Trimethylamine-N-Oxide Promotes Osteoclast Differentiation and Bone Loss via Activating ROS-Dependent NF-κB Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. In Vitro Experiments
2.4. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.5. F-Actin Ring Formation Assay
2.6. Bone Resorption Assay
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Western Blot
2.9. Intracellular ROS Assay
2.10. In Vivo Experiments
2.11. Micro-CT Analysis
2.12. HE, TRAP, and Immunohistochemical Staining
2.13. RNA and Protein Extraction
2.14. Statistical Analysis
3. Results
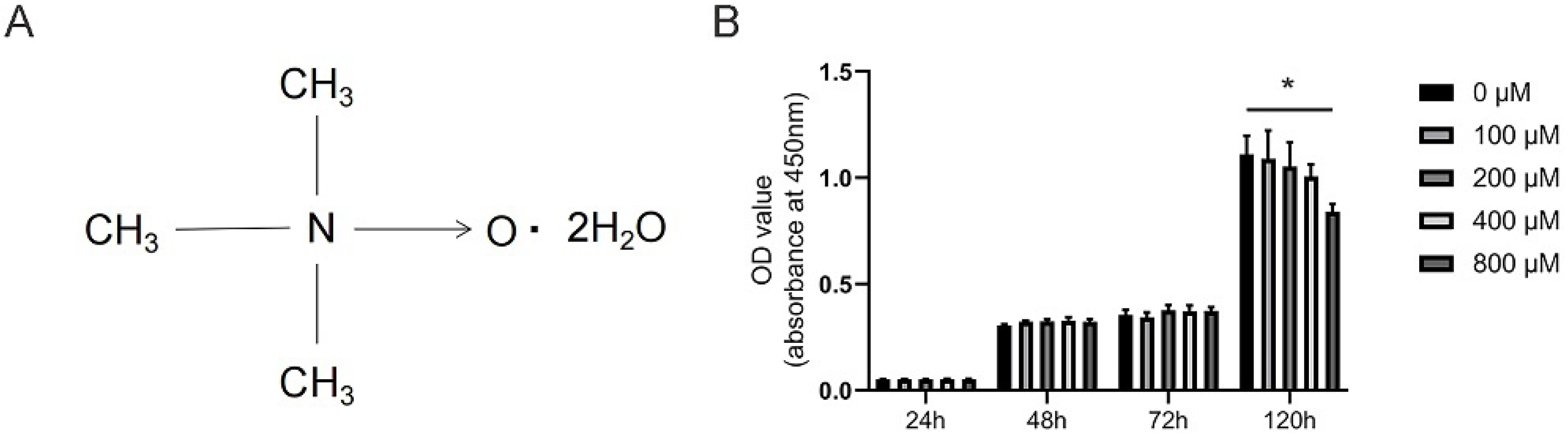
3.1. Effects of TMAO on the Viability of BMMs
3.2. TMAO Promoted Osteoclast Differentiation
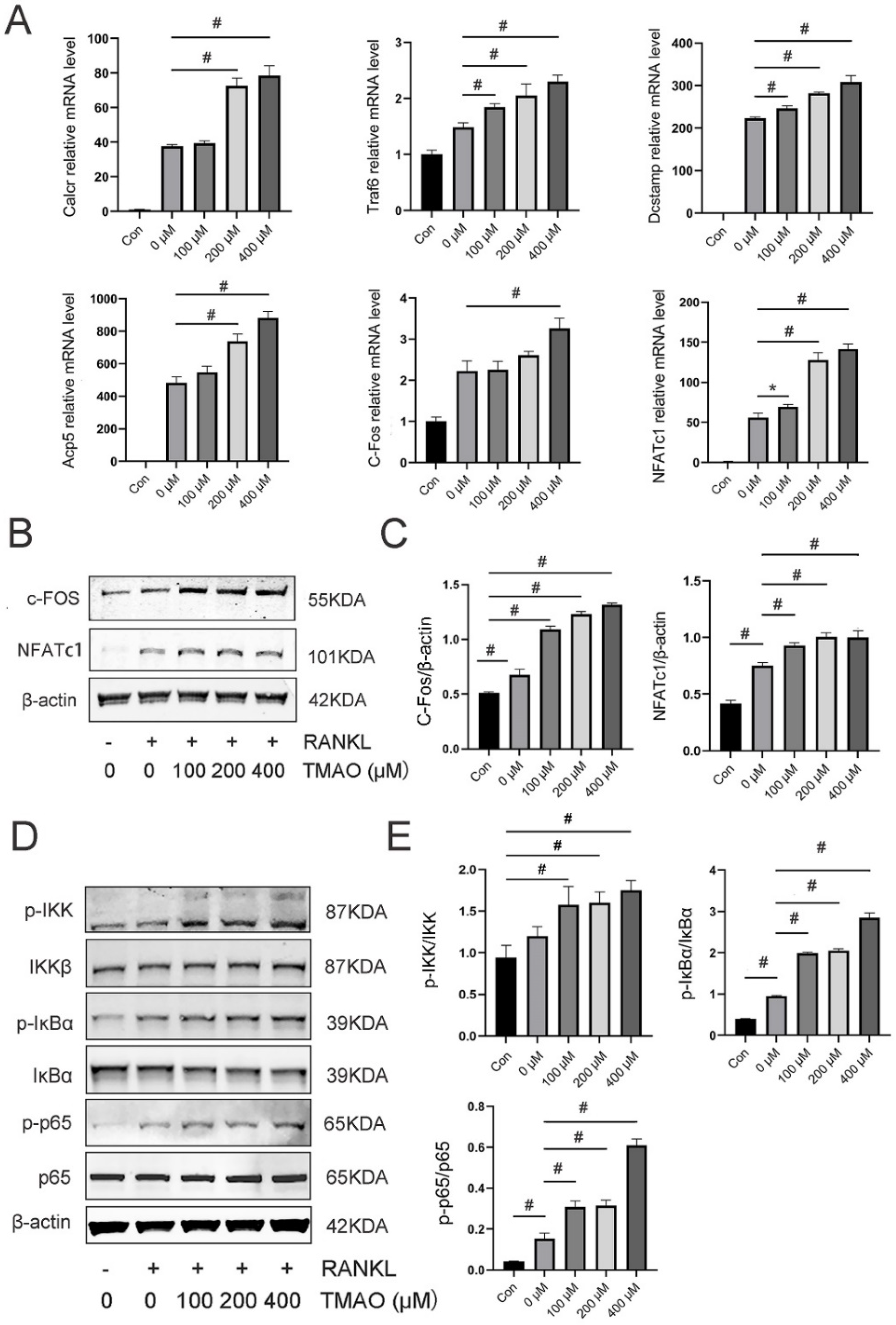
3.3. TMAO Enhanced Osteoclast Gene Expression and NF-κB Signaling Pathway
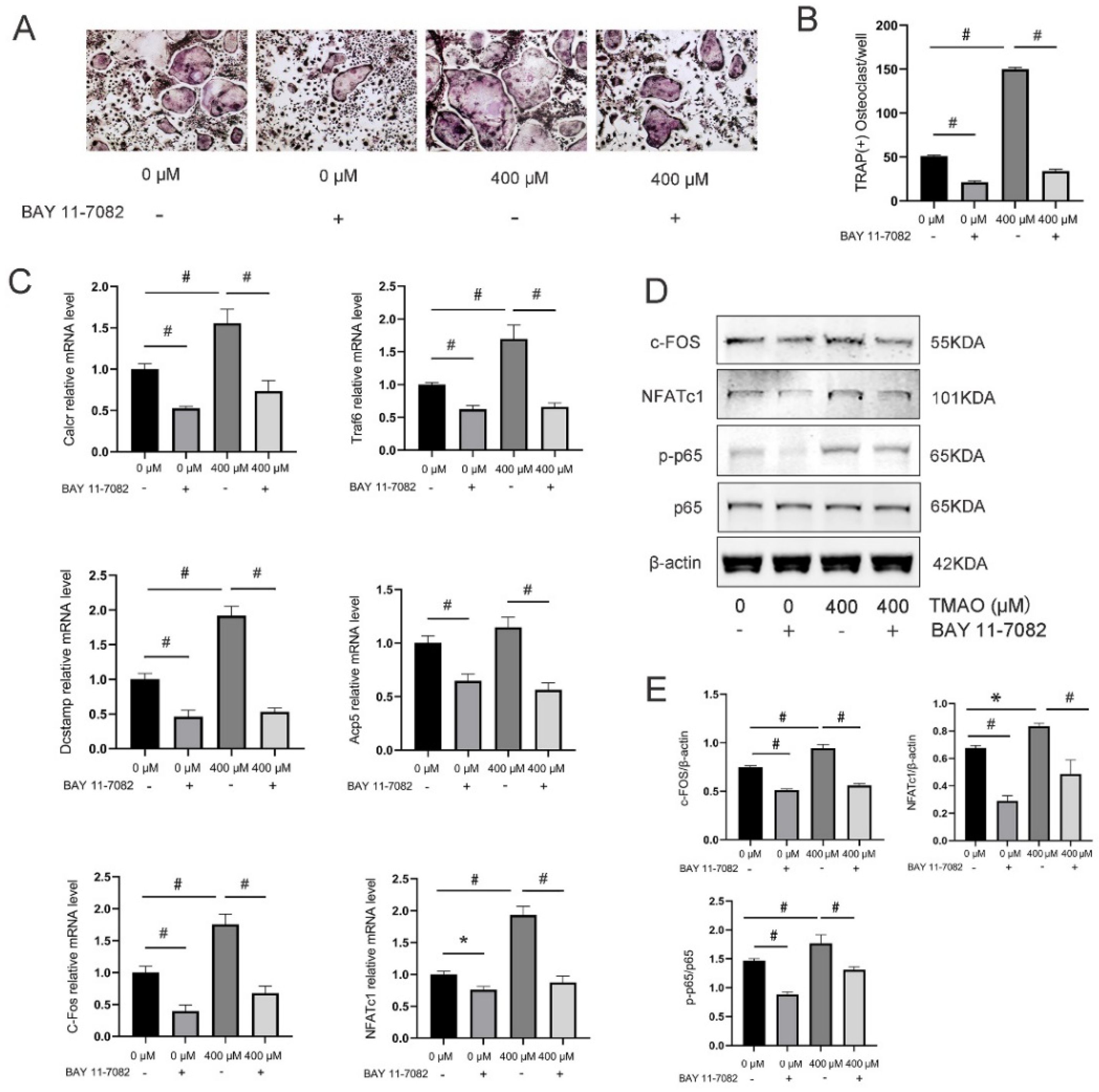
3.4. Inhibition of the NF-κB Signaling Pathway with BAY 11-7082 Reversed the Effect of TMAO on Osteoclast Differentiation
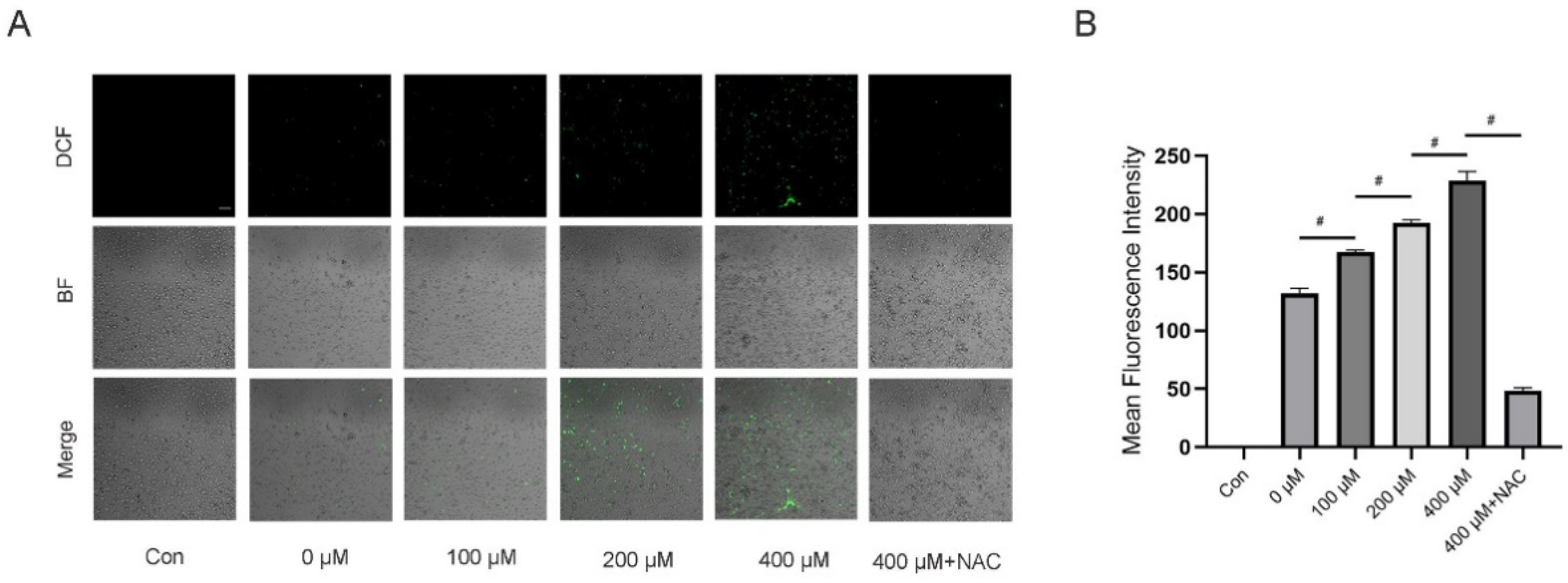
3.5. TMAO Increased ROS Levels during Osteoclast Differentiation
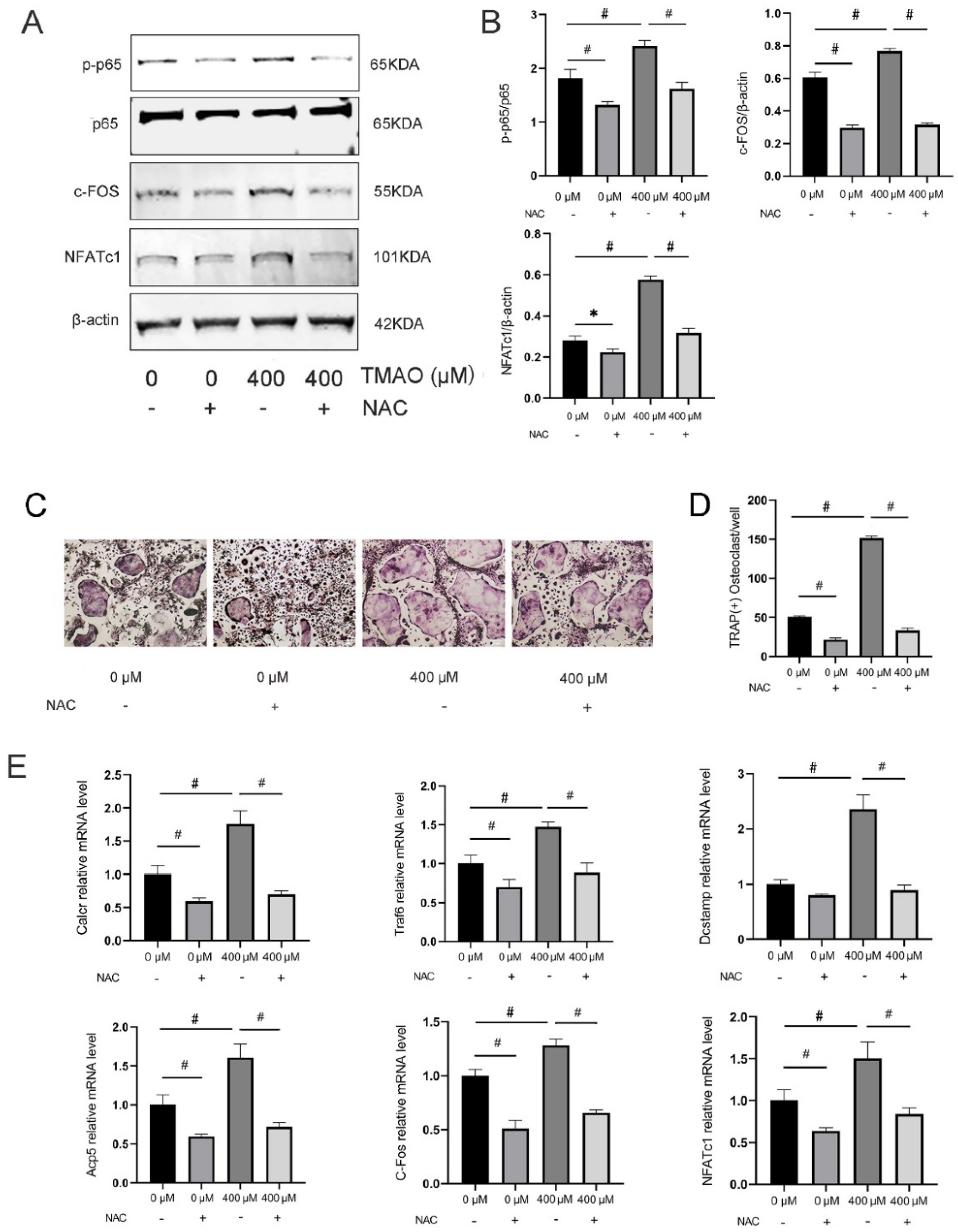
3.6. Inhibition of ROS by NAC Suppressed TMAO-Induced NF-κB Activation
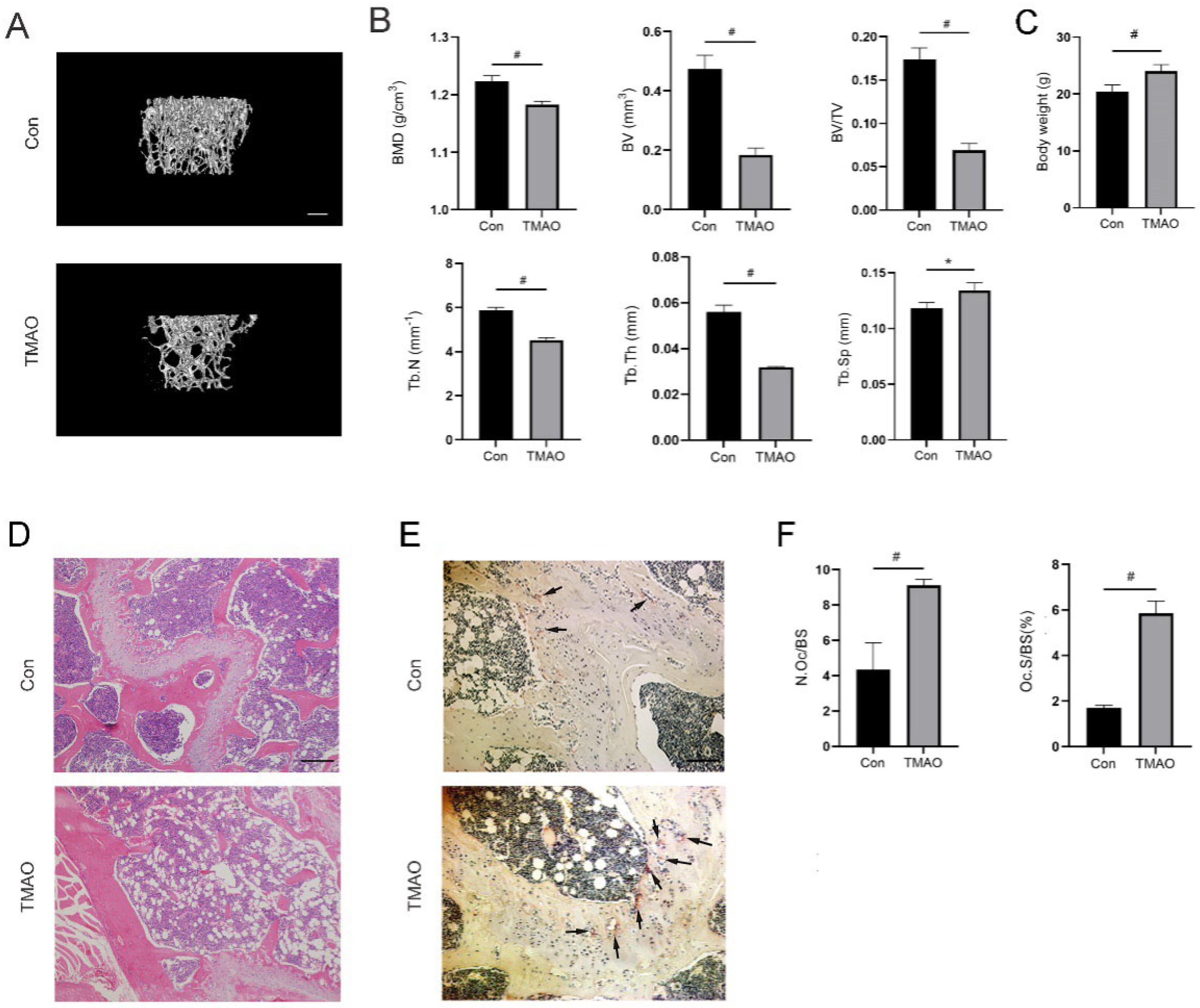
3.7. TMAO Induced Bone Loss in Mice
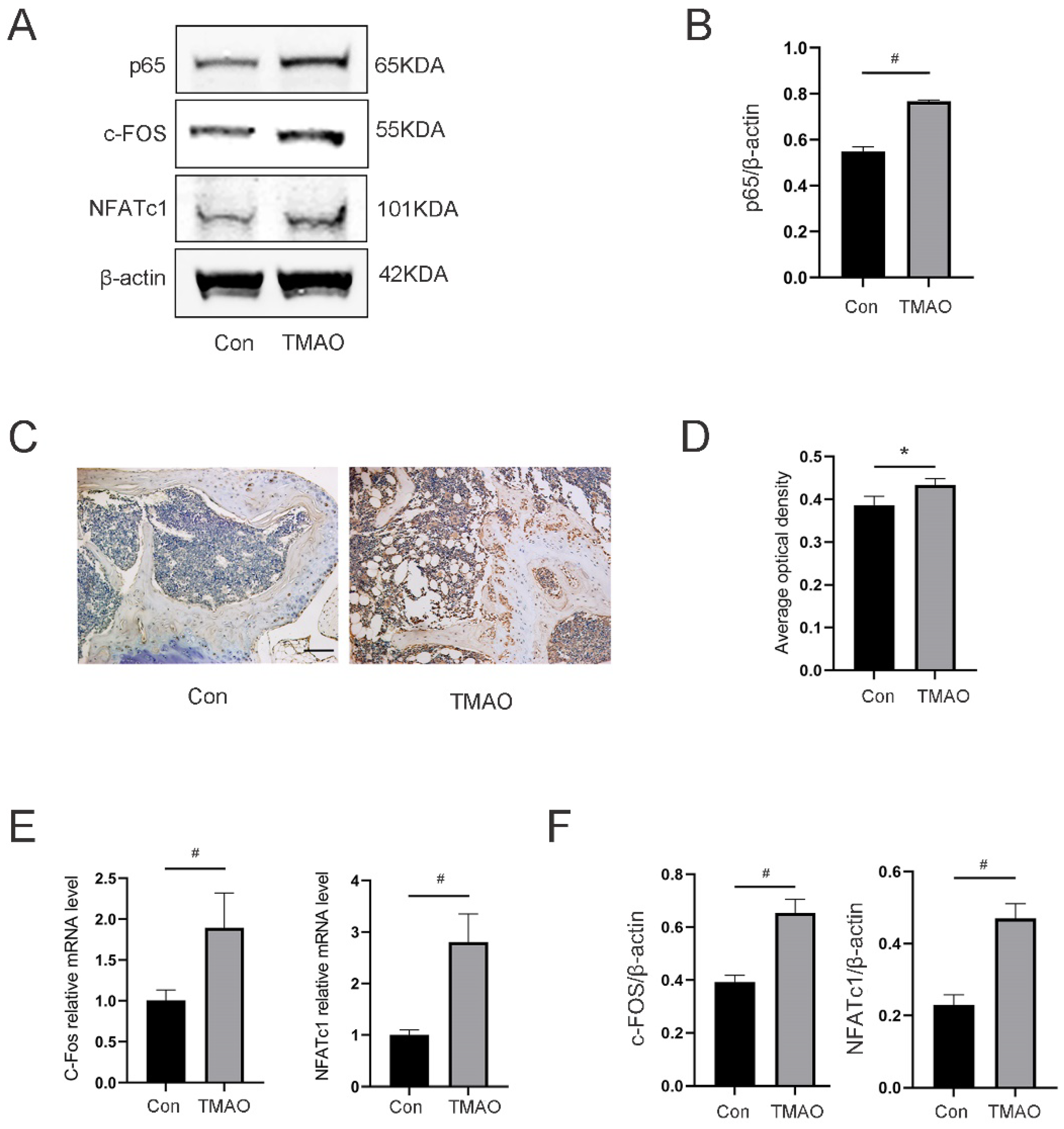
3.8. TMAO Increased the Expression of Osteoclast Genes and NF-κB Signaling in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-Oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Kang, S. The Role of the Gut Microbiome in Energy Balance with a Focus on the Gut-Adipose Tissue Axis. Front. Genet. 2020, 11, 297. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S. Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J. Stroke 2019, 21, 151–159. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Lu, J.; Ye, C.; Huang, Y.; Huang, D.; Tang, L.; Hou, W.; Kuang, Z.; Chen, Y.; Xiao, S.; Yishake, M.; et al. Corilagin Suppresses RANKL-Induced Osteoclastogenesis and Inhibits Oestrogen Deficiency-Induced Bone Loss via the NF-ΚB and PI3K/AKT Signalling Pathways. J. Cell. Mol. Med. 2020, 24, 10444–10457. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, B.; Zhu, R.; Li, R.; Tian, Y.; Liu, C.; Jia, Q.; Wang, L.; Tang, J.; Zhao, D.; et al. Fructus Ligustri Lucidi Preserves Bone Quality through the Regulation of Gut Microbiota Diversity, Oxidative Stress, TMAO and Sirt6 Levels in Aging Mice. Aging (Albany NY) 2019, 11, 9348–9368. [Google Scholar] [CrossRef]
- Zhou, T.; Heianza, Y.; Chen, Y.; Li, X.; Sun, D.; DiDonato, J.A.; Pei, X.; LeBoff, M.S.; Bray, G.A.; Sacks, F.M.; et al. Circulating Gut Microbiota Metabolite Trimethylamine N-Oxide (TMAO) and Changes in Bone Density in Response to Weight Loss Diets: The POUNDS Lost Trial. Diabetes Care 2019, 42, 1365–1371. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.-L.; Meng, S.; Gao, H.; Sui, L.-J.; Jin, S.; Li, Y.; Fan, S.-G. Gut Microbiota-Dependent Trimethylamine N-Oxide Are Related with Hip Fracture in Postmenopausal Women: A Matched Case-Control Study. Aging 2020, 12, 10633–10641. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the Immune System and Bone Metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef]
- Bar-Shavit, Z. The Osteoclast: A Multinucleated, Hematopoietic-Origin, Bone-Resorbing Osteoimmune Cell. J. Cell. Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Yuan, K.; Mei, J.; Shao, D.; Zhou, F.; Qiao, H.; Liang, Y.; Li, K.; Tang, T. Cerium Oxide Nanoparticles Regulate Osteoclast Differentiation Bidirectionally by Modulating the Cellular Production of Reactive Oxygen Species. Int. J. Nanomed. 2020, 15, 6355–6372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Yan, J.; Li, M.; Liu, T.; Zhu, C.; Pan, G.; Guo, Q.; Yang, H.; Pei, M.; et al. Melatonin at Pharmacological Concentrations Suppresses Osteoclastogenesis via the Attenuation of Intracellular ROS. Osteoporos. Int. 2017, 28, 3325–3337. [Google Scholar] [CrossRef]
- Lin, H.; Liu, T.; Li, X.; Gao, X.; Wu, T.; Li, P. The Role of Gut Microbiota Metabolite Trimethylamine N-Oxide in Functional Impairment of Bone Marrow Mesenchymal Stem Cells in Osteoporosis Disease. Ann. Transl. Med. 2020, 8, 1009. [Google Scholar] [CrossRef]
- Narahara, S.; Matsushima, H.; Sakai, E.; Fukuma, Y.; Nishishita, K.; Okamoto, K.; Tsukuba, T. Genetic Backgrounds and Redox Conditions Influence Morphological Characteristics and Cell Differentiation of Osteoclasts in Mice. Cell Tissue Res. 2012, 348, 81–94. [Google Scholar] [CrossRef]
- Sang, S.; Zhang, Z.; Qin, S.; Li, C.; Dong, Y. MicroRNA-16-5p Inhibits Osteoclastogenesis in Giant Cell Tumor of Bone. Biomed Res. Int. 2017, 2017, 3173547. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Yuan, L.; Yang, X. Protective Effects of Tartary Buckwheat Flavonoids on High TMAO Diet-Induced Vascular Dysfunction and Liver Injury in Mice. Food Funct. 2015, 6, 3359–3372. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wu, W.; Sun, X.; Zhang, P. Glucocorticoids Enhanced Osteoclast Autophagy Through the PI3K/Akt/MTOR Signaling Pathway. Calcif. Tissue Int. 2020, 107, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Strickson, S.; Campbell, D.G.; Emmerich, C.H.; Knebel, A.; Plater, L.; Ritorto, M.S.; Shpiro, N.; Cohen, P. The Anti-Inflammatory Drug BAY 11-7082 Suppresses the MyD88-Dependent Signalling Network by Targeting the Ubiquitin System. Biochem. J. 2013, 451, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.-L.; Wang, G.-Y.; Zuo, X.-Q.; Qu, C.-J.; Yao, B.-C.; Wang, D.-L. Gut Microbiota: An Overlooked Factor That Plays a Significant Role in Osteoporosis. J. Int. Med. Res. 2019, 47, 4095–4103. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-ΚB (Nuclear Factor ΚB) Signals. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef]
- Mondal, J.; Stirnemann, G.; Berne, B.J. When Does Trimethylamine N-Oxide Fold a Polymer Chain and Urea Unfold It? J. Phys. Chem. B 2013, 117, 8723–8732. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Paccou, J.; Edwards, M.H.; Ward, K.A.; Jameson, K.A.; Moss, C.L.; Harvey, N.C.; Dennison, E.M.; Cooper, C. Ischemic Heart Disease Is Associated with Lower Cortical Volumetric Bone Mineral Density of Distal Radius. Osteoporos. Int. 2015, 26, 1893–1901. [Google Scholar] [CrossRef]
- Gupta, G.; Aronow, W.S. Atherosclerotic Vascular Disease May Be Associated with Osteoporosis or Osteopenia in Postmenopausal Women: A Preliminary Study. Arch. Gerontol. Geriatr. 2006, 43, 285–288. [Google Scholar] [CrossRef]
- Crepaldi, G.; Maggi, S. Epidemiologic Link between Osteoporosis and Cardiovascular Disease. J. Endocrinol. Investig. 2009, 32, 2–5. [Google Scholar]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-ΚB Signaling Pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-ΚB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xu, Y.J.; Zhang, Z.L.; Li, K.; Li, B.; Zhang, W.; Yang, H. Ferric Ion Could Facilitate Osteoclast Differentiation and Bone Resorption through the Production of Reactive Oxygen Species. J. Orthop. Res. 2012, 30, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Babelova, A.; Trummer, O.; Erben, R.G.; Rauner, M.; Rammelt, S.; Weissmann, N.; Weinberger, V.; Benkhoff, S.; Kampschulte, M.; et al. NADPH Oxidase 4 Limits Bone Mass by Promoting Osteoclastogenesis. J. Clin. Investig. 2013, 123, 4731–4738. [Google Scholar] [CrossRef]
- Cheon, Y.-H.; Lee, C.H.; Jeong, D.H.; Kwak, S.C.; Kim, S.; Lee, M.S.; Kim, J.-Y. Dual Oxidase Maturation Factor 1 Positively Regulates RANKL-Induced Osteoclastogenesis via Activating Reactive Oxygen Species and TRAF6-Mediated Signaling. Int. J. Mol. Sci. 2020, 21, 6416. [Google Scholar] [CrossRef]
- Koh, J.-M.; Lee, Y.-S.; Kim, Y.S.; Kim, D.J.; Kim, H.-H.; Park, J.-Y.; Lee, K.-U.; Kim, G.S. Homocysteine Enhances Bone Resorption by Stimulation of Osteoclast Formation and Activity through Increased Intracellular ROS Generation. J. Bone Miner. Res. 2006, 21, 1003–1011. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Zhang, H.; Jie, Q.; Li, X.; Shi, Q.; Huang, Q.; Gao, B.; Han, Y.; Guo, K.; et al. Glucocorticoids: Dose-Related Effects on Osteoclast Formation and Function via Reactive Oxygen Species and Autophagy. Bone 2015, 79, 222–232. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, S.; Yang, L.; Huang, X.; Zou, L.; Huang, Y.; Dong, S.; Zhou, X.; Yang, X. Cerium Ion Promotes the Osteoclastogenesis through the Induction of Reactive Oxygen Species. J. Trace Elem. Med. Biol. 2019, 52, 126–135. [Google Scholar] [CrossRef]
- Wu, P.; Chen, J.; Chen, J.; Tao, J.; Wu, S.; Xu, G.; Wang, Z.; Wei, D.; Yin, W. Trimethylamine N-Oxide Promotes ApoE-/- Mice Atherosclerosis by Inducing Vascular Endothelial Cell Pyroptosis via the SDHB/ROS Pathway. J. Cell. Physiol. 2020, 235, 6582–6591. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-Oxide Induces Inflammation and Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells via Activating ROS-TXNIP-NLRP3 Inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhu, X.-H.; Ran, L.; Lang, H.-D.; Yi, L.; Mi, M.-T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-MtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Li, D.; Zhao, M.; Liu, C.; Liu, J.; Zeng, A.; Shi, X.; Cheng, S.; Pan, B.; Zheng, L.; et al. Gut Flora-Dependent Metabolite Trimethylamine-N-Oxide Accelerates Endothelial Cell Senescence and Vascular Aging through Oxidative Stress. Free Radic. Biol. Med. 2018, 116, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, L.; Wang, Y.; Zhai, J.; Chen, G.; Hu, K. Gambogic Acid Induces Heme Oxygenase-1 through Nrf2 Signaling Pathway and Inhibits NF-ΚB and MAPK Activation to Reduce Inflammation in LPS-Activated RAW264.7 Cells. Biomed. Pharmacother. 2019, 109, 555–562. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, C.; Chen, Z.; Yang, Z.; Liu, K.; Gong, S. Sirtuin-3 Protects Cochlear Hair Cells Against Noise-Induced Damage via the Superoxide Dismutase 2/Reactive Oxygen Species Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 766512. [Google Scholar] [CrossRef]
- Ma, S.; Qin, J.; Hao, Y.; Shi, Y.; Fu, L. Structural and Functional Changes of Gut Microbiota in Ovariectomized Rats and Their Correlations with Altered Bone Mass. Aging 2020, 12, 10736–10753. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Meng, F.; Ma, S.; Fu, L. Species-Level Gut Microbiota Analysis in Ovariectomized Osteoporotic Rats by Shallow Shotgun Sequencing. Gene 2022, 817, 146205. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yu, M.; Pal, S.; Tyagi, A.M.; Dar, H.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. Parathyroid Hormone-Dependent Bone Formation Requires Butyrate Production by Intestinal Microbiota. J. Clin. Investig. 2020, 130, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Schepper, J.D.; Collins, F.; Rios-Arce, N.D.; Kang, H.J.; Schaefer, L.; Gardinier, J.D.; Raghuvanshi, R.; Quinn, R.A.; Britton, R.; Parameswaran, N.; et al. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J. Bone Miner. Res. 2020, 35, 801–820. [Google Scholar] [CrossRef]
- Liu, J.-H.; Chen, C.-Y.; Liu, Z.-Z.; Luo, Z.-W.; Rao, S.-S.; Jin, L.; Wan, T.-F.; Yue, T.; Tan, Y.-J.; Yin, H.; et al. Extracellular Vesicles from Child Gut Microbiota Enter into Bone to Preserve Bone Mass and Strength. Adv. Sci. 2021, 8, 2004831. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Hao, Y.; Fu, L. Trimethylamine-N-Oxide Promotes Osteoclast Differentiation and Bone Loss via Activating ROS-Dependent NF-κB Signaling Pathway. Nutrients 2022, 14, 3955. https://doi.org/10.3390/nu14193955
Wang N, Hao Y, Fu L. Trimethylamine-N-Oxide Promotes Osteoclast Differentiation and Bone Loss via Activating ROS-Dependent NF-κB Signaling Pathway. Nutrients. 2022; 14(19):3955. https://doi.org/10.3390/nu14193955
Chicago/Turabian StyleWang, Ning, Yongqiang Hao, and Lingjie Fu. 2022. "Trimethylamine-N-Oxide Promotes Osteoclast Differentiation and Bone Loss via Activating ROS-Dependent NF-κB Signaling Pathway" Nutrients 14, no. 19: 3955. https://doi.org/10.3390/nu14193955
APA StyleWang, N., Hao, Y., & Fu, L. (2022). Trimethylamine-N-Oxide Promotes Osteoclast Differentiation and Bone Loss via Activating ROS-Dependent NF-κB Signaling Pathway. Nutrients, 14(19), 3955. https://doi.org/10.3390/nu14193955





