Could the Majority of the Greek and Cypriot Population Be Vitamin D Deficient?
Abstract
:1. Introduction
2. Material and Methods
2.1. Design of the Study
2.2. Vitamin D Laboratory Assays
2.3. Statistical Analysis
3. Results
3.1. Descriptive Characteristics
3.1.1. Greek Database
3.1.2. Cypriot Database
3.2. Associations between Variables and Vitamin D Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamshchikov, A.V.; Nirali, D.S.; Blumberg, H.M.; Ziegler, T.R.; Tangpricha, V. Vitamin D for the treatment of infectious diseases: A systematic review. Endocr. Pract. 2009, 15, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- Ramnemark, A.; Norberg, M.; Pettersson-Kymmer, U.; Eliasson, M. Adequate vitamin D levels in a Swedish population living above latitude 63°N: The 2009 Northern Sweden MONICA study. Int. J. Circumpolar Health 2015, 74, 27963. [Google Scholar] [CrossRef] [PubMed]
- Ardesia, M.; Ferlazzo, G.; Fries, W. Vitamin D and Inflammatory Bowel Disease. BioMed Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Nicolaidou, P.; Kakourou, T.; Papadopoulou, A.; Kavadias, G.; Dimitriou, E.; Georgouli, H.; Tsapra, H.; Giannoulia-Karantana, A.; Fretzayas, A.; Tsiftis, G.; et al. Low vitamin D status in preschool children in Greece. Nutr. Res. 2006, 26, 620–625. [Google Scholar] [CrossRef]
- Medeiros, J.F.P.; Borges, M.V.D.O.; Soares, A.A.; dos Santos, J.C.; de Oliveira, A.B.B.; da Costa, C.H.B.; Cruz, M.S.; Bortolin, R.H.; de Freitas, R.C.C.; Dantas, P.M.S.; et al. The impact of vitamin D supplementation on VDR gene expression and body composition in monozygotic twins: Randomized controlled trial. Sci. Rep. 2020, 10, 11943. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of Vitamin D Status and Vitamin D3 Supplementation on Genome Wide Expression of White Blood Cells: A Randomised Double-Blind Clinical Trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef]
- Pinnawala, N.U.; Thrastardottir, T.O.; Constantinou, C. Keeping a Balance During the Pandemic: A Narrative Review on the Important Role of Micronutrients in Preventing Infection and Reducing Complications of COVID-19. Curr. Nutr. Rep. 2021, 10, 200–210. [Google Scholar] [CrossRef]
- Maruotti, N.; Cantatore, F.P. Vitamin D and the immune system. J. Rheumatol. 2010, 37, 491–495. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Rosella, G.; Wu, Y.; Lubel, J.S.; Gibson, P.R. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin. Nutr. 2017, 25, 6–13. [Google Scholar] [CrossRef]
- Barbáchano, A.; Fernández-Barral, A.; Ferrer-Mayorga, G.; Costales-Carrera, A.; Larriba, M.J.; Muñoz, A. The endocrine vitamin D system in the gut. Mol. Cell Endocrinol. 2017, 453, 79–87. [Google Scholar] [CrossRef]
- Israel, A.; Cicurel, A.; Feldhamer, I.; Dror, Y.; Giveon, S.M.; Gillis, D.; Strich, D.; Lavie, G. The link between vitamin D deficiency and COVID-19 in a large population. medRxiv 2020. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Van De Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021, 97, 442–447. [Google Scholar] [CrossRef]
- Watad, A.; Azrielant, S.; Bragazzi, N.L.; Sharif, K.; David, P.; Katz, I.; Aljadeff, G.; Quaresma, M.; Tanay, G.; Adawi, M.; et al. Seasonality and autoimmune diseases: The contribution of the four seasons to the mosaic of autoimmunity. J. Autoimmun. 2017, 82, 13–30. [Google Scholar] [CrossRef]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G. Vitamin D Intake Is Inversely Associated with Rheumatoid Arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef]
- Borges, M.C.; Martini, L.A.; Rogero, M.M. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition 2011, 27, 399–404. [Google Scholar] [CrossRef]
- Kolokotroni, O.; Papadopoulou, A.; Middleton, N.; Kouta, C.; Raftopoulos, V. Vitamin D levels and status amongst asthmatic and non-asthmatic adolescents in Cyprus: A comparative cross-sectional study. BMC Public Health 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Lambrinou, C.-P.; Tsoutsoulopoulou, K.; Binou, P.; Karachaliou, A.; Breidenassel, C.; Gonzalez-Gross, M.; Kiely, M.; Cashman, K.D. A Systematic Review of Vitamin D Status in Southern European Countries; Springer: Berlin/Heidelberg, Germany, 2018; Volume 57. [Google Scholar] [CrossRef]
- Olmos, J.M.; Hernández, J.L.; García-Velasco, P.; Martínez, J.; Llorca, J.; González-Macías, J. Serum 25-hydroxyvitamin D, parathyroid hormone, calcium intake, and bone mineral density in Spanish adults. Osteoporos. Int. 2016, 27, 105–113. [Google Scholar] [CrossRef]
- Lippi, G.; Nouvenne, A.; Ticinesi, A.; Bonelli, P.; Salvagno, G.L.; Cervellin, G.; Guidi, G.C. The burden of vitamin D deficiency in a mediterranean country without a policy of food fortification. Acta Biomed. 2015, 86, 59–62. [Google Scholar]
- Karras, S.; A Paschou, S.; Kandaraki, E.; Anagnostis, P.; Annweiler, C.; Tarlatzis, B.C.; Hollis, B.W.; Grant, W.B.; Goulis, D. Hypovitaminosis D in pregnancy in the Mediterranean region: A systematic review. Eur. J. Clin. Nutr. 2016, 70, 979–986. [Google Scholar] [CrossRef]
- Ritu, G.; Gupta, A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients 2014, 6, 729–775. [Google Scholar] [CrossRef]
- Rovner, A.J.; O’Brien, K.O. Hypovitaminosis D among Healthy Children in the United States: A Review of the Current Evidence. Arch. Pediatr. Adolesc. Med. 2008, 162, 513–519. [Google Scholar] [CrossRef]
- Singhellakis, P.N.; Malandrinou, F.C.; Psarrou, C.J.; Danelli, A.M.; Tsalavoutas, S.D.; Constandellou, E.S. Vitamin D deficiency in white, apparently healthy, free-living adults in a temperate region. Hormones 2011, 10, 131–143. [Google Scholar] [CrossRef]
- Kolokotroni, O.; Papadopoulou, A.; Yiallouros, P.K.; Raftopoulos, V.; Kouta, C.; Lamnisos, D.; Nicolaidou, P.; Middleton, N. Association of vitamin D with adiposity measures and other determinants in a cross-sectional study of Cypriot adolescents. Public Health Nutr. 2015, 18, 112–121. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Hulshof, T.; Bourhis, A.-S.; Hull, G.L.J.; Dowling, K.G.; Kiely, M.E.; Cashman, K.D. Prevalence of vitamin D deficiency and insufficiency among schoolchildren in Greece: The role of sex, degree of urbanisation and seasonality. Br. J. Nutr. 2017, 118, 550–558. [Google Scholar] [CrossRef]
- Karras, S.N.; Anagnostis, P.; Annweiler, C.; Naughton, D.P.; Petroczi, A.; Bili, E.; Harizopoulou, V.; Tarlatzis, B.C.; Persinaki, A.; Papadopoulou, F.; et al. Maternal vitamin D status during pregnancy: The Mediterranean reality. Eur. J. Clin. Nutr. 2014, 68, 864–869. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Kouis, P.; Middleton, N.; Kolokotroni, O.; Karpathios, T.; Nicolaidou, P.; Yiallouros, P.K. Association of vitamin D receptor gene polymorphisms and vitamin D levels with asthma and atopy in Cypriot adolescents: A case-control study. Multidiscip. Respir. Med. 2015, 10, 26. [Google Scholar] [CrossRef]
- Divanoglou, N.; Komninou, D.; Stea, E.A.; Argiriou, A.; Papatzikas, G.; Tsakalof, A.; Pazaitou-Panayiotou, K.; Georgakis, M.K.; Petridou, E. Association of Vitamin D Receptor Gene Polymorphisms with Serum Vitamin D Levels in a Greek Rural Population (Velestino Study). Lifestyle Genom. 2021, 14, 81–90. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Nolan, V.G.; Nottage, K.A.; Cole, E.W.; Hankins, J.S.; Gurney, J.G. Prevalence of Vitamin D deficiency in sickle cell disease: A systematic review. PLoS ONE 2015, 10, e0119908. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Fakhoury, H.; Karras, S.N.; Al Anouti, F.; Bhattoa, H.P. Variations in 25-Hydroxyvitamin D in Countries from the Middle East and Europe: The Roles of UVB Exposure and Diet. Nutrients 2019, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
- Hwalla, N.; Al Dhaheri, A.S.; Radwan, H.; Alfawaz, H.A.; Fouda, M.A.; Al-Daghri, N.M.; Zaghloul, S.; Blumberg, J.B. The prevalence of micronutrient deficiencies and inadequacies in the middle east and approaches to interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; Munger, K.L.; Köchert, K.; Arnason, B.G.W.; Comi, G.; Cook, S.D.; Goodin, D.S.; Filippi, M.; Hartung, H.-P.; Jeffery, D.R.; et al. Association of Vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon Beta-1b. JAMA Neurol. 2015, 72, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.-P.; Miller, D.H.; Montalban, X.; et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef]
- Feketea, G.M.; Bocsan, I.C.; Tsiros, G.; Voila, P.; Stanciu, L.A.; Zdrenghea, M. Vitamin D Status in Children in Greece and Its Relationship with Sunscreen Application. Children 2021, 8, 111. [Google Scholar] [CrossRef]
| Greek Database N = 8780 | Cypriot Database N = 2594 | |||
|---|---|---|---|---|
| (Mean ± SD) | p-Value | (Mean ± SD) | p-Value | |
| Age (years old) | 49.3 (± 20.0) | 39.9 (± 19.4) | ||
| Females, N (%): | 6040(69) | 1668(64) | ||
| Males, N (%): | 2628(30) | 907(35) | ||
| Vitamin D levels (ng/mL) | 25.1 ± 14.4 | 25.8 ± 10.9 | ||
| insufficiency (21–29 ng/mL): | 32.8% | 38.3% | ||
| deficiency (≤20 ng/mL) | 39.9% | 31.0% | ||
| Vitamin D levels in each age group (ng/mL) | ||||
| <12 years old | 26.3 ± 12.4 | 28.4 ± 8.9 | ||
| 12–17 years old | 27.5 ± 14.6 | 25.6 ± 8.9 | ||
| 18–74 years old | 25.6 ± 14.9 | 25.2 ± 10.8 | ||
| ≥75 years old | 23.6 ± 13.8 | 27.8 ± 17.0 | ||
| Vitamin D levels by sex (ng/mL) | ||||
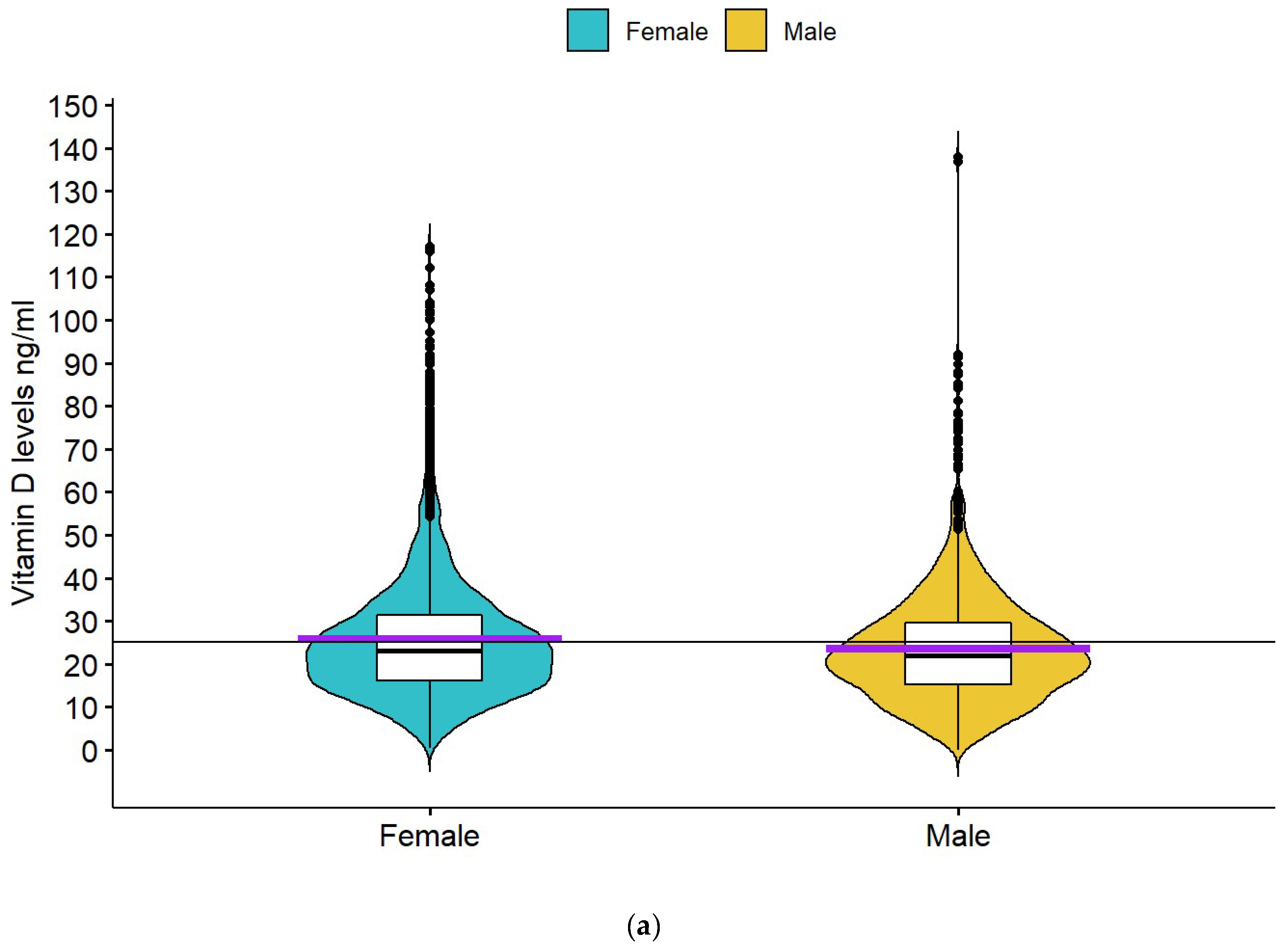
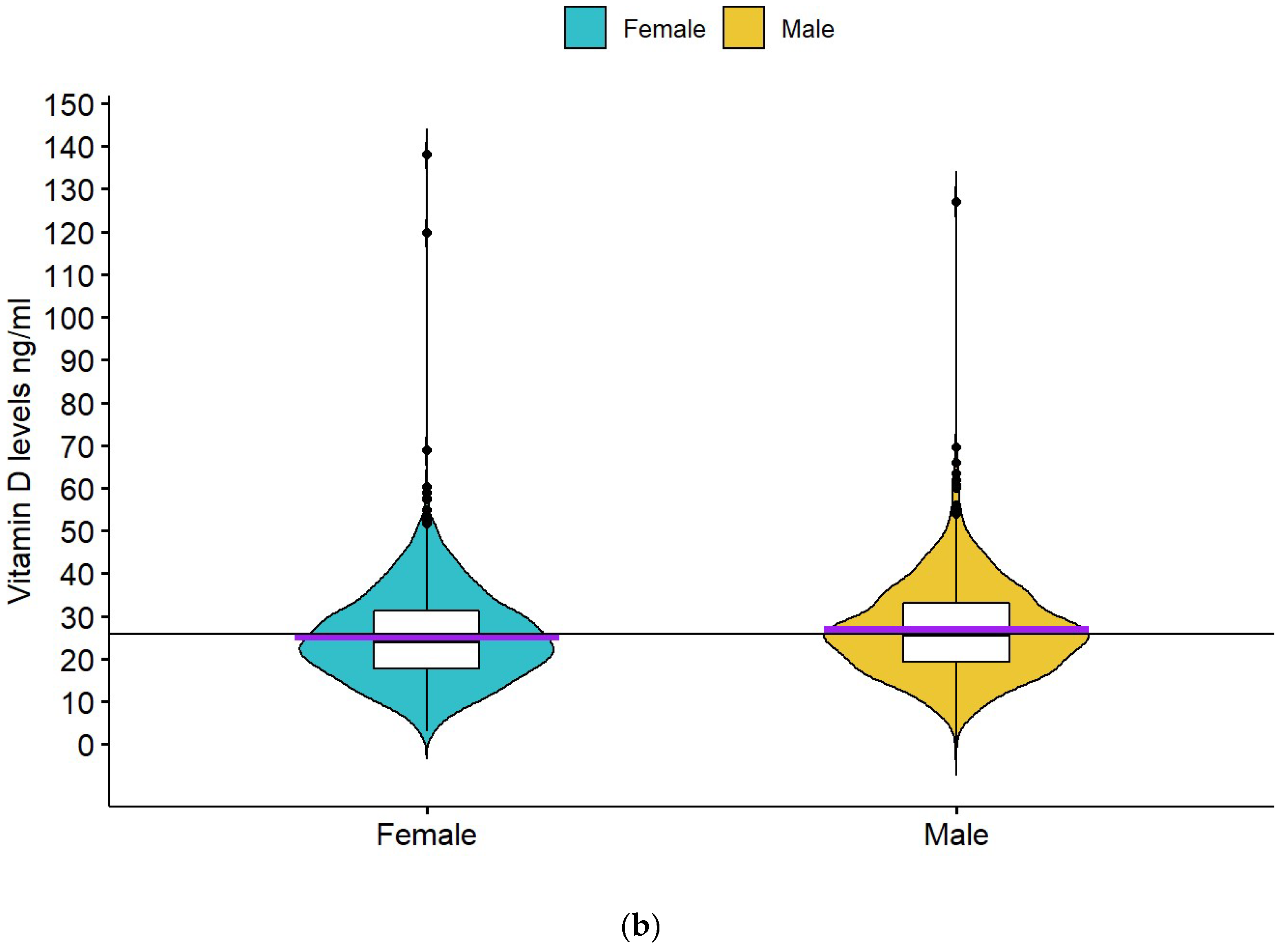
| Females | 25.7 ± 15.0 | p < 0.001 | 25.2 ± 10.8 | p = 0.002 |
| Males | 23.6 ± 13.0 | 26.8 ± 10.9 | ||
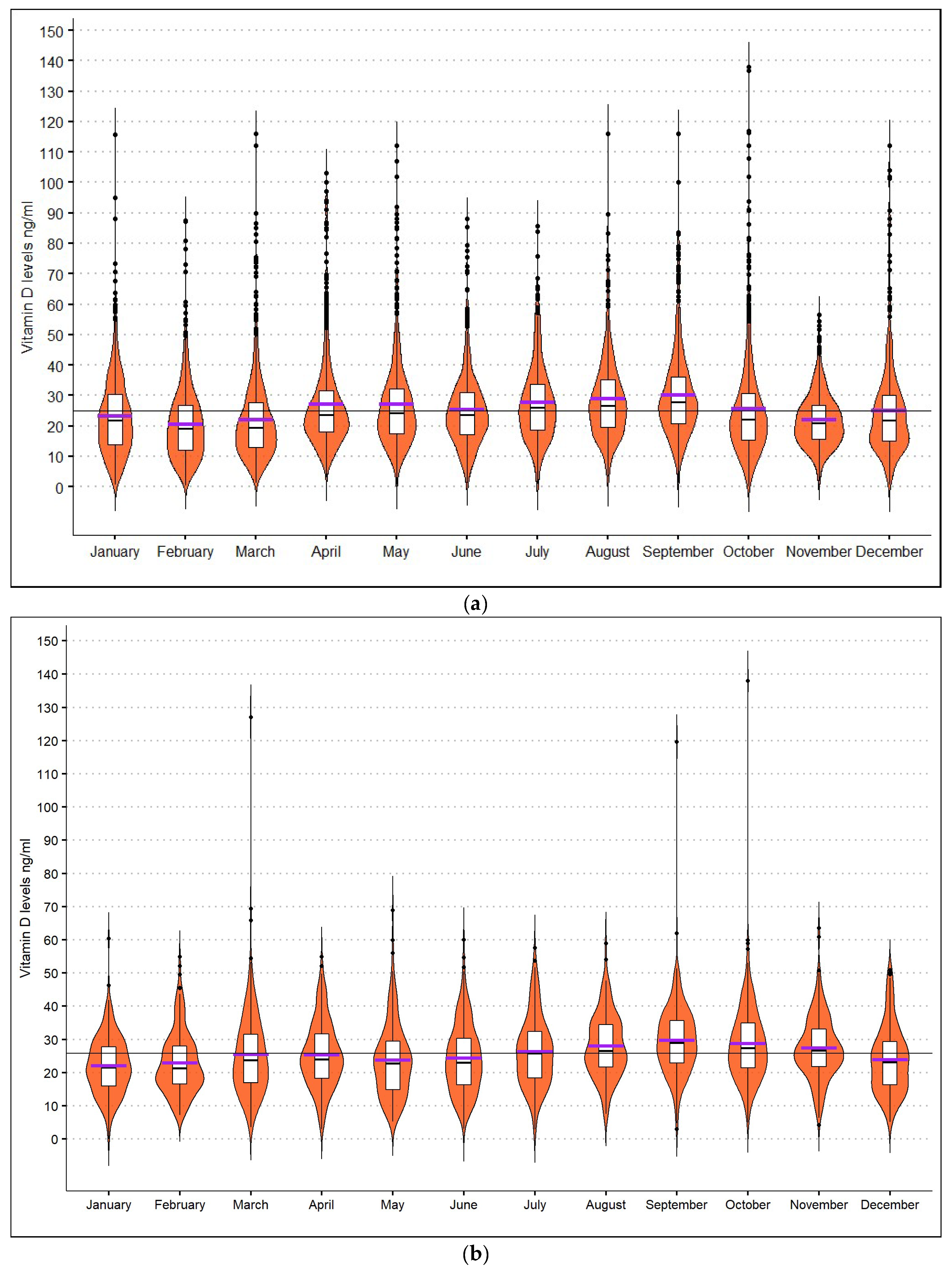
| Month with the highest vitamin D mean levels (ng/mL): | September: 30.4 ± 17.4 | p < 0.001 | September: 29.7 ± 10.8 | p < 0.001 |
| Month with the lowest vitamin D mean levels (ng/mL): | February: 20.6 ± 11.9 | January: 22.1 ± 8.7 | ||
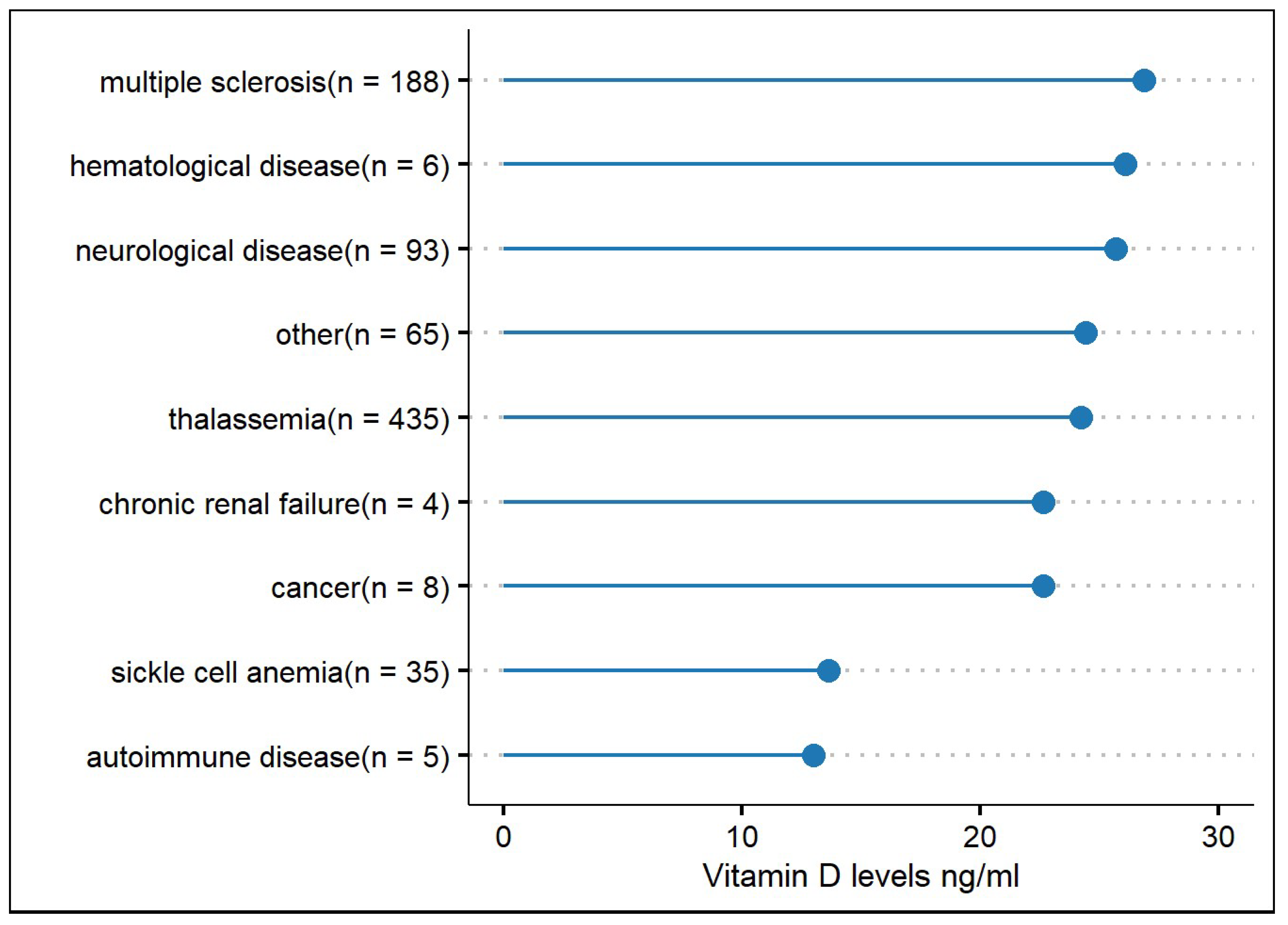
| Levels of vitamin D for most frequently recorded diseases. Greek database only (ng/mL): | ||||
| Thalassemia | 24.2 ± 12.9 | |||
| Multiple sclerosis | 26.9 ± 15.4 | |||
| Other neurological diseases * | 25.7 ± 15.8 | |||
| Sickle cell anaemia | 13.6 ± 10.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xyda, S.E.; Kotsa, K.; Doumas, A.; Papanastasiou, E.; Garyfallos, A.A.; Samoutis, G. Could the Majority of the Greek and Cypriot Population Be Vitamin D Deficient? Nutrients 2022, 14, 3778. https://doi.org/10.3390/nu14183778
Xyda SE, Kotsa K, Doumas A, Papanastasiou E, Garyfallos AA, Samoutis G. Could the Majority of the Greek and Cypriot Population Be Vitamin D Deficient? Nutrients. 2022; 14(18):3778. https://doi.org/10.3390/nu14183778
Chicago/Turabian StyleXyda, Souzana E., Kalliopi Kotsa, Argyrios Doumas, Emmanouil Papanastasiou, Alexandros A. Garyfallos, and George Samoutis. 2022. "Could the Majority of the Greek and Cypriot Population Be Vitamin D Deficient?" Nutrients 14, no. 18: 3778. https://doi.org/10.3390/nu14183778
APA StyleXyda, S. E., Kotsa, K., Doumas, A., Papanastasiou, E., Garyfallos, A. A., & Samoutis, G. (2022). Could the Majority of the Greek and Cypriot Population Be Vitamin D Deficient? Nutrients, 14(18), 3778. https://doi.org/10.3390/nu14183778






