloric intakes within the first 72 h | |||
| Day 1, Kcal/kg, median (IQR) | 8.22 (7.11–10) | 8.45 (7.24–11) | 0.21644 |
| Day 2, Kcal/kg, median (IQR) | 14.54 (11.93–18.11) | 16.08 (12.85–20) | 0.018 |
| Day 3, Kcal/kg, median (IQR) | 20.36 (15.71–25.08) | 21.80 (16.97–25.8) | 0.144 |
| Mechanical ventilation in-hospital, n (%) | 46 (55.4%) | 163 (41.3%) | 0.018 |
| Length of hospital stay, days, median (IQR) | 20 (11–33) | 18 (12–29) | 0.848 |
| Length of NCU stay, days, median (IQR) | 11 (6–18) | 8 (5–14) | 0.021 |
| 30-day mortality, n (%) | 27 (32.5%) | 66 (16.7%) | 0.001 |
| 6-month mortality, n (%) | 33 (39.8%) | 77 (19.5%) | <0.001 |
| 6-month poor outcome (mRS > 3), n (%) | 62 (73.8%) | 207 (52.5%) | <0.001 |
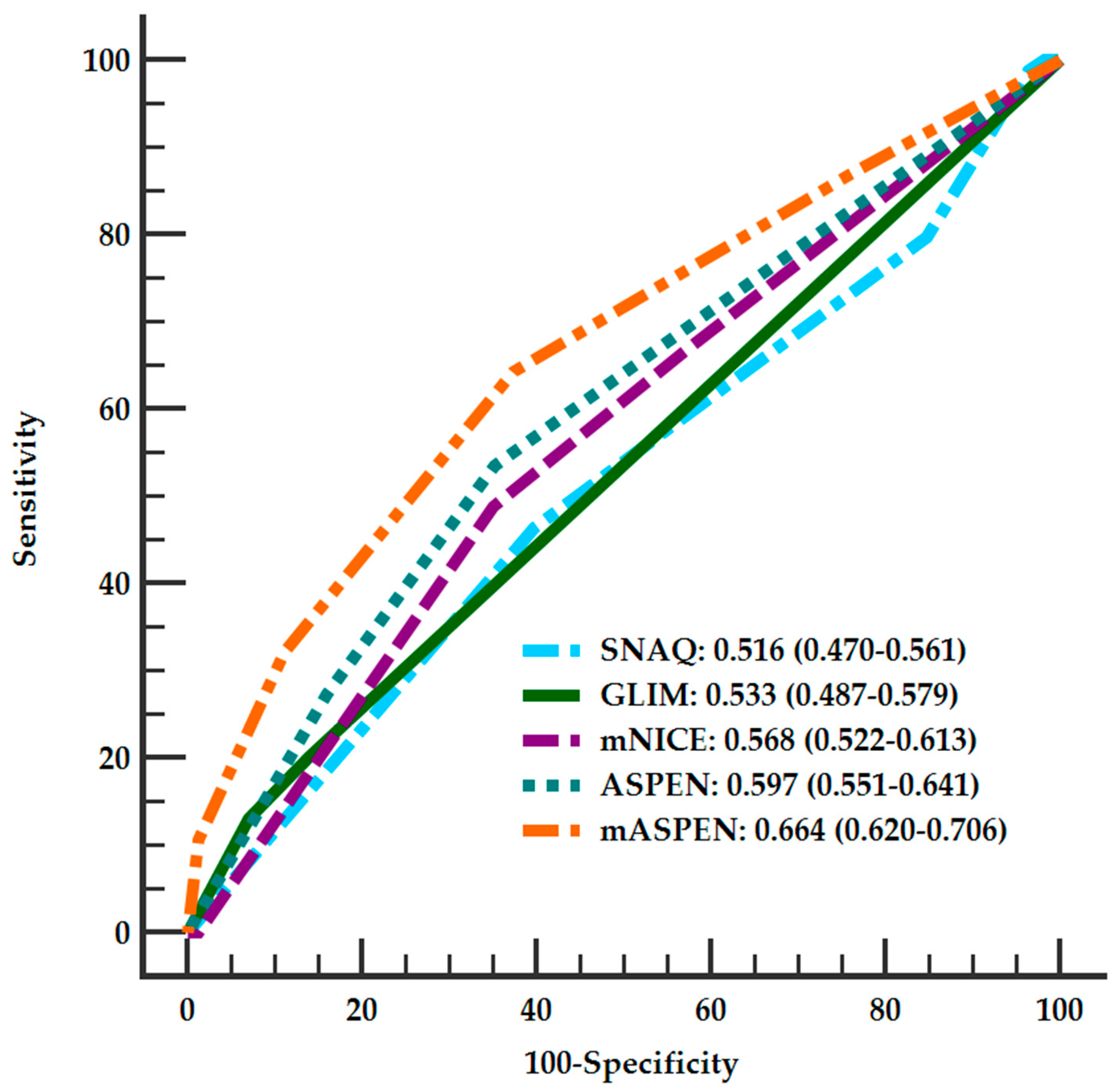
| SNAQ (95%CI) | GLIM (95%CI) | mNICE (95%CI) | ASPEN (95%CI) | mASPEN (95%CI) | |
|---|---|---|---|---|---|
| Sensitivity | 20.2 (12.6–30.7) | 20.2 (12.6–30.7) | 48.8 (37.8–59.9) | 53.6 (42.4–64.4) | 63.1 (51.8–73.2) |
| Specificity | 84.8 (80.8–88.1) | 86.0 (82.1–89.2) | 65 (60.0–69.6) | 64.7 (59.8–69.4) | 0.64 (58.9–68.7) |
| PPV | 22.1 (13.7–33.2) | 23.6 (14.7–35.3) | 22.9 (17.1–29.9) | 24.5 (18.6–31.4) | 27.2 (21.2–34.1) |
| NPV | 83.3 (79.2–86.7) | 83.5 (79.4–87.0) | 85.6 (81.0–89.3) | 86.7 (82.2–90.3) | 89 (76.8–92.3) |
| Accuracy | 73 | 75 | 62 | 63 | 64 |
| к | 5.2 | 6.7 | 9.5 | 12.5 | 17.8 |
| AUCs | 0.516 (0.470–0.561) | 0.533 (0.487–0.579) | 0.568 (0.522–0.613) | 0.597 (0.551–0.641) | 0.664 * (0.620–0.706) |
| Parameter | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Hypertension | 1.745 (1.074–2.834) | 0.025 | - | - |
| Heart disease | 1.921 (1.023–3.606) | 0.042 | - | - |
| Day2 (Kcal/kg) | 0.957 (0.919–0.996) | 0.033 | - | - |
| GCS | 0.913 (0.852–0.979) | 0.010 | 0.910 (0.848–0.978) | 0.010 |
| Age | 1.025 (1.008–1.042) | 0.004 | 1.027 (1.010–1.044) | 0.002 |
| ASPEN | 1.573 (1.184–2.089) | 0.002 | 1.597 (1.194–2.136) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Zhao, X.-L.; Xiong, R.-Q.; Chen, Q.-F.; Wu, Y.-M.; Lin, Z.-Z.; Wang, S.-N.; Wu, T.; Pan, S.-Y.; Huang, K.-B. The Performances of SNAQ, GLIM, mNICE, and ASPEN for Identification of Neurocritically Ill Patients at High Risk of Developing Refeeding Syndrome. Nutrients 2022, 14, 4032. https://doi.org/10.3390/nu14194032
Liu N, Zhao X-L, Xiong R-Q, Chen Q-F, Wu Y-M, Lin Z-Z, Wang S-N, Wu T, Pan S-Y, Huang K-B. The Performances of SNAQ, GLIM, mNICE, and ASPEN for Identification of Neurocritically Ill Patients at High Risk of Developing Refeeding Syndrome. Nutrients. 2022; 14(19):4032. https://doi.org/10.3390/nu14194032
Chicago/Turabian StyleLiu, Na, Xiao-Lin Zhao, Rui-Qi Xiong, Quan-Feng Chen, Yong-Ming Wu, Zhen-Zhou Lin, Sheng-Nan Wang, Tong Wu, Su-Yue Pan, and Kai-Bin Huang. 2022. "The Performances of SNAQ, GLIM, mNICE, and ASPEN for Identification of Neurocritically Ill Patients at High Risk of Developing Refeeding Syndrome" Nutrients 14, no. 19: 4032. https://doi.org/10.3390/nu14194032
APA StyleLiu, N., Zhao, X.-L., Xiong, R.-Q., Chen, Q.-F., Wu, Y.-M., Lin, Z.-Z., Wang, S.-N., Wu, T., Pan, S.-Y., & Huang, K.-B. (2022). The Performances of SNAQ, GLIM, mNICE, and ASPEN for Identification of Neurocritically Ill Patients at High Risk of Developing Refeeding Syndrome. Nutrients, 14(19), 4032. https://doi.org/10.3390/nu14194032





