The Lactobacillus gasseri G098 Strain Mitigates Symptoms of DSS-Induced Inflammatory Bowel Disease in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Strain
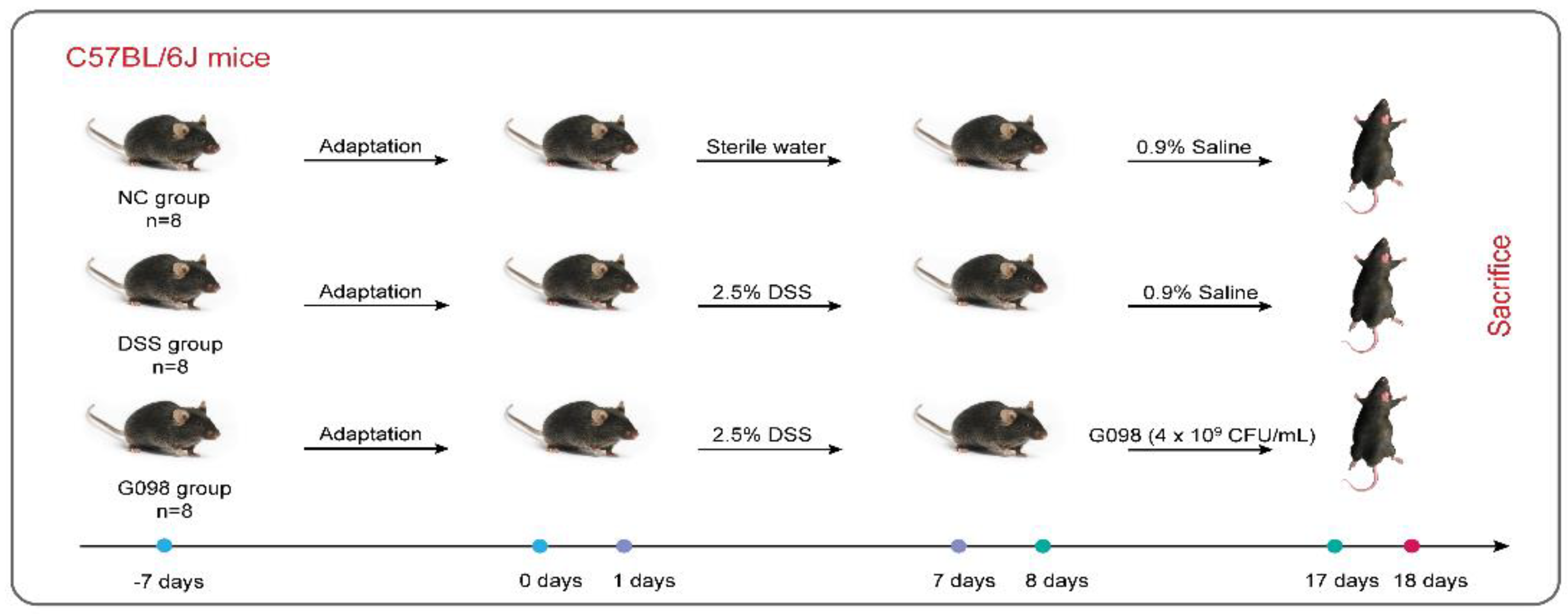
2.2. Experimental Design
2.3. Histological Analysis
2.4. Quantification of Inflammatory Cytokines in the Serum
2.5. Fecal DNA Extraction, Sequencing, and Analysis
2.6. Statistical Analysis
3. Results
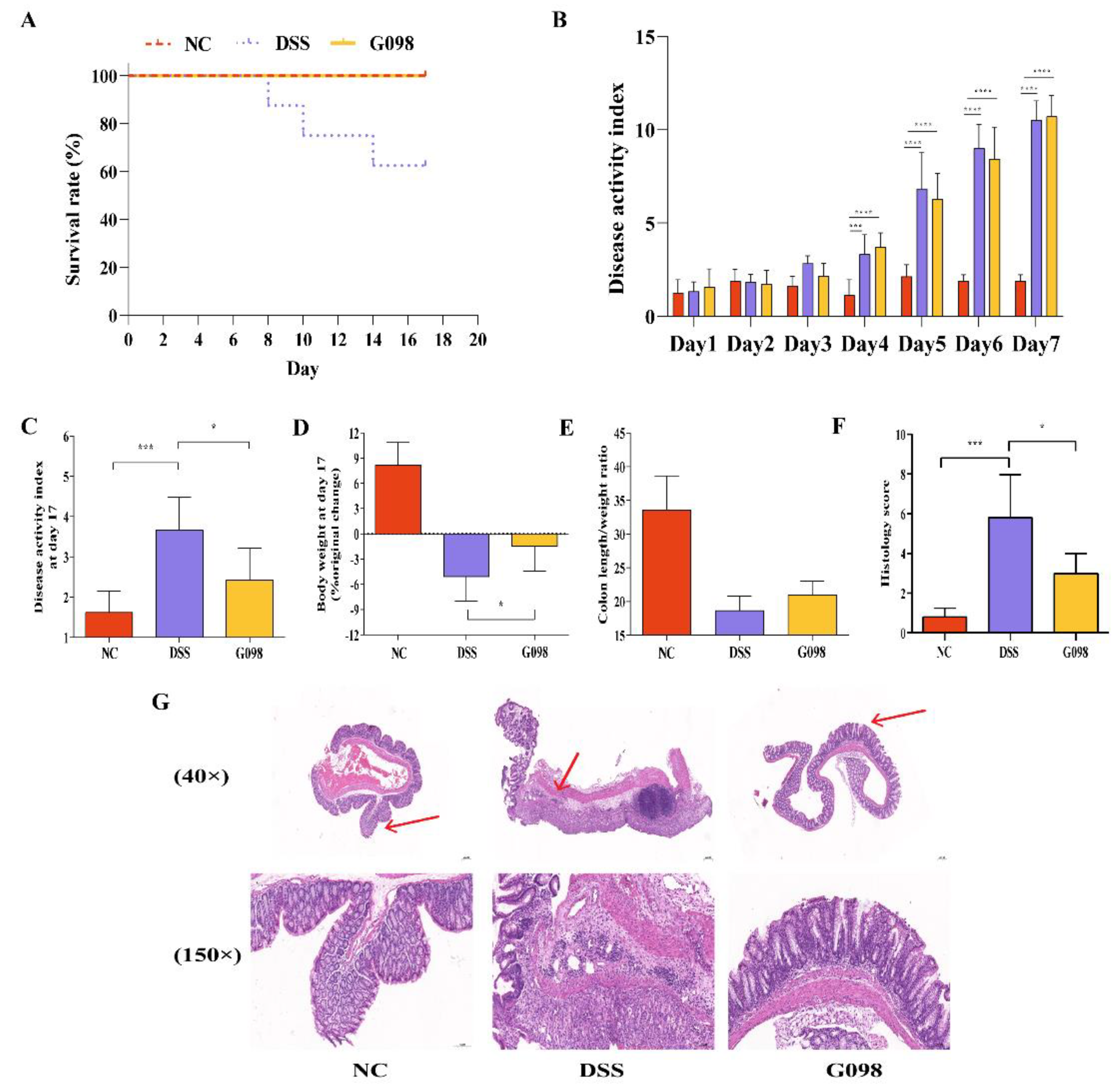
3.1. Lactobacillus Gasseri G098 Alleviated Inflammatory Manifestations in Mice with Colitis
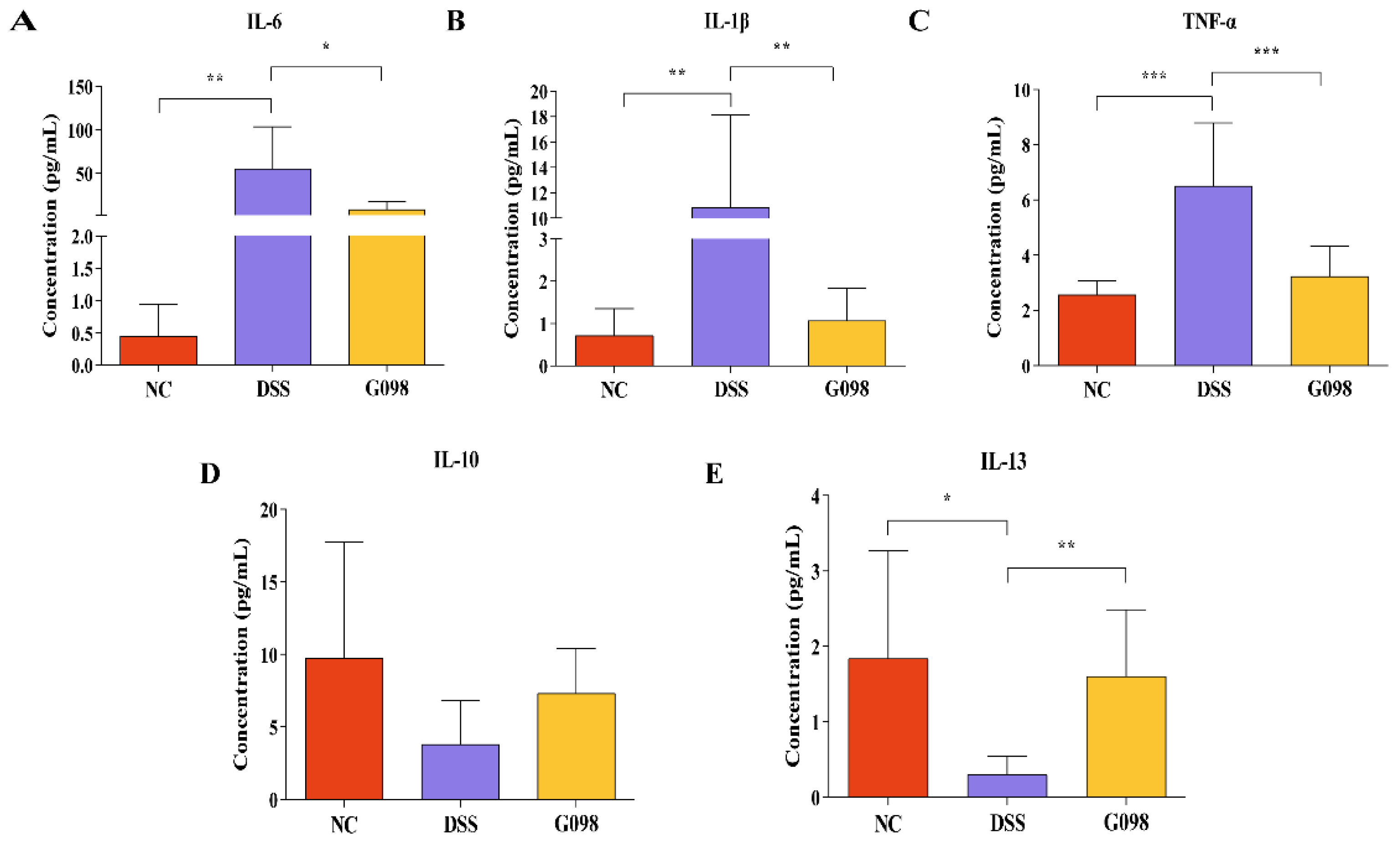
3.2. Lactobacillus Gasseri G098 Intake Reversed DSS-Induced Changes in Serum Pro-/Anti-Inflammatory Cytokine Levels
3.3. Effect of Lactobacillus Gasseri G098 on the Gut Microbiota Composition
3.4. Effect of Lactobacillus Gasseri G098 on the Gut Microbiota Metabolic Pathways
3.5. Correlation between Significant Differential Gut Microbiota with Gut Metabolic Pathways, Serum Cytokines, and DAI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113. [Google Scholar] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.M.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The gut microbiome and diet in psychiatry: Focus on depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2021, 12, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Durant, L.; Hoyles, L.; McCartney, A.L.; Man, R.; Segal, J.; Costello, S.P.; Hendy, P.; Reddi, D.; Bouri, S. Deficient resident memory T cell and CD8 T cell response to commensals in inflammatory bowel disease. J. Crohn’s Colitis 2020, 14, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Van Assche, G.; Vermeire, S. Infliximab therapy for inflammatory bowel disease–seven years on. Aliment. Pharmacol. Ther. 2006, 23, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.D.; Flanagan, M.E.; Telliez, J.-B. Discovery and development of Janus Kinase (JAK) inhibitors for inflammatory diseases: Miniperspective. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- D’haens, G.R.; Panaccione, R.; Higgins, P.D.; Vermeire, S.; Gassull, M.; Chowers, Y.; Hanauer, S.B.; Herfarth, H.; Hommes, D.W.; Kamm, M. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: When to start, when to stop, which drug to choose, and how to predict response? Off. J. Am. Coll. Gastroenterol.|ACG 2011, 106, 199–212. [Google Scholar] [CrossRef]
- Raine, T.; Danese, S. Breaking through the therapeutic ceiling: What will it take? Gastroenterology 2022, 162, 1507–1511. [Google Scholar] [CrossRef]
- Hawkey, C. Hematopoietic stem cell transplantation in Crohn’s disease: State-of-the-art treatment. Dig. Dis. 2017, 35, 107–114. [Google Scholar] [CrossRef]
- Derwa, Y.; Gracie, D.; Hamlin, P.; Ford, A. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.K.; Han, D.H.; Jang, Y.J.; Park, S.; Jang, S.J.; Lee, G.; Han, H.S.; Ko, G. Alleviation of DSS-induced colitis via Lactobacillus acidophilus treatment in mice. Food Funct. 2021, 12, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, W.K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zuo, Z.-X.; Mao, A.-P. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: Meta-analysis of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Lauer, E.; Kandler, O. Lactobacillus gasseri sp. nov., a new species of the subgenus Thermobacterium. Zent. Bakteriol. I. Abt. Orig. C Allg. Angew. Ökologische Mikrobiol. 1980, 1, 75–78. [Google Scholar] [CrossRef]
- Olivares, M.; Díaz-Ropero, M.P.; Gómez, N.; Lara-Villoslada, F.; Sierra, S.; Maldonado, J.A.; Martín, R.; López-Huertas, E.; Rodríguez, J.M.; Xaus, J. Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int. J. Food Microbiol. 2006, 107, 104–111. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Jan, R.-L.; Wu, L.S.-H.; Chen, P.-C.; Kao, H.-F.; Kuo, W.-S.; Wang, J.-Y. Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells. J. Mol. Med. 2018, 96, 39–51. [Google Scholar] [CrossRef]
- Mallick, H.; Franzosa, E.A.; Mclver, L.J.; Banerjee, S.; Sirota-Madi, A.; Kostic, A.D.; Clish, C.B.; Vlamakis, H.; Xavier, R.J.; Huttenhower, C. Predictive metabolomic profiling of microbial communities using amplicon or metagenomic sequences. Nat. Commun. 2019, 10, 3136. [Google Scholar] [CrossRef]
- Kumar, J.; Kumar, M.; Gupta, S.; Ahmed, V.; Bhambi, M.; Pandey, R.; Chauhan, N.S. An improved methodology to overcome key issues in human fecal metagenomic DNA extraction. Genom. Proteom. Bioinform. 2016, 14, 371–378. [Google Scholar] [CrossRef]
- Solomon, L.; Mansor, S.; Mallon, P.; Donnelly, E.; Hoper, M.; Loughrey, M.; Kirk, S.; Gardiner, K. The dextran sulphate sodium (DSS) model of colitis: An overview. Comp. Clin. Pathol. 2010, 19, 235–239. [Google Scholar] [CrossRef]
- Münch, A.; Langner, C. Microscopic colitis: Clinical and pathologic perspectives. Clin. Gastroenterol. Hepatol. 2015, 13, 228–236. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011, 22, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kitani, A.; Strober, W.; Fuss, I.J. The role of NLRP3 and IL-1β in the pathogenesis of inflammatory bowel disease. Front. Immunol. 2018, 9, 2566. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Toyonaga, A.; Sasaki, E.; Ishida, O.; Ikeda, H.; Tsuruta, O.; Harada, K.; Tateishi, H.; Nishiyama, T.; Tanikawa, K. Soluble interleukin-6 receptors in inflammatory bowel disease: Relation to circulating interleukin-6. Gut 1995, 36, 45–49. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.; Felice, C.; Papa, A.; Gasbarrini, A.; Rapaccini, G.L.; Guidi, L.; Armuzzi, A. Anti TNF-α therapy for ulcerative colitis: Current status and prospects for the future. Expert Rev. Clin. Immunol. 2017, 13, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Casteele, N.V.; Khanna, R.; Levesque, B.G.; Stitt, L.; Zou, G.; Singh, S.; Lockton, S.; Hauenstein, S.; Ohrmund, L.; Greenberg, G.R. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015, 64, 1539–1545. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Caviglia, G.P.; Abdulle, A.; Pellicano, R.; Ditto, M.C.; Morino, M.; Fusaro, E.; Saracco, G.M.; Bugianesi, E.; Astegiano, M. Adalimumab therapy improves intestinal dysbiosis in Crohn’s disease. J. Clin. Med. 2019, 8, 1646. [Google Scholar] [CrossRef]
- Kunz, C.; Kuntz, S.; Rudloff, S. Intestinal Flora. In Breast-Feeding: Early Influences on Later Health; Springer: Dordrecht, The Netherlands, 2009; pp. 67–79. [Google Scholar]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef] [Green Version]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shao, D.; Shi, J. Lactobacillus rhamnosus from human breast milk ameliorates ulcerative colitis in mice via gut microbiota modulation. Food Funct. 2021, 12, 5171–5186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-E.; Jeong, J.-J.; Kim, J.-K.; Han, M.J.; Kim, D.-H. Simultaneous amelioratation of colitis and liver injury in mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Sci. Rep. 2018, 8, 7500. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, Y.; Song, Y.; Gao, Y.; Zhao, F.; Luo, Y.; Qian, F.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020, 11, 5205–5222. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining gut microbial Dysbiosis: A review of applied indexes for assessment of intestinal microbiota imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Wang, Y.; Wang, D.; Ke, Y.; Zeng, X. Propionate and Butyrate Produced by Gut Microbiota after Probiotic Supplementation Attenuate Lung Metastasis of Melanoma Cells in Mice. Mol. Nutr. Food Res. 2021, 65, 2100096. [Google Scholar] [CrossRef] [PubMed]
- Serino, M. SCFAs—the thin microbial metabolic line between good and bad. Nat. Rev. Endocrinol. 2019, 15, 318–319. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am. J. Physiol. -Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef]
- Cabrera, G.; Pérez, R.; Gomez, J.; Abalos, A.; Cantero, D. Toxic effects of dissolved heavy metals on Desulfovibrio vulgaris and Desulfovibrio sp. strains. J. Hazard. Mater. 2006, 135, 40–46. [Google Scholar] [CrossRef]
- Zicola, E.; Arrigo, E.; Mancardi, D. H2S Pretreatment Is Promigratory and Decreases Ischemia/Reperfusion Injury in Human Microvascular Endothelial Cells. Oxid. Med. Cell. Longev. 2021, 2021, 8886666. [Google Scholar] [CrossRef] [PubMed]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Gouya, M.M.; Abbasi, M.; Delavari, A.; Alikhani, S.; Alaedini, F.; Safaie, A.; Forouzanfar, M.; Gregg, E.W. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care 2008, 31, 96–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-Q.; Quan, K.-Y.; Feng, C.-J.; Zhang, T.; He, Q.-W.; Kwok, L.-Y.; Chen, Y.-F. The Lactobacillus gasseri G098 Strain Mitigates Symptoms of DSS-Induced Inflammatory Bowel Disease in Mice. Nutrients 2022, 14, 3745. https://doi.org/10.3390/nu14183745
Zhang W-Q, Quan K-Y, Feng C-J, Zhang T, He Q-W, Kwok L-Y, Chen Y-F. The Lactobacillus gasseri G098 Strain Mitigates Symptoms of DSS-Induced Inflammatory Bowel Disease in Mice. Nutrients. 2022; 14(18):3745. https://doi.org/10.3390/nu14183745
Chicago/Turabian StyleZhang, Wei-Qin, Ke-Yu Quan, Cui-Jiao Feng, Tao Zhang, Qiu-Wen He, Lai-Yu Kwok, and Yong-Fu Chen. 2022. "The Lactobacillus gasseri G098 Strain Mitigates Symptoms of DSS-Induced Inflammatory Bowel Disease in Mice" Nutrients 14, no. 18: 3745. https://doi.org/10.3390/nu14183745
APA StyleZhang, W.-Q., Quan, K.-Y., Feng, C.-J., Zhang, T., He, Q.-W., Kwok, L.-Y., & Chen, Y.-F. (2022). The Lactobacillus gasseri G098 Strain Mitigates Symptoms of DSS-Induced Inflammatory Bowel Disease in Mice. Nutrients, 14(18), 3745. https://doi.org/10.3390/nu14183745





