Impact of Preoperative Visceral Fat Area Measured by Bioelectrical Impedance Analysis on Clinical and Oncologic Outcomes of Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
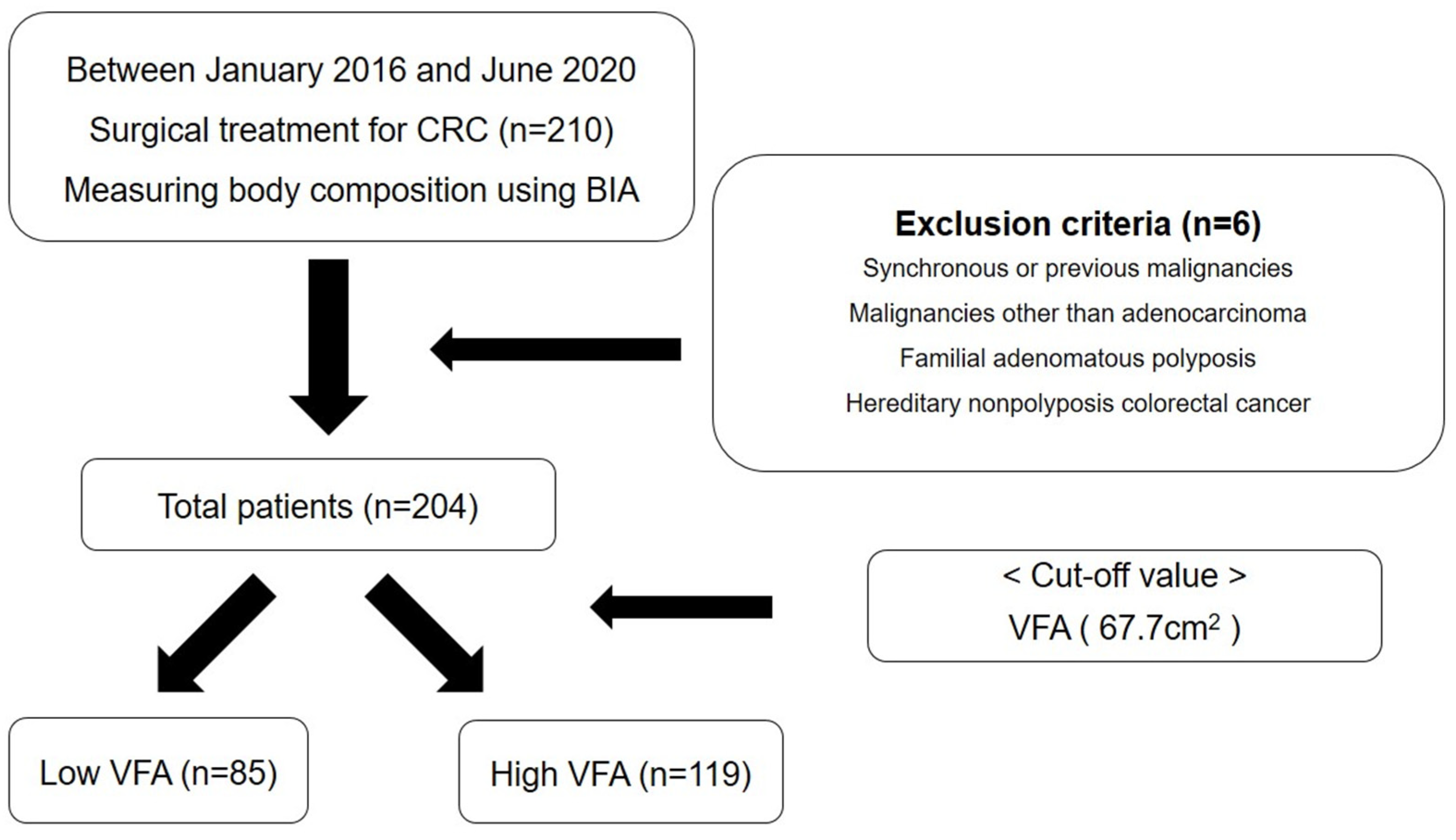
2.2. Patients and Data Collection
2.3. Data Collection and Definitions
2.4. Preoperative Evaluation and Surgical Treatment
2.5. Bioelectrical Impedance Analysis
2.6. Assessment of Hematologic Parameters and Inflammation-Based Prognostic Scores
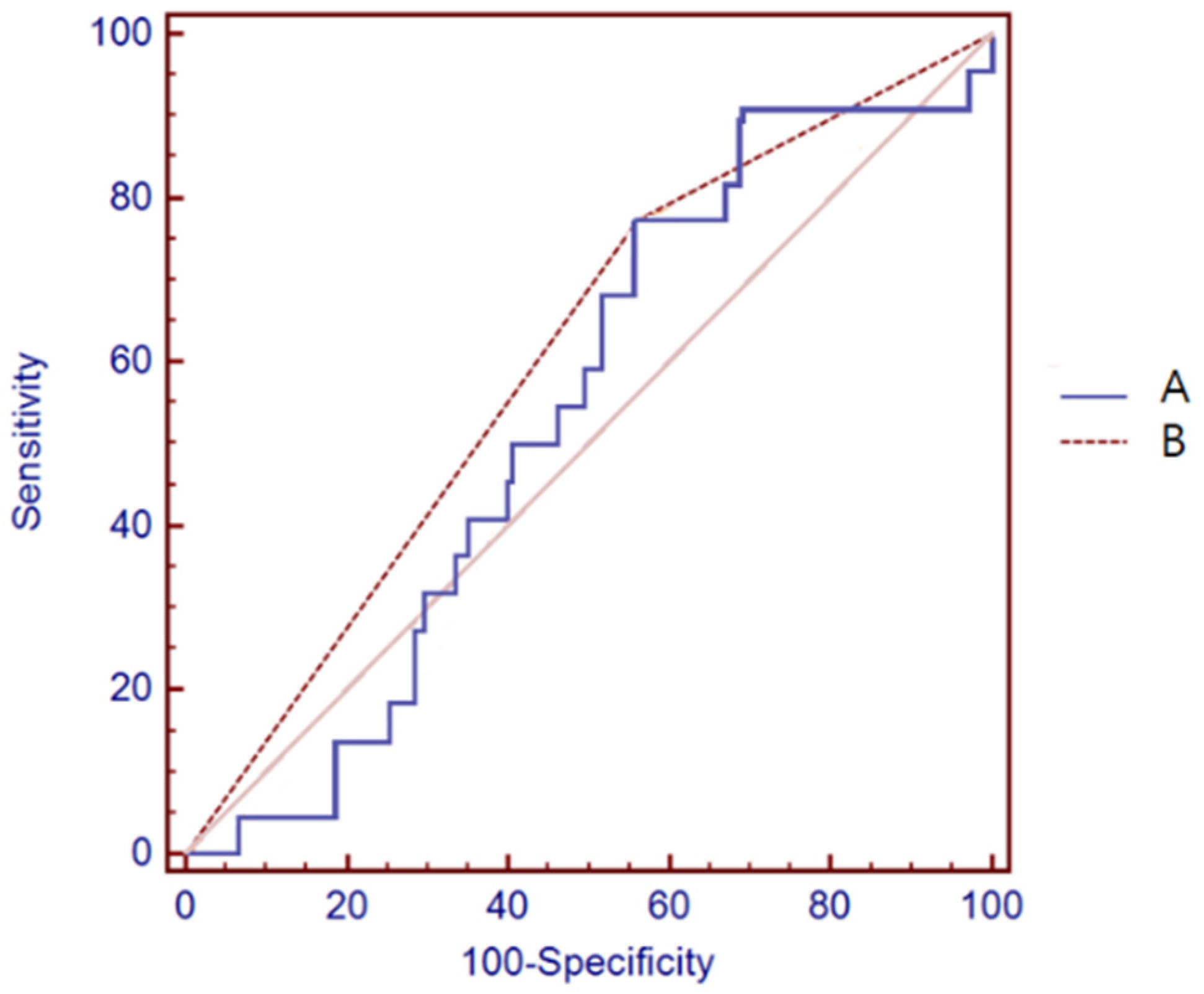
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Perioperative Clinical Outcomes
3.3. Postoperative Pathologic Outcomes
3.4. Body Composition Analysis Using BIA
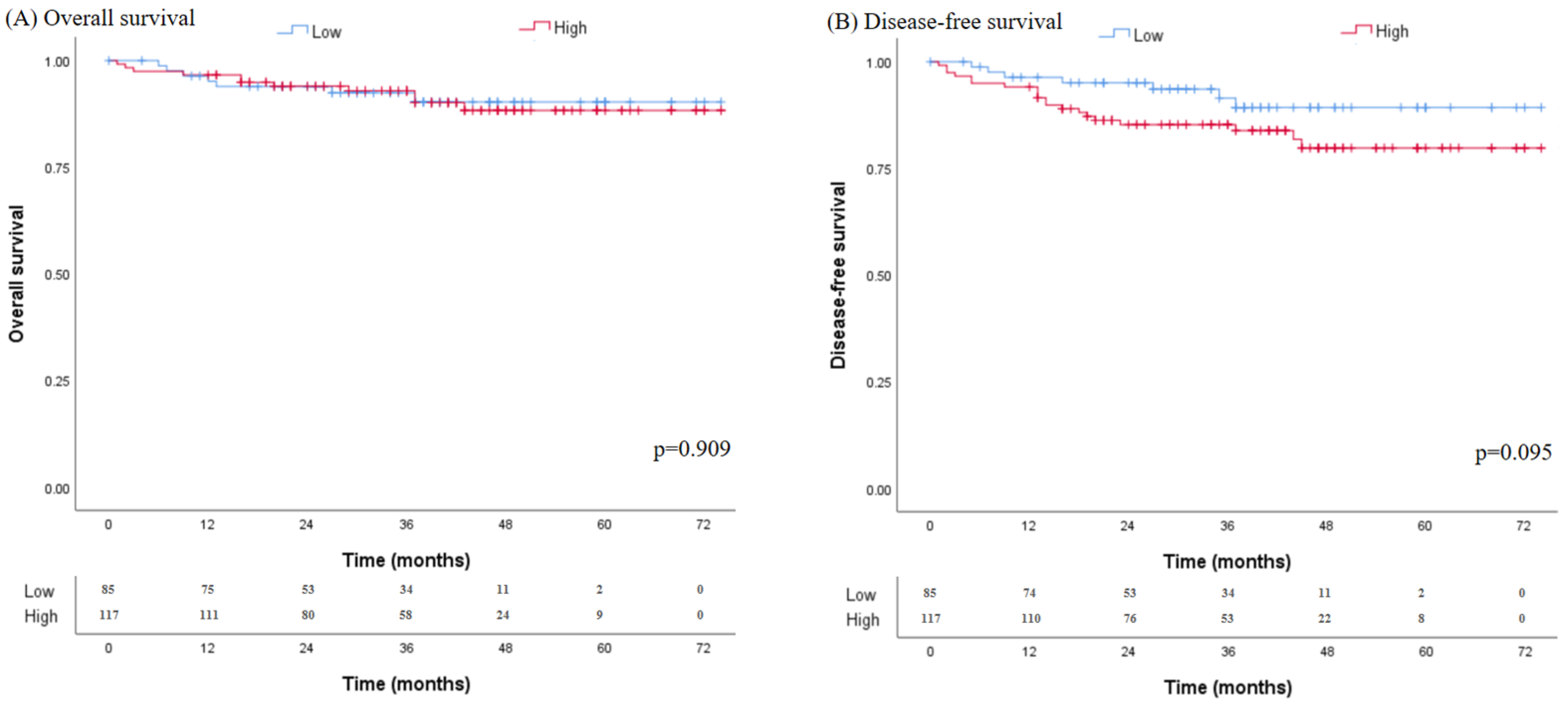
3.5. Oncologic Outcomes
3.6. Univariate and Multivariate Survival Analyses of Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islami, F.; Ward, E.M.; Sung, H.; A Cronin, K.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. JNCI J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Seo, I.; Jeong, H.; Byun, S.J.; Kim, S.; Bae, S.U.; Kwon, S.Y.; Lee, H.W. Prognostic Impact of Tumor-Associated Macrophages on Long-Term Oncologic Outcomes in Colorectal Cancer. Life 2021, 11, 1240. [Google Scholar] [CrossRef]
- Seo, I.; Lee, H.W.; Byun, S.J.; Park, J.Y.; Min, H.; Lee, S.H.; Lee, J.-S.; Kim, S.; Bae, S.U. Neoadjuvant chemoradiation alters biomarkers of anticancer immunotherapy responses in locally advanced rectal cancer. J. Immunother. Cancer 2021, 9, e001610. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.U.; Jeong, W.K.; Baek, S.K.; Kim, N.-K.; Hwang, I. Prognostic impact of programmed cell death ligand 1 expression on long-term oncologic outcomes in colorectal cancer. Oncol. Lett. 2018, 16, 5214–5222. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Choi, Y.J.; Kim, D.W.; Park, E.-C.; Kang, J.-G. Risk Factors for Colorectal Cancer in Korea: A Population-Based Retrospective Cohort Study. Ann. Coloproctology 2019, 35, 347–356. [Google Scholar] [CrossRef]
- Perez-Hernandez, A.I.; Catalan, V.; Gomez-Ambrosi, J.; Rodriguez, A.; Fruhbeck, G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front. Endocrinol 2014, 5, 65. [Google Scholar]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Goulart, A.; Malheiro, N.; Ríos, H.; Sousa, N.; Leao, P. Influence of Visceral Fat in the Outcomes of Colorectal Cancer. Dig. Surg. 2019, 36, 33–40. [Google Scholar] [CrossRef]
- Martinez-Useros, J.; Garcia-Foncillas, J. Obesity and colorectal cancer: Molecular features of adipose tissue. J. Transl. Med. 2016, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Ballian, N.; Lubner, M.G.; Munoz, A.; Harms, B.A.; Heise, C.P.; Foley, E.F.; Kennedy, G. Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. J. Surg. Oncol. 2012, 105, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lee, H.L.; Doo, E.Y.; Lee, K.N.; Jun, D.W.; Lee, O.Y.; Han, D.S.; Yoon, B.C.; Choi, H.S. Visceral Obesity Predicts Fewer Lymph Node Metastases and Better Overall Survival in Colon Cancer. J. Gastrointest. Surg. 2015, 19, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Fernandez, O.; Trejo-Avila, M.; Salgado-Nesme, N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J. Clin. Cases 2020, 8, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Charette, N.; Vandeputte, C.; Ameye, L.; Van Bogaert, C.; Krygier, J.; Guiot, T.; Deleporte, A.; Delaunoit, T.; Geboes, K.; Van Laethem, J.-L.; et al. Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: A post hoc analysis of two non-randomized phase II trials. BMC Cancer 2019, 19, 134. [Google Scholar] [CrossRef]
- Hurwitz, E.E.; Simon, M.; Vinta, S.R.; Zehm, C.F.; Shabot, S.M.; Minhajuddin, A.; Abouleish, A.E. Adding Examples to the ASA-Physical Status Classification Improves Correct Assignment to Patients. Anesthesiology 2017, 126, 614–622. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef]
- Geiger, J.T.; Aquina, C.T.; Esce, A.; Zhao, P.; Glocker, R.; Fleming, F.; Iannuzzi, J.; Stoner, M.; Doyle, A. One-year patient survival correlates with surgeon volume after elective open abdominal aortic surgery. J. Vasc. Surg. 2021, 73, 108–116.e1. [Google Scholar] [CrossRef]
- Yang, T.; Wei, M.; He, Y.; Deng, X.; Wang, Z. Impact of visceral obesity on outcomes of laparoscopic colorectal surgery: A meta-analysis. ANZ J. Surg. 2015, 85, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Joh, Y.-G.; Son, G.-M.; Kim, H.-S.; Jo, H.-J.; Kim, H.-Y. Distribution and Impact of the Visceral Fat Area in Patients With Colorectal Cancer. Ann. Coloproctology 2016, 32, 20–26. [Google Scholar] [CrossRef]
- Saijo, Y.; Kiyota, N.; Kawasaki, Y.; Miyazaki, Y.; Kashimura, J.; Fukuda, M.; Kishi, R. Relationship between C-reactive protein and visceral adipose tissue in healthy Japanese subjects. Diabetes Obes. Metab. 2004, 6, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Tsuriya, D.; Morita, H.; Morioka, T.; Takahashi, N.; Ito, T.; Oki, Y.; Nakamura, H. Significant Correlation Between Visceral Adiposity and High-sensitivity C-reactive Protein (hs-CRP) in Japanese Subjects. Intern. Med. 2011, 50, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, A.; Kardys, I.; de Maat, M.P.; Uitterlinden, A.G.; Sijbrands, E.J.; Bootsma, A.H.; Stijnen, T.; Hofman, A.; Schram, M.T.; Witteman, J.C. Genetic variation, c-reactive protein levels, and incidence of diabetes. Diabetes 2007, 56, 872–878. [Google Scholar] [CrossRef]
- Santa-Paavola, R.; Lehtinen-Jacks, S.; Jääskeläinen, T.; Männistö, S.; Lundqvist, A. The association of high-sensitivity C-reactive protein with future weight gain in adults. Int. J. Obes. 2022, 46, 1234–1240. [Google Scholar] [CrossRef]
- Liao, C.-K.; Yu, Y.-L.; Lin, Y.-C.; Hsu, Y.-J.; Chern, Y.-J.; Chiang, J.-M.; You, J.-F. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: An updated systematic review and meta-analysis. World J. Surg. Oncol. 2021, 19, 139. [Google Scholar] [CrossRef]
- Liesenfeld, D.B.; Grapov, D.; Fahrmann, J.F.; Salou, M.; Scherer, D.; Toth, R.; Habermann, N.; Böhm, J.; Schrotz-King, P.; Gigic, B.; et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: The ColoCare study. Am. J. Clin. Nutr. 2015, 102, 433–443. [Google Scholar] [CrossRef]
- Guiu, B.; Petit, J.M.; Bonnetain, F.; Ladoire, S.; Guiu, S.; Cercueil, J.-P.; Krausé, D.; Hillon, P.; Borg, C.; Chauffert, B.; et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010, 59, 341–347. [Google Scholar] [CrossRef]
- Basile, D.; Bartoletti, M.; Polano, M.; Bortot, L.; Gerratana, L.; Di Nardo, P.; Borghi, M.; Fanotto, V.; Pelizzari, G.; Lisanti, C.; et al. Prognostic role of visceral fat for overall survival in metastatic colorectal cancer: A pilot study. Clin. Nutr. 2021, 40, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Wu, K.; Liu, Y.; Shi, L.; Wang, D.; Li, G.; Tao, K.; Wang, G. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int. J. Biochem. Cell Biol. 2017, 84, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chang, S.Y.; Lim, J.S.; Park, S.J.; Park, J.J.; Cheon, J.H.; Kim, W.H.; Kim, A.T.I. Impact of Visceral Fat on Survival and Metastasis of Stage III Colorectal Cancer. Gut Liver 2022, 16, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Doyon, C.Y.; Brochu, M.; Messier, V.; Lavoie, M.; Faraj, M.; Doucet, É.; Rabasa-Lhoret, R.; Dionne, I.J. Association between Abdominal Fat (DXA) and Its Subcomponents (CT Scan) before and after Weight Loss in Obese Postmenopausal Women: A MONET Study. J. Obes. 2011, 2011, 239516. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Dahlk, S.L.; King, J.; Vashi, P.G.; Grutsch, J.F.; A Lammersfeld, C. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr. J. 2008, 7, 19. [Google Scholar] [CrossRef]
- Brändstedt, J.; Wangefjord, S.; Nodin, B.; Gaber, A.; Manjer, J.; Jirström, K. Gender, anthropometric factors and risk of colorectal cancer with particular reference to tumour location and TNM stage: A cohort study. Biol. Sex Differ. 2012, 3, 23. [Google Scholar] [CrossRef]
- Kim, M.; Shinkai, S.; Murayama, H.; Mori, S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr. Gerontol. Int. 2015, 15, 1013–1022. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, K.S.; Ahn, S.; Ku, E.J.; Jung, K.Y.; Kim, Y.J.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Park, K.S.; et al. Comparison of Abdominal Visceral Adipose Tissue Area Measured by Computed Tomography with That Estimated by Bioelectrical Impedance Analysis Method in Korean Subjects. Nutrients 2015, 7, 10513–10524. [Google Scholar] [CrossRef]

| Low VFA (n = 85) | High VFA (n = 119) | p Value | |
|---|---|---|---|
| Age (year) | 65.9 ± 9.7 | 66.0 ± 10.2 | 0.929 |
| Sex | 0.019 | ||
| Male | 66 (77.6) | 74 (62.2) | |
| Female | 19 (22.4) | 45 (37.8) | |
| Preoperative CEA (ng/mL) | 7.0 ± 20.7 | 5.4 ± 16.0 | 0.552 |
| Preoperative CRP | 0.4 ± 0.7 | 0.8 ± 1.7 | 0.047 |
| ASA groups | 0.827 | ||
| I | 26 (30.6) | 33 (27.7) | |
| II | 49 (57.6) | 69 (58.0) | |
| III | 26 (30.6) | 33 (27.7) | |
| BMI (kg/m2) | 21.3 ± 1.8 | 25.0 ± 2.6 | <0.001 |
| Sideness of tumor | 0.599 | ||
| Right | 22 (25.9) | 27 (22.7) | |
| Left | 63 (74.1) | 92 (77.3) | |
| Location of tumor | 0.740 | ||
| Colon | 43 (50.6) | 63 (52.9) | |
| Rectum | 42 (49.4) | 56 (47.1) | |
| Hemoglobin (g/dL) | 12.6 ± 2.0 | 12.4 ± 1.7 | 0.609 |
| Platelet (×103) | 246.2 ± 71.8 | 241.4 ± 72.3 | 0.636 |
| WBC (×103) | 6.4 ± 2.1 | 6.0 ± 1.9 | 0.105 |
| PLR | 181.7 ± 114.6 | 188.2 ± 102.2 | 0.677 |
| NLR | 3.3 ± 3.8 | 3.1 ± 2.5 | 0.636 |
| PNI | 66.9 ± 27.7 | 71.2 ± 30.8 | 0.305 |
| PIV | 383.1 ± 710.2 | 276.9 ± 294.7 | 0.196 |
| Albumin (g/dL) | 4.2 ± 0.5 | 4.2 ± 0.4 | 0.603 |
| Neoadjuvant CCRT | 17 (20.0) | 28 (23.5) | 0.549 |
| Low VFA (n = 85) | High VFA (n = 119) | p Value | |
|---|---|---|---|
| Operation time (min) | 209.3 ± 112.1 | 204.0 ± 86.2 | 0.711 |
| Time to gas out (d) | 3.2 ± 2.2 | 4.0 ± 4.8 | 0.319 |
| Time to sips of water (d) | 4.0 ± 3.1 | 4.0 ± 4.8 | 0.983 |
| Time to soft diet (d) | 6.3 ± 3.2 | 6.6 ± 5.1 | 0.603 |
| Time to hospital stay (d) | 10.4 ± 6.4 | 10.2 ± 6.2 | 0.773 |
| Morbidity within 30 days after surgery | 28 (32.9) | 40 (33.6) | 0.920 |
| Clavien–Dindo classifications > 3a | 17 (20.0) | 25 (21.0) | 0.861 |
| Low VFA (n = 85) | High VFA (n = 119) | p Value | |
|---|---|---|---|
| Tumor stage | 0.114 | ||
| T1 | 16 (18.8) | 33 (24.0) | |
| T2 | 16 (18.8) | 42 (20.6) | |
| T3 | 43 (50.6) | 99 (48.5) | |
| T4 | 10 (11.8) | 14 (6.9) | |
| Nodal stage | 0.945 | ||
| N0 | 55 (64.7) | 79 (66.4) | |
| N1 | 21 (24.7) | 27 (22.7) | |
| N2 | 9 (10.6) | 13 (10.9) | |
| Histology | 0.027 | ||
| Well differentiated | 10 (11.9) | 3 (2.6) | |
| Moderately differentiated | 70 (83.3) | 106 (90.6) | |
| Poorly differentiated | 4 (4.8) | 8 (6.8) | |
| Retrieved LNs | 19.5 ± 9.4 | 18.1 ± 9.2 | 0.310 |
| LN > 12 | 77 (90.6) | 99 (83.2) | 0.130 |
| Positive LNs | 1.0 ± 2.0 | 0.9 ± 2.1 | 0.807 |
| Tumor size (cm) | 3.9 ± 2.1 | 3.5 ± 2.1 | 0.211 |
| Lymphovascular invasion | 27 (31.8) | 27 (23.5) | 0.192 |
| Perineural invasion | 16 (19.3) | 25 (22.5) | 0.584 |
| Low VFA (n = 85) | High VFA (n = 119) | p Value | |
|---|---|---|---|
| Height (cm) | 162.3 ± 8.6 | 162.4 ± 9.5 | 0.980 |
| Weight (kg) | 56.4 ± 7.8 | 66.2 ± 11.2 | <0.001 |
| Phase angle (′) | 5.1 ± 0.6 | 5.0 ± 0.7 | 0.629 |
| ASM (kg) | 7.0 ± 1.1 | 7.1 ± 1.1 | 0.650 |
| SMI (kg/m2) | 2.7 ± 0.5 | 2.7 ± 0.4 | 0.749 |
| Body fluid | 33.1 ± 5.3 | 33.9 ± 6.7 | 0.347 |
| ICF (%) | 20.3 ± 3.4 | 20.8 ± 4.2 | 0.362 |
| ECF (%) | 12.8 ± 2.0 | 13.1 ± 2.6 | 0.328 |
| BFM (kg) | 11.6 ± 2.6 | 20.3 ± 4.8 | <0.001 |
| Low VFA (n = 85) | High VFA (n = 119) | p Value | |
|---|---|---|---|
| Median follow-up (months) | 35.6 ± 16.2 | 40.0 ± 18.0 | 0.073 |
| 5 yr OS (%) | 90.3 | 88.3 | 0.909 |
| 5 yr DFS (%) | 89.3 | 79.8 | 0.105 |
| Recurrence | 3 | 14 | |
| Recurrence pattern | 0.070 | ||
| Systemic recurrence | 3 | 9 | |
| Local recurrence | 0 | 5 |
| Prognostic Factor | N | OS (5 Years, %) | Log Rank p-Value | DFS (5 Years, %) | Log Rank p-Value |
|---|---|---|---|---|---|
| Visceral fat area | 0.909 | 0.105 | |||
| Low | 85 | 90.3 | 89.3 | ||
| High | 119 | 88.3 | 79.8 | ||
| Age | 0.689 | 0.917 | |||
| ≤65 | 89 | 90.2 | 84.7 | ||
| >65 | 115 | 87.8 | 82.1 | ||
| Sex | 0.060 | 0.016 | |||
| Male | 140 | 85.5 | 79.5 | ||
| Female | 64 | 96.9 | 92.0 | ||
| BMI | 0.332 | 0.327 | |||
| High (>25) | 52 | 92.8 | 90.2 | ||
| Low (<25) | 152 | 87.5 | 80.8 | ||
| ASA score | 0.253 | 0.571 | |||
| 1 | 59 | 94.9 | 81.9 | ||
| 2 and 3 | 145 | 86.6 | 84.0 | ||
| Sideness | 0.431 | 0.687 | |||
| Right sided | 49 | 84.2 | 79.4 | ||
| Left sided | 155 | 90.6 | 84.7 | ||
| Pre-op CEA (ng/mL) | 0.164 | 0.072 | |||
| <5 | 162 | 90.6 | 85.0 | ||
| ≥5 | 42 | 82.2 | 76.7 | ||
| Pre-op CRP (mg/L) | 0.043 | 0.623 | |||
| <0.3 | 99 | 90.0 | 86.6 | ||
| ≥0.3 | 55 | 80.3 | 83.7 | ||
| Tumor stage | 0.119 | 0.037 | |||
| T1 and T2 | 91 | 92.8 | 92.0 | ||
| T3 and T4 | 113 | 85.6 | 76.0 | ||
| Nodal stage | <0.001 | 0.001 | |||
| Nodal negative | 133 | 94.5 | 90.4 | ||
| Nodal positive | 71 | 79.0 | 69.5 | ||
| Differentiation | 0.822 | 0.488 | |||
| Well | 15 | 92.9 | 92.9 | ||
| Moderate and poor | 188 | 89.1 | 83.0 | ||
| Lymphovascular invasion | 0.085 | 0.089 | |||
| No | 146 | 90.8 | 84.8 | ||
| Yes | 54 | 83.3 | 78.3 | ||
| Perineural invasion | 0.030 | 0.004 | |||
| No | 153 | 92.1 | 85.5 | ||
| Yes | 41 | 80.6 | 72.6 | ||
| LN harvest | 0.314 | 0.363 | |||
| ≥12 | 176 | 88.3 | 82.2 | ||
| <12 | 28 | 92.3 | 92.9 | ||
| PIV | 0.010 | 0.298 | |||
| Low | 145 | 94.1 | 86.1 | ||
| High | 59 | 77.3 | 77.1 | ||
| Phase angle | 0.215 | 0.944 | |||
| Low | 117 | 92.1 | 85.3 | ||
| High | 87 | 84.3 | 82.4 | ||
| Sarcopenia | 0.311 | 0.313 | |||
| No | 143 | 90.3 | 85.0 | ||
| Yes | 61 | 85.6 | 79.4 |
| Variables | Reference Category | Overall Survival | Disease-Free Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| VFA | |||||
| High | Low | 1.67 (0.50–5.56) | 0.401 | 4.26 (1.28–14.20) | 0.018 |
| Sex | |||||
| Female | Male | 0.59 (0.12–2.87) | 0.509 | 0.11 (0.01–0.91) | 0.040 |
| Sarcopenia | |||||
| Yes | No | 1.57 (0.49–5.08) | 0.451 | 2.31 (0.79–6.77) | 0.126 |
| Pre-OP CEA | |||||
| ≥5 | <5 | 0.96 (0.29–3.16) | 0.942 | 0.92 (0.28–3.04) | 0.890 |
| CRP | |||||
| ≥0.3 | <0.3 | 3.88 (1.00–15.05) | 0.050 | 1.38 (0.44–4.35) | 0.585 |
| PIV | |||||
| High | Low | 1.17 (0.316–4.356) | 0.811 | 0.62 (0.19–2.03) | 0.426 |
| Tumor stage | |||||
| T3, T4 | T1, T2 | 0.91 (0.14–6.08) | 0.926 | 1.11 (0.27–4.63) | 0.889 |
| Nodal stage | |||||
| N1, N2 | N0 | 8.00 (1.41–45.21) | 0.019 | 1.28 (0.37–4.45) | 0.702 |
| Lymphovascular invasion | |||||
| Yes | No | 3.06 (0.88–10.63) | 0.078 | 3.56 (1.10–11.54) | 0.034 |
| Perineural invasion | |||||
| Yes | No | 1.10 (0.31–3.95) | 0.880 | 2.46 (0.73–8.25) | 0.144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.E.; Bae, S.U.; Jeong, W.K.; Baek, S.K. Impact of Preoperative Visceral Fat Area Measured by Bioelectrical Impedance Analysis on Clinical and Oncologic Outcomes of Colorectal Cancer. Nutrients 2022, 14, 3971. https://doi.org/10.3390/nu14193971
Kim KE, Bae SU, Jeong WK, Baek SK. Impact of Preoperative Visceral Fat Area Measured by Bioelectrical Impedance Analysis on Clinical and Oncologic Outcomes of Colorectal Cancer. Nutrients. 2022; 14(19):3971. https://doi.org/10.3390/nu14193971
Chicago/Turabian StyleKim, Kyeong Eui, Sung Uk Bae, Woon Kyung Jeong, and Seong Kyu Baek. 2022. "Impact of Preoperative Visceral Fat Area Measured by Bioelectrical Impedance Analysis on Clinical and Oncologic Outcomes of Colorectal Cancer" Nutrients 14, no. 19: 3971. https://doi.org/10.3390/nu14193971
APA StyleKim, K. E., Bae, S. U., Jeong, W. K., & Baek, S. K. (2022). Impact of Preoperative Visceral Fat Area Measured by Bioelectrical Impedance Analysis on Clinical and Oncologic Outcomes of Colorectal Cancer. Nutrients, 14(19), 3971. https://doi.org/10.3390/nu14193971





