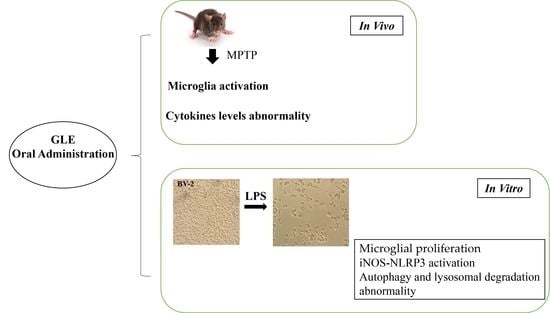
Ganoderma lucidum Modulates Inflammatory Responses following 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Administration in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Experimental Procedure
2.4. Immunohistochemistry (IHC) and Imaging Analysis
2.5. Multiple Assays of 23 Cytokines in Brain Tissues
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
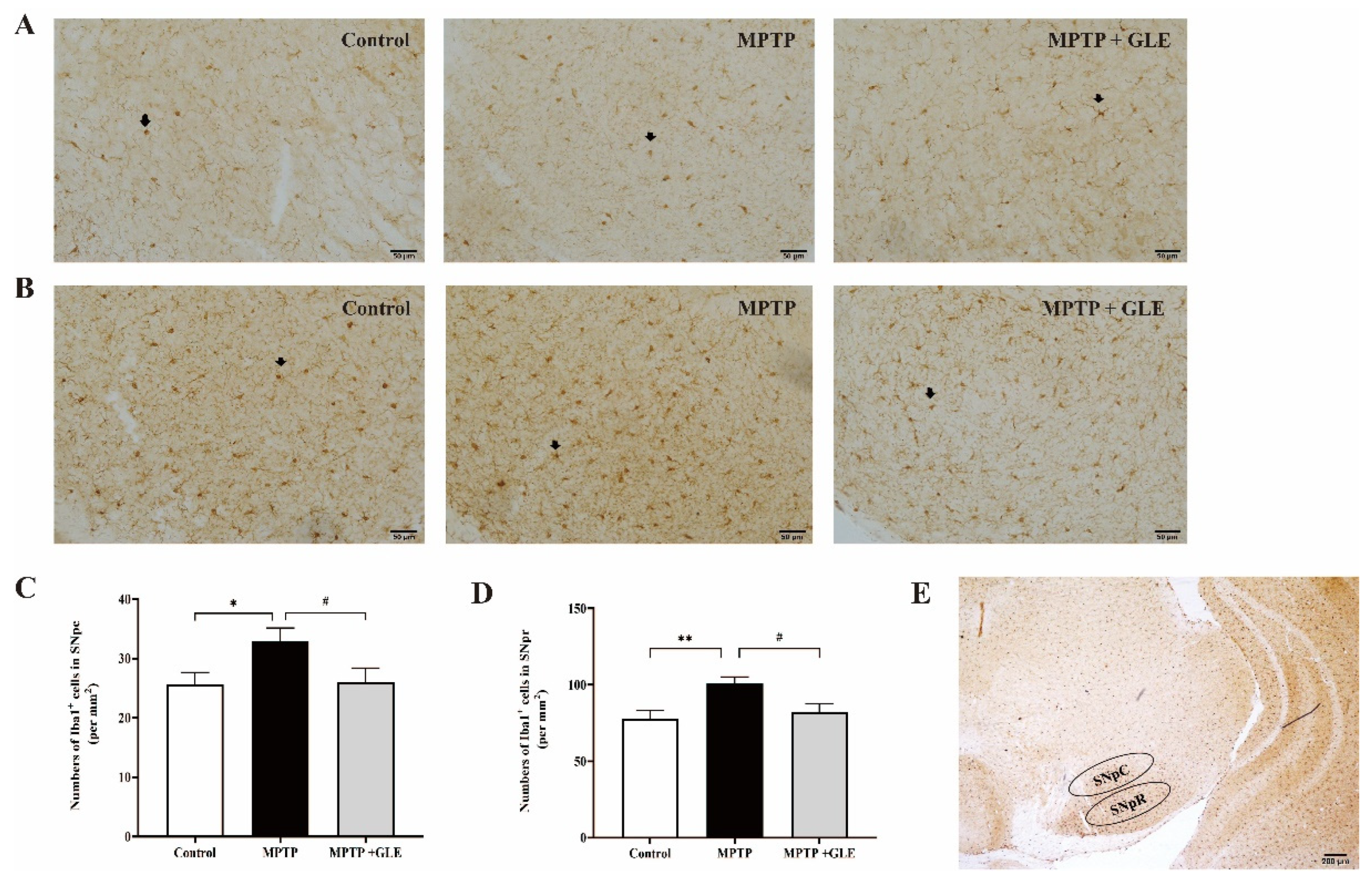
3.1. GLE Treatment Attenuated Microglia Activation in MPTP-Lesioned Mice
3.2. GLE Treatment Modulated Cytokine and Chemokine Levels in MPTP-Lesioned Mice
3.3. GLE Inhibited LPS-Induced BV2 Microglia Proliferation
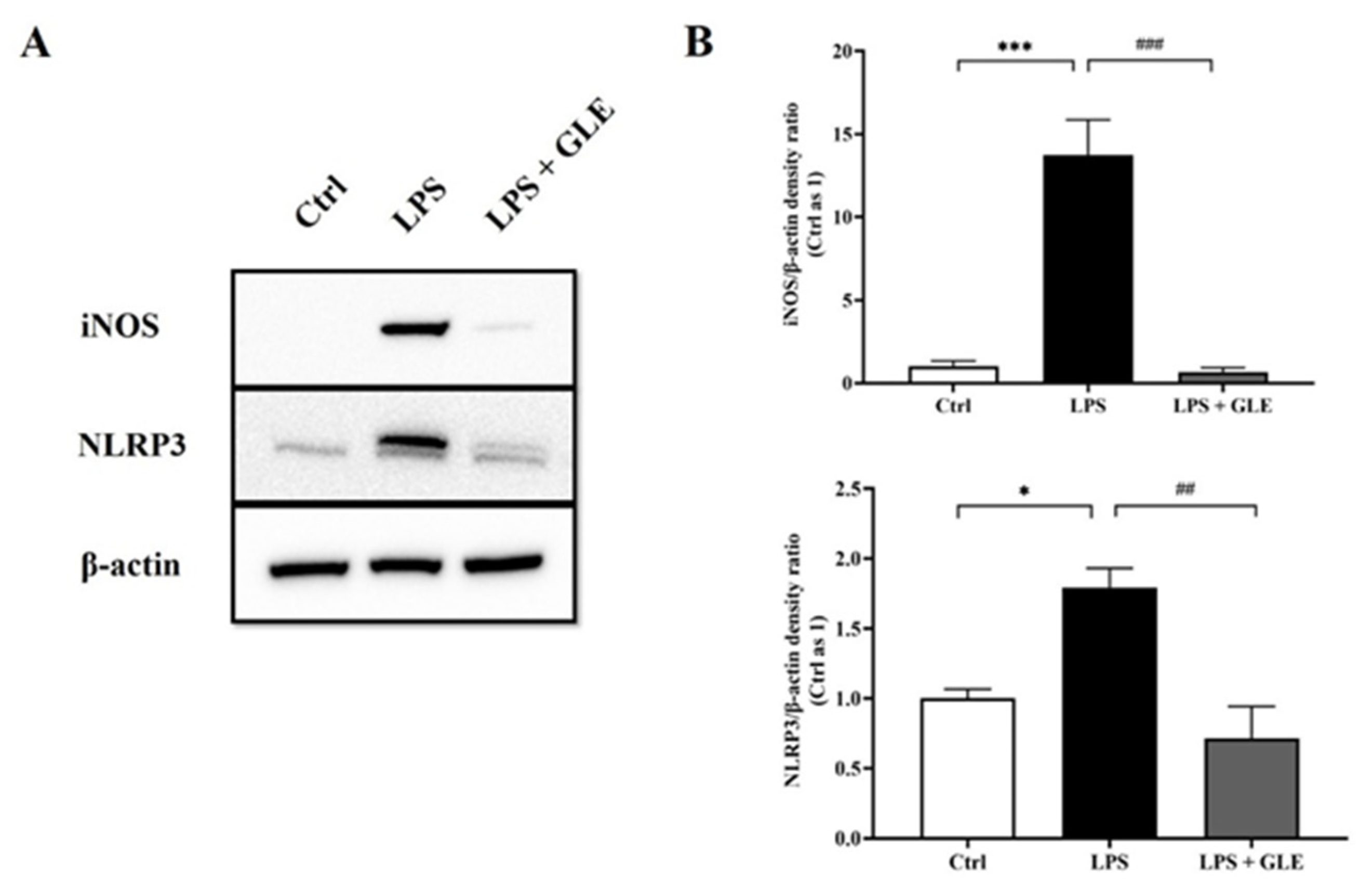
3.4. GLE Suppressed LPS-Stimulated iNOS-NLRP3 Activation in BV-2 Cells
3.5. GLE Inhibited Autophagy and Lysosomal Degradation in LPS-Induced BV-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Athauda, D.; Foltynie, T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015, 11, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A Mechanistic Review on Medicinal Mushrooms-Derived Bioactive Compounds: Potential Mycotherapy Candidates for Alleviating Neurological Disorders. Planta Med. 2020, 86, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Liu, M.M.; Liu, T.; Yeung, S.; Wang, Z.; Andresen, B.; Parsa, C.; Orlando, R.; Zhou, B.; Wu, W.; Li, X.; et al. Inhibitory activity of medicinal mushroom Ganoderma lucidum on colorectal cancer by attenuating inflammation. Precis. Clin. Med. 2021, 4, 231–245. [Google Scholar] [CrossRef]
- Mi, X.; Zeng, G.R.; Liu, J.Q.; Luo, Z.S.; Zhang, L.; Dai, X.M.; Fang, W.T.; Zhang, J.; Chen, X.C. Ganoderma Lucidum Triterpenoids Improve Maternal Separation-Induced Anxiety- and Depression-like Behaviors in Mice by Mitigating Inflammation in the Periphery and Brain. Nutrients 2022, 14, 2268. [Google Scholar] [CrossRef]
- Taufek, N.M.; Harith, H.H.; Rahim, M.H.A.; Ilham, Z.; Rowan, N.; Wan-Mohtar, W.A.A.Q.I. Performance of mycelial biomass and exopolysaccharide from Malaysian Ganoderma lucidum for the fungivore red hybrid Tilapia (Oreochromis sp.) in Zebrafish embryo. Aquac. Rep. 2020, 17, 100322. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; Rowan, N. Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications. Int. J. Mol. Sci. 2021, 22, 1675. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, S.; Cai, Y.; Zhou, M.; Zuo, X.; Chan, P. Ganoderma lucidum Protects Dopaminergic Neuron Degeneration through Inhibition of Microglial Activation. Evid. Based Complement. Alternat. Med. 2011, 2011, 156810. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, M.; Zhang, R.P.; Xu, S.L. Ganoderma lucidum extract protects dopaminergic neurons through inhibiting the production of inflammatory mediators by activated microglia. Sheng Li Xue Bao 2010, 62, 547–554. [Google Scholar] [PubMed]
- Ren, Z.L.; Wang, C.D.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Mustapha, M.; Mat Taib, C.N. MPTP-induced mouse model of Parkinson’s disease: A promising direction of therapeutic strategies. Bosn. J. Basic Med. Sci. 2021, 21, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Buneeva, O.; Kopylov, A.; Kapitsa, I.; Ivanova, E.; Zgoda, V.; Medvedev, A. The Effect of Neurotoxin MPTP and Neuroprotector Isatin on the Profile of Ubiquitinated Brain Mitochondrial Proteins. Cells 2018, 7, 91. [Google Scholar] [CrossRef]
- Cao, J.J.; Li, K.S.; Shen, Y.Q. Activated immune cells in Parkinson’s disease. J. Neuroimmune Pharmacol. 2011, 6, 323–329. [Google Scholar] [CrossRef]
- Sadasivan, S.; Sharp, B.; Schultz-Cherry, S.; Smeyne, R.J. Synergistic effects of influenza and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: Experimental evidence for the multi-hit hypothesis. NPJ Parkinsons Dis. 2017, 3, 18. [Google Scholar] [CrossRef]
- Martens, L.H.; Zhang, J.; Barmada, S.J.; Zhou, P.; Kamiya, S.; Sun, B.; Min, S.W.; Gan, L.; Finkbeiner, S.; Huang, E.J.; et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J. Clin. Invest. 2012, 122, 3955–3959. [Google Scholar] [CrossRef]
- Kostuk, E.W.; Cai, J.; Iacovitti, L. Regional microglia are transcriptionally distinct but similarly exacerbate neurodegeneration in a culture model of Parkinson’s disease. J. Neuroinflammation 2018, 15, 139. [Google Scholar] [CrossRef]
- Coleman, M.P. The challenges of axon survival: Introduction to the special issue on axonal degeneration. Exp. Neurol. 2013, 246, 1–5. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, S.; Feinberg, P.A.; Johnson, K.M.; Schafer, D.P. A microglia-cytokine axis to modulate synaptic connectivity and function. Curr. Opin. Neurobiol. 2017, 47, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, A.; Mendonca, P.; Soliman, K.F.A. Involvement of NFkB and MAPK signaling pathways in the preventive effects of Ganoderma lucidum on the inflammation of BV-2 microglial cells induced by LPS. J. Neuroimmunol. 2020, 345, 577269. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zeng, K.; Liao, L.; Yu, Q.; Tu, P.; Wang, X. Schizandrin A Inhibits Microglia-Mediated Neuroninflammation through Inhibiting TRAF6-NF-kappaB and Jak2-Stat3 Signaling Pathways. PLoS ONE 2016, 11, e0149991. [Google Scholar] [CrossRef]
- Zhou, D.; Jiang, Y. Sirtuin 3 attenuates neuroinflammation-induced apoptosis in BV-2 microglia. Aging 2019, 11, 9075–9089. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Cheng, J.; Liao, Y.; Dong, Y.; Hu, H.; Yang, N.; Kong, X.; Li, S.; Li, X.; Guo, J.; Qin, L.; et al. Microglial autophagy defect causes parkinson disease-like symptoms by accelerating inflammasome activation in mice. Autophagy 2020, 16, 2193–2205. [Google Scholar] [CrossRef]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia clear neuron-released alpha-synuclein via selective autophagy and prevent neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef]
- Xu, Y.; Propson, N.E.; Du, S.; Xiong, W.; Zheng, H. Autophagy deficiency modulates microglial lipid homeostasis and aggravates tau pathology and spreading. Proc. Natl. Acad. Sci. USA 2021, 118, e2023418118. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Zabala, A.; Sierra-Torre, V.; Sierra, A. Assessing Autophagy in Microglia: A Two-Step Model to Determine Autophagosome Formation, Degradation, and Net Turnover. Front. Immunol. 2020, 11, 620602. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Li, Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflammation 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Song, L.; Xue, J.; Wang, X.; Song, S.; Wang, S. Polysaccharide from Ganoderma lucidum ameliorates cognitive impairment by regulating the inflammation of the brain-liver axis in rats. Food Funct. 2021, 12, 6900–6914. [Google Scholar] [CrossRef]
- Kahveci, R.; Kahveci, F.O.; Gokce, E.C.; Gokce, A.; Kisa, U.; Sargon, M.F.; Fesli, R.; Gurer, B. Effects of Ganoderma lucidum Polysaccharides on Different Pathways Involved in the Development of Spinal Cord Ischemia Reperfusion Injury: Biochemical, Histopathologic, and Ultrastructural Analysis in a Rat Model. World Neurosurg. 2021, 150, e287–e297. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, D.; Yin, H.; Li, H.; Du, J.; Bao, H. Ganoderic Acid A Attenuates LPS-Induced Neuroinflammation in BV2 Microglia by Activating Farnesoid X Receptor. Neurochem. Res. 2021, 46, 1725–1736. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, D.; Li, H.; Luo, S.; Xiao, Y.; Han, L.; Zhou, F.; Wang, C.; Feng, L.; Wang, G.; et al. Activation of FXR by ganoderic acid A promotes remyelination in multiple sclerosis via anti-inflammation and regeneration mechanism. Biochem. Pharmacol. 2021, 185, 114422. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Sui, R. Ganoderic Acid A-Mediated Modulation of Microglial Polarization is Involved in Depressive-Like Behaviors and Neuroinflammation in a Rat Model of Post-Stroke Depression. Neuropsychiatr. Dis. Treat. 2021, 17, 2671–2681. [Google Scholar] [CrossRef]
- Kou, R.W.; Gao, Y.Q.; Xia, B.; Wang, J.Y.; Liu, X.N.; Tang, J.J.; Yin, X.; Gao, J.M. Ganoderterpene A, a New Triterpenoid from Ganoderma lucidum, Attenuates LPS-Induced Inflammation and Apoptosis via Suppressing MAPK and TLR-4/NF-kappaB Pathways in BV-2 Cells. J. Agric. Food Chem. 2021, 69, 12730–12740. [Google Scholar] [CrossRef]
- Sheng, F.; Zhang, L.; Wang, S.; Yang, L.; Li, P. Deacetyl Ganoderic Acid F Inhibits LPS-Induced Neural Inflammation via NF-kappaB Pathway Both In Vitro and In Vivo. Nutrients 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Aguirre Moreno, A.C.; Campos Pena, V.; Villeda Hernandez, J.; Leon Rivera, I.; Montiel Arcos, E. Ganoderma lucidum reduces kainic acid-induced hippocampal neuronal damage via inflammatory cytokines and glial fibrillary acid protein expression. Proc. West Pharmacol. Soc. 2011, 54, 78–79. [Google Scholar] [PubMed]
- Ren, Z.L.; Zuo, P.P. Neural regeneration: Role of traditional Chinese medicine in neurological diseases treatment. J. Pharmacol. Sci. 2012, 120, 139–145. [Google Scholar] [CrossRef] [PubMed]



| Cytokine/Chemokine | Control | MPTP | MPTP + GLE |
|---|---|---|---|
| IL-1α | 59.31 ± 7.63 | 69.06 ± 8.72 | 67.96 ± 4.83 |
| IL-1β | 5985.88 ± 513.24 | 7048.02 ± 329.99 * | 6534.95 ± 176.21 |
| IL-2 | 2006.17 ± 393.29 | 2437.60 ± 332.68 | 2421.81 ± 169.47 |
| IL-3 | 162.65 ± 11.88 | 183.67 ± 8.04 | 169.14 ± 7.93 |
| IL-4 | 53.63 ± 6.27 | 65.46 ± 8.04 | 63.02 ± 3.38 |
| IL-5 | 123.02 ± 14.17 | 133.25 ± 9.50 | 135.11 ± 5.32 |
| IL-6 | 174.53 ± 15.20 | 209.65 ± 7.87 | 198.62 ± 7.90 |
| IL-9 | 26,312.90 ± 4622.58 | 30,082.75 ± 3523.41 | 28,480.24 ± 2334.98 |
| IL-10 | 254.47 ± 26.70 | 280.79 ± 26.42 | 271.50 ± 16.68 |
| IL-12(p40) | 115.18 ± 8.51 | 135.87 ± 7.89 * | 127.55 ± 3.78 |
| IL-12(p70) | 781.60 ± 84.75 | 794.76 ± 39.17 | 847.94 ± 30.29 |
| IL-13 | 30,195.89 ± 2496.90 | 34,524.49 ± 1708.82 | 34,031.25 ± 871.92 |
| IL-17 | 2384.74 ± 199.21 | 2820.02 ± 122.31 * | 2737.16 ± 48.81 |
| Eotaxin | 14,680.31 ± 757.97 | 14,612.08 ± 289.31 | 13,475.91 ± 330.17 |
| G-CSF | 102.73 ± 11.11 | 116.59 ± 10.21 | 114.11 ± 5.11 |
| GM-CSF | 1555.74 ± 36.43 | 1651.85 ± 24.38 * | 1514.10 ± 24.59 ## |
| KC | 295.41 ± 16.34 | 367.05 ± 14.78 ** | 355.82 ± 15.63 |
| MCP-1 | 1952.11 ± 128.24 | 2134.07 ± 128.52 | 2165.10 ± 86.53 |
| MIP-1α | 447.26 ± 39.74 | 441.25 ± 26.90 | 433.75 ± 21.50 |
| MIP-1β | 755.20 ± 89.44 | 936.23 ± 57.13 * | 904.77 ± 42.74 |
| RANTES | 319.19 ± 33.62 | 341.21 ± 11.75 | 332.40 ± 6.73 |
| TNF-α | 5008.07 ± 156.57 | 6403.28 ± 334.58 * | 6319.66 ± 253.61 |
| IFN-γ | 398.57 ± 38.14 | 481.55 ± 24.00 * | 439.06 ± 26.87 |
| Cytokine/Chemokine | Control | MPTP | MPTP + GLE |
|---|---|---|---|
| IL-1α | 61.11 ± 3.82 | 75.43 ± 2.94 ** | 65.93 ± 2.18 # |
| IL-1β | 4895.35 ± 459.92 | 5895.72 ± 161.64 * | 4927.78 ± 138.20 # |
| IL-2 | 894.40 ± 69.15 | 1486.18 ± 218.39 * | 1328.81 ± 69.23 |
| IL-3 | 115.38 ± 8.88 | 138.77 ± 8.18 | 109.21 ± 8.20 # |
| IL-4 | 41.76 ± 3.42 | 54.33 ± 2.97 * | 55.00 ± 4.52 |
| IL-5 | 102.39 ± 10.97 | 120.48 ± 9.47 | 129.13 ± 9.16 |
| IL-6 | 157.80 ± 18.02 | 183.73 ± 6.73 | 180.29 ± 7.09 |
| IL-9 | 15,997.54 ± 1410.37 | 18,889.61 ± 1096.69 | 19,500.01 ± 878.82 |
| IL-10 | 197.01 ± 16.39 | 244.15 ± 12.87 * | 233.61 ± 16.56 |
| IL-12(p40) | 94.06 ± 7.31 | 115.49 ± 4.13 * | 107.81 ± 5.11 |
| IL-12(p70) | 446.91 ± 34.71 | 531.65 ± 26.58 * | 468.48 ± 18.29 |
| IL-13 | 23,491.19 ± 2188.84 | 26,769.18 ± 1134.10 | 26,409.85 ± 939.14 |
| IL-17 | 1787.37 ± 151.17 | 1921.06 ± 84.15 | 1990.46 ± 59.05 |
| Eotaxin | 7062.60 ± 160.99 | 7494.43 ± 295.88 | 7187.90 ± 159.94 |
| G-CSF | 80.60 ± 9.07 | 105.89 ± 7.23 * | 100.30 ± 4.43 |
| GM-CSF | 725.62 ± 14.80 | 788.56 ± 14.17 ** | 771.08 ± 11.42 |
| KC | 250.23 ± 24.36 | 304.47 ± 14.22 * | 306.95 ± 13.88 |
| MCP-1 | 1448.53 ± 112.57 | 1592.19 ± 64.50 | 1660.90 ± 65.06 |
| MIP-1α | 262.39 ± 19.93 | 341.37 ± 17.93 ** | 311.37 ± 13.78 # |
| MIP-1β | 361.47 ± 25.75 | 428.31 ± 18.00 * | 388.70 ± 10.01 |
| RANTES | 227.98 ± 22.43 | 261.88 ± 11.39 | 242.62 ± 11.30 |
| TNF-α | 4535.07 ± 360.77 | 5566.64 ± 313.77 * | 4606.82 ± 209.94 # |
| IFN-γ | 339.27 ± 30.05 | 431.28 ± 20.21 * | 390.81 ± 18.97 |
| Extracts | Models | Underlying Mechanisms | References | |
|---|---|---|---|---|
| In Vivo | In Vitro | |||
| Ganoderic acid A (GAA) | D-galactose mice | —— | Regulating the imbalance of the Th17/Tregs axis | Zhang Y et al., 2021 [35] |
| GAA | —— | LPS-stimulated BV-2 | Activating farnesoid X receptor (FXR) | Jia Y et al., 2021 [37] |
| GAA | Multiple sclerosis animal | —— | Activating farnesoid X receptor (FXR) | Jia Y et al., 2021 [38] |
| GAA | Post-stroke depression | —— | Regulating M1/M2 microglial polarization by activating the ERK/CREB pathway | Zhang L et al., 2021 [39] |
| Ganoderterpene A | —— | LPS-stimulated BV-2 | Suppressing the activation of MAPK and TLR-4/NF-κB signaling pathways | Kou RW et al., 2021 [40] |
| Ganoderma lucidum polysaccharides (GLPs) | —— | LPS- and Aβ42-stimulated BV-2 and primary mouse microglia | Modulate microglial phagocytosis and behavioral response | Cai Q et al., 2017 [34] |
| GLPs | D-galactose rats | —— | Regulating inflammation of the brain–liver axis | Zhang Y et al., 2021 [35] |
| GLPs | Spinal cord ischemia–reperfusion injury | —— | Reducing lipid peroxidation, inflammatory cytokine production | Kahveci R et al., 2021 [36] |
| Ganoderma lucidum triterpenoids (GLTs) | Maternal separation-induced anxiety and depression | —— | Reversing up-regulation of pro-inflammatory markers in the periphery and brain, and activating microglia in the prefrontal cortex and hippocampus | Mi X et al., 2022 [7] |
| The aqueous extract of GL | Kainic acid-induced seizures | —— | Decreasing immunoreactivity for GFAP as well as TNF-alpha and IL-1beta in the CA3 region | Aguirre Moreno AC et al., 2022 [42] |
| GL extracts | —— | LPS- and MPP(+)-treated MES23.5 cell | Preventing the production of microglia-derived proinflammatory and cytotoxic factors | Ding H et al., 2010 [11] |
| GL extracts | —— | LPS and MPP(+)-treated co-cultures of microglia and MES 23.5 Cells | Preventing the production of proinflammatory factors | Zhang R et al., 2011 [10] |
| Deacetyl ganoderic acid F | LPS-stimulated Zebrafish and mice | LPS-stimulated BV-2 | Suppression of NO production and pro-inflammatory cytokine secretion, modulation of the NF-κB pathway | Sheng F et al., 2019 [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Ding, H.; Zhou, M.; Chan, P. Ganoderma lucidum Modulates Inflammatory Responses following 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Administration in Mice. Nutrients 2022, 14, 3872. https://doi.org/10.3390/nu14183872
Ren Z, Ding H, Zhou M, Chan P. Ganoderma lucidum Modulates Inflammatory Responses following 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Administration in Mice. Nutrients. 2022; 14(18):3872. https://doi.org/10.3390/nu14183872
Chicago/Turabian StyleRen, Zhili, Hui Ding, Ming Zhou, and Piu Chan. 2022. "Ganoderma lucidum Modulates Inflammatory Responses following 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Administration in Mice" Nutrients 14, no. 18: 3872. https://doi.org/10.3390/nu14183872
APA StyleRen, Z., Ding, H., Zhou, M., & Chan, P. (2022). Ganoderma lucidum Modulates Inflammatory Responses following 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Administration in Mice. Nutrients, 14(18), 3872. https://doi.org/10.3390/nu14183872