Sixteen-Week Vitamin D3 Supplementation Increases Peripheral T Cells in Overweight Black Individuals: Post hoc Analysis of a Randomized, Double-Blinded, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Anthropometry Measurements
2.4. Biochemical Measurements
2.5. Statistical Analysis
3. Results
3.1. General Demographics
3.2. Effects of Vitamin D3 Supplementation on Serum 25(OH)D
3.3. Changes in T Cells after Vitamin D3 Supplementation
3.4. Adjusted Effects of Vitamin D3 Supplementation on T Cells
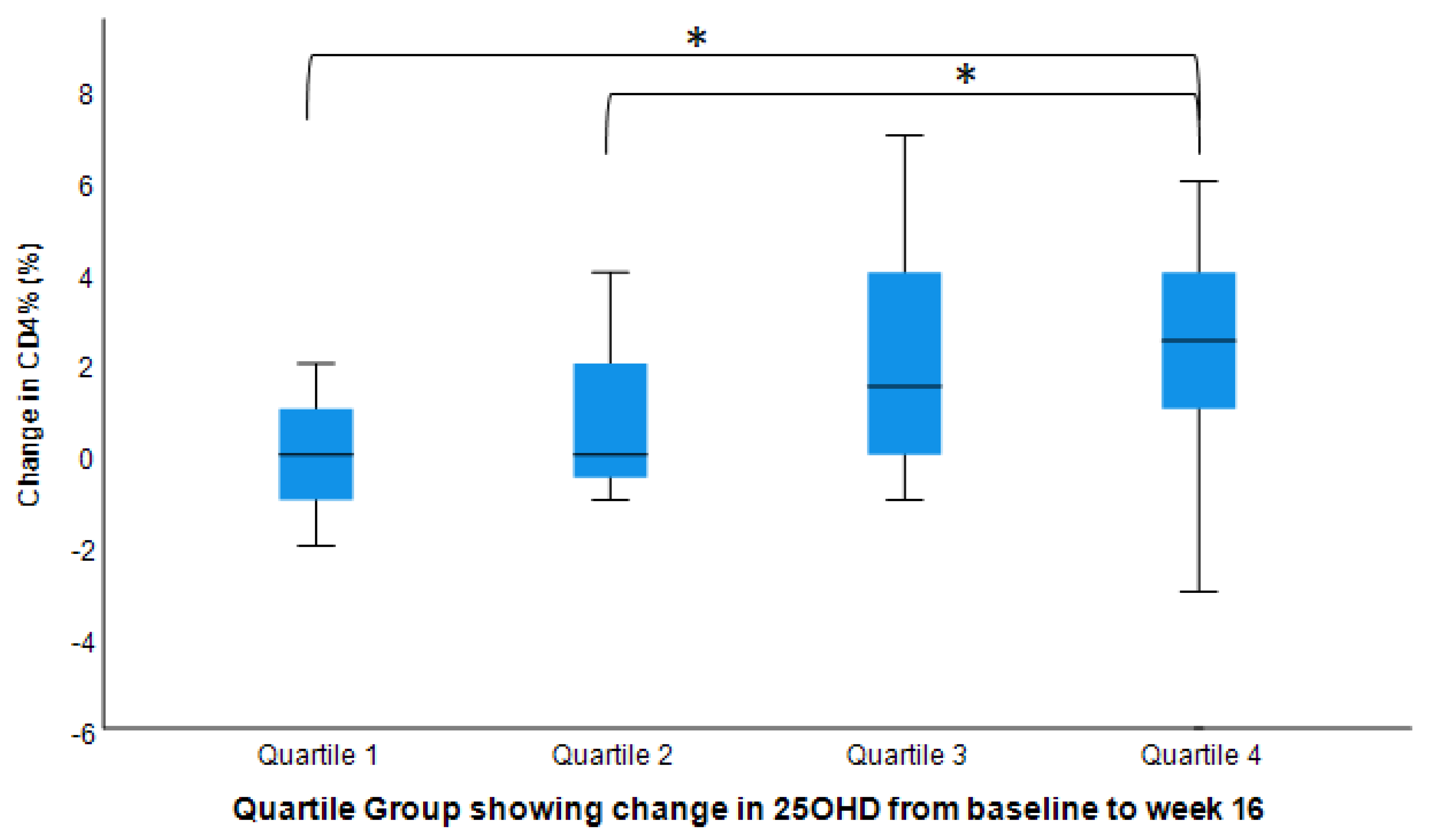
3.5. Post hoc Pairwise Analysis: Post-Interventional Changes in CD3+, CD4+, and CD8+ by Dosage Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Oren, Y.; Shapira, Y.; Agmon-Levin, N.; Kivity, S.; Zafrir, Y.; Altman, A.; Lerner, A.; Shoenfeld, Y. Vitamin D insufficiency in a sunny environment: A demographic and seasonal analysis. Isr. Med. Assoc. J. IMAJ 2010, 12, 751–756. [Google Scholar] [PubMed]
- Haimi, M.; Kremer, R. Vitamin D deficiency/insufficiency from childhood to adulthood: Insights from a sunny country. World J. Clin. Pediatrics 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Johnson, C.L.; Lacher, D.A.; Pfeiffer, C.M.; Schleicher, R.L.; Sempos, C.T. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011, 59, 1–8. [Google Scholar]
- Bhagatwala, J.; Zhu, H.; Parikh, S.J.; Guo, D.-H.; Kotak, I.; Huang, Y.; Havens, R.; Pham, M.; Afari, E.; Kim, S.; et al. Dose and time responses of vitamin D biomarkers to monthly vitamin D (3) supplementation in overweight/obese African Americans with suboptimal vitamin d status: A placebo controlled randomized clinical trial. BMC Obes. 2015, 2, 27. [Google Scholar] [CrossRef]
- Forrest, K.Y.Z.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The Effect Of Vitamin D Supplementation On Serum 25OHD In Thin And Obese Women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef]
- Souberbielle, J.-C.; Body, J.-J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef]
- Liao, Y.; Greenlund, K.J.; Croft, J.B.; Keenan, N.L.; Giles, W.H. Factors Explaining Excess Stroke Prevalence in the US Stroke Belt. Stroke 2009, 40, 3336–3341. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)(2)D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Konijeti, G.G.; Arora, P.; Boylan, M.R.; Song, Y.; Huang, S.; Harrell, F.; Newton-Cheh, C.; O’Neill, D.; Korzenik, J.; Wang, T.J.; et al. Vitamin D Supplementation Modulates T Cell–Mediated Immunity in Humans: Results from a Randomized Control Trial. J. Clin. Endocrinol. Metab. 2016, 101, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Zerofsky, M.; Jacoby, B.; Pedersen, T.L.; Stephensen, C.B. Effects of a Randomized, Controlled Trial of Daily Vitamin D3 Supplementation During Pregnancy on Regulatory Immunity and Inflammation. FASEB J. 2016, 30, 296–297. [Google Scholar] [CrossRef]
- Gorman, S.; Geldenhuys, S.; Judge, M.; Weeden, C.E.; Waithman, J.; Hart, P.H. Dietary Vitamin D Increases Percentages and Function of Regulatory T Cells in the Skin-Draining Lymph Nodes and Suppresses Dermal Inflammation. J. Immunol. Res. 2016, 2016, 1426503. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.E.; Hubler, S.L.; Moore, J.R.; Barta, L.E.; Praska, C.E.; Nashold, F.E. Vitamin D Actions on CD4(+) T Cells in Autoimmune Disease. Front. Immunol. 2015, 6, 100. [Google Scholar] [CrossRef]
- Hulgan, T.; Shepherd, B.E.; Raffanti, S.P.; Fusco, J.S.; Beckerman, R.; Barkanic, G.; Sterling, T.R. Absolute Count and Percentage of CD4+ Lymphocytes Are Independent Predictors of Disease Progression in HIV-Infected Persons Initiating Highly Active Antiretroviral Therapy. J. Infect. Dis. 2007, 195, 425–431. [Google Scholar] [CrossRef]
- Gompels, M.; Dunn, D.T.; Phillips, A.; Dooley, D.; De Burgh Thomas, A.; Anderson, J.; Post, F.; Pillay, D.; Gazzard, B.; Hill, T.; et al. Does Discordancy Between the CD4 Count and CD4 Percentage in HIV-Positive Individuals Influence Outcomes on Highly Active Antiretroviral Therapy? J. Infect. Dis. 2012, 205, 540–547. [Google Scholar] [CrossRef][Green Version]
- Strindhall, J.; Skog, M.; Ernerudh, J.; Bengner, M.; Löfgren, S.; Matussek, A.; Nilsson, B.O.; Wikby, A. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: The Swedish HEXA immune study. Age 2013, 35, 985–991. [Google Scholar] [CrossRef]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef]
- Raed, A.; Bhagatwala, J.; Zhu, H.; Pollock, N.K.; Parikh, S.J.; Huang, Y.; Havens, R.; Kotak, I.; Guo, D.H.; Dong, Y. Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: A placebo controlled randomized trial. PLoS ONE 2017, 12, e0188424. [Google Scholar] [CrossRef]
- Bofill, M.; Janossy, G.; Lee, C.A.; MacDonald-Burns, D.; Phillips, A.N.; Sabin, C.; Timms, A.; Johnson, M.A.; Kernoff, P.B. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin. Exp. Immunol. 1992, 88, 243–252. [Google Scholar] [CrossRef]
- Valiathan, R.; Deeb, K.; Diamante, M.; Ashman, M.; Sachdeva, N.; Asthana, D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology 2014, 219, 487–496. [Google Scholar] [CrossRef]
- Dybul, M.; Fauci, A.S.; Bartlett, J.G.; Kaplan, J.E.; Pau, A.K. Guidelines for using antiretroviral agents among hiv-infected adults and adolescents: The panel on clinical practices for treatment of hiv*. Ann. Intern. Med. 2002, 137, 381–433. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Livak, B.; Burke-Miller, J.; French, A.L.; Glesby, M.J.; Sharma, A.; Young, M.; Villacres, M.C.; Tien, P.C.; Golub, E.T.; et al. Vitamin D insufficiency may impair CD4 recovery among Women’s Interagency HIV Study participants with advanced disease on HAART. AIDS 2013, 27, 573–578. [Google Scholar] [CrossRef]
- Petrie, H.T.; Hugo, P.; Scollay, R.; Shortman, K. Lineage relationships and developmental kinetics of immature thymocytes: CD3, CD4, and CD8 acquisition in vivo and in vitro. J. Exp. Med. 1990, 172, 1583–1588. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Marcus, J.L.; Leyden, W.A.; Chao, C.R.; Chow, F.C.; Horberg, M.A.; Hurley, L.B.; Klein, D.B.; Quesenberry, C.P.J.; Towner, W.J.; Silverberg, M.J. HIV infection and incidence of ischemic stroke. AIDS 2014, 28, 1911–1919. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Karnchanasorn, R.; Ou, H.-Y.; Chiu, K.C. Plasma 25-Hydroxyvitamin D Levels Are Favorably Associated With β-Cell Function. Pancreas 2012, 41, 863–868. [Google Scholar] [CrossRef]
- Kamen, D.L.; Tangpricha, V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. J. Mol. Med. 2010, 88, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011, 14, 938–939. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Intervention Groups | p-Value | |||
|---|---|---|---|---|---|
| Placebo | 600 IU/Day | 2000 IU/Day | 4000 IU/Day | ||
| Number of subjects (n) | 13 | 14 | 15 | 14 | |
| Age (year) | 30 ± 11 | 27 ± 10 | 25 ± 8.3 | 23 ± 6.9 | 0.23 |
| Male/Female Ratio | 4/9 | 2/12 | 2/13 | 1/13 | 0.39 |
| Height (m) | 1.64 ± 0.08 | 1.65 ± 0.08 | 1.63 ± 0.09 | 1.63 ± 0.08 | 0.83 |
| Weight (kg) | 99 ± 26 | 95 ± 15 | 92 ± 18 | 91 ± 21 | 0.73 |
| BMI (kg/m2) | 37 ± 7.6 | 35 ± 5.8 | 35 ± 7 | 34 ± 7.7 | 0.81 |
| Serum 25(OH)D (ng/mL) | 35 ± 10 | 33 ± 9.1 | 35 ± 9.9 | 32 ± 10 | 0.89 |
| T Cells | |||||
| CD3+% | 75 ± 6.7 | 76 ± 4.2 | 75 ± 6.8 | 76 ± 5.6 | 0.97 |
| CD3+ Count (cells/uL) | 1533 ± 515 | 1214 ± 271 | 1344 ± 454 | 1452 ± 536 | 0.30 |
| CD4+% | 44 ± 5.9 | 48 ± 7.2 | 45 ± 6.2 | 47 ± 7.0 | 0.49 |
| CD4+ Count (cells/uL) | 911 ± 321 | 767 ± 227 | 812 ± 290 | 885 ± 298 | 0.54 |
| CD8+% | 27 ± 8.5 | 25 ± 6.4 | 26 ± 3.7 | 25 ± 7.4 | 0.87 |
| CD8+ Count (cells/uL) | 561 ± 252 | 390 ± 126 | 467 ± 171 | 509 ± 311 | 0.25 |
| T Cell | Intervention Groups | ||||
|---|---|---|---|---|---|
| Placebo | 600 IU/Day | 2000 IU/Day | 4000 IU/Day | p | |
| CD3+% (%) | |||||
| Model 1 | 76 (74, 77) | 76 (74, 78) | 78 (76, 80) | 77 (75, 79) | 0.01 |
| Model 2 | 76 (74, 77) | 76 (74, 78) | 78 (76, 80) | 77 (75, 79) | 0.02 |
| CD3+ Count (cells/uL) | |||||
| Model 1 | 1375 (1258, 1492) | 1382 (1185, 1579) | 1555 (1350, 1761) | 1469 (1280, 1658) | 0.18 |
| Model 2 | 1384 (1272, 1496) | 1388 (1194, 1582) | 1552 (1349, 1755) | 1468 (1282, 1654) | 0.25 |
| CD4+% (%) | |||||
| Model 1 | 46 (45, 48) | 47 (45, 49) | 49 (47, 52) | 47 (45, 50) | <0.001 |
| Model 2 | 47 (45, 48) | 47 (45, 49) | 50 (47, 52) | 47 (46, 50) | <0.001 |
| CD4+ Count (cells/uL) | |||||
| Model 1 | 841 (769, 914) | 798 (661, 936) | 994 (851, 1138) | 917 (784, 1049) | 0.05 |
| Model 2 | 847 (781, 914) | 804 (669, 939) | 995 (854, 1136) | 913 (784, 1043) | 0.08 |
| CD8+% (%) | |||||
| Model 1 | 26 (25, 28) | 26 (24, 28) | 26 (24, 28) | 26 (24, 28) | 0.57 |
| Model 2 | 26 (25, 28) | 26 (24, 28) | 26 (24, 28) | 26 (24, 28) | 0.42 |
| CD8+ Count (cells/uL) | |||||
| Model 1 | 474 (417, 531) | 482 (398, 565) | 510 (422, 597) | 490 (409, 571) | 0.80 |
| Model 2 | 476 (421, 532) | 481 (401, 561) | 506 (422, 590) | 493 (416, 570) | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Chen, L.; Huang, Y.; Raed, A.; Havens, R.; Dong, Y.; Zhu, H. Sixteen-Week Vitamin D3 Supplementation Increases Peripheral T Cells in Overweight Black Individuals: Post hoc Analysis of a Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2022, 14, 3922. https://doi.org/10.3390/nu14193922
Dong Y, Chen L, Huang Y, Raed A, Havens R, Dong Y, Zhu H. Sixteen-Week Vitamin D3 Supplementation Increases Peripheral T Cells in Overweight Black Individuals: Post hoc Analysis of a Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients. 2022; 14(19):3922. https://doi.org/10.3390/nu14193922
Chicago/Turabian StyleDong, Yutong, Li Chen, Ying Huang, Anas Raed, Robyn Havens, Yanbin Dong, and Haidong Zhu. 2022. "Sixteen-Week Vitamin D3 Supplementation Increases Peripheral T Cells in Overweight Black Individuals: Post hoc Analysis of a Randomized, Double-Blinded, Placebo-Controlled Trial" Nutrients 14, no. 19: 3922. https://doi.org/10.3390/nu14193922
APA StyleDong, Y., Chen, L., Huang, Y., Raed, A., Havens, R., Dong, Y., & Zhu, H. (2022). Sixteen-Week Vitamin D3 Supplementation Increases Peripheral T Cells in Overweight Black Individuals: Post hoc Analysis of a Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients, 14(19), 3922. https://doi.org/10.3390/nu14193922






