Can Artificial Sweeteners Increase the Risk of Cancer Incidence and Mortality: Evidence from Prospective Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources and Methods of Data Retrieval
2.2. Inclusion Criteria
2.3. Data Extraction and Risk of Bias within Individual Studies
2.4. Statistical Analysis
3. Results
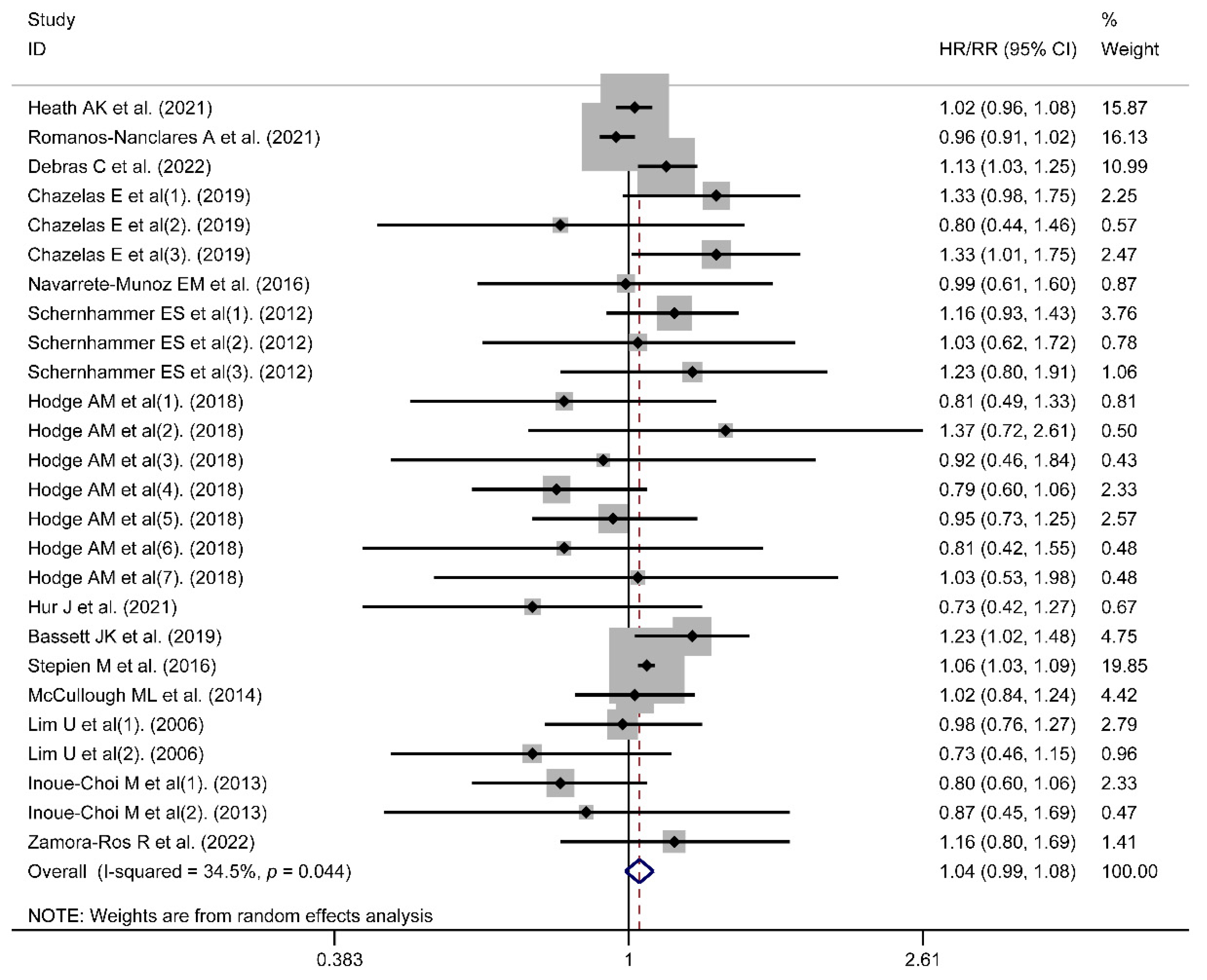
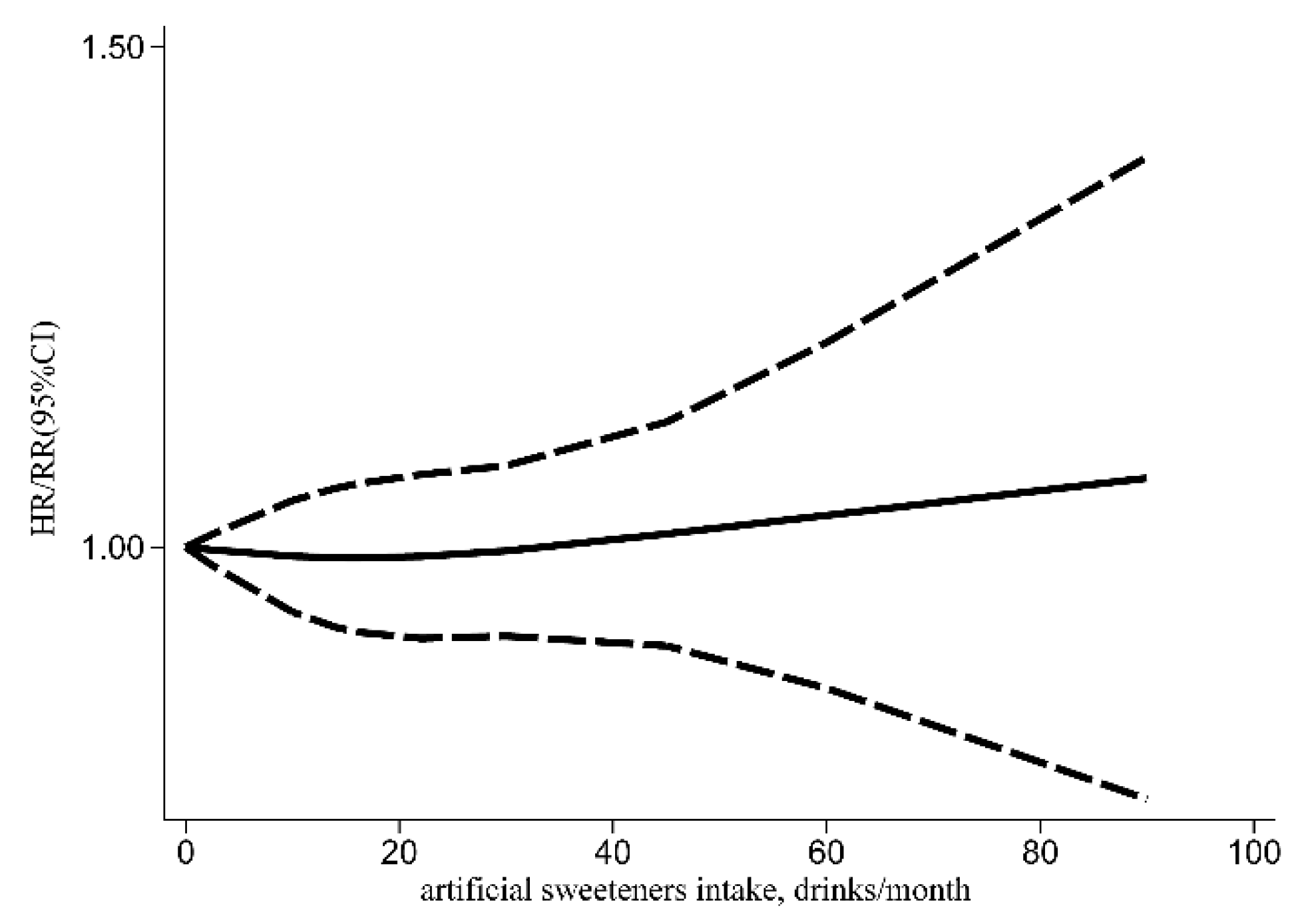
3.1. Risk of Cancer Incidence
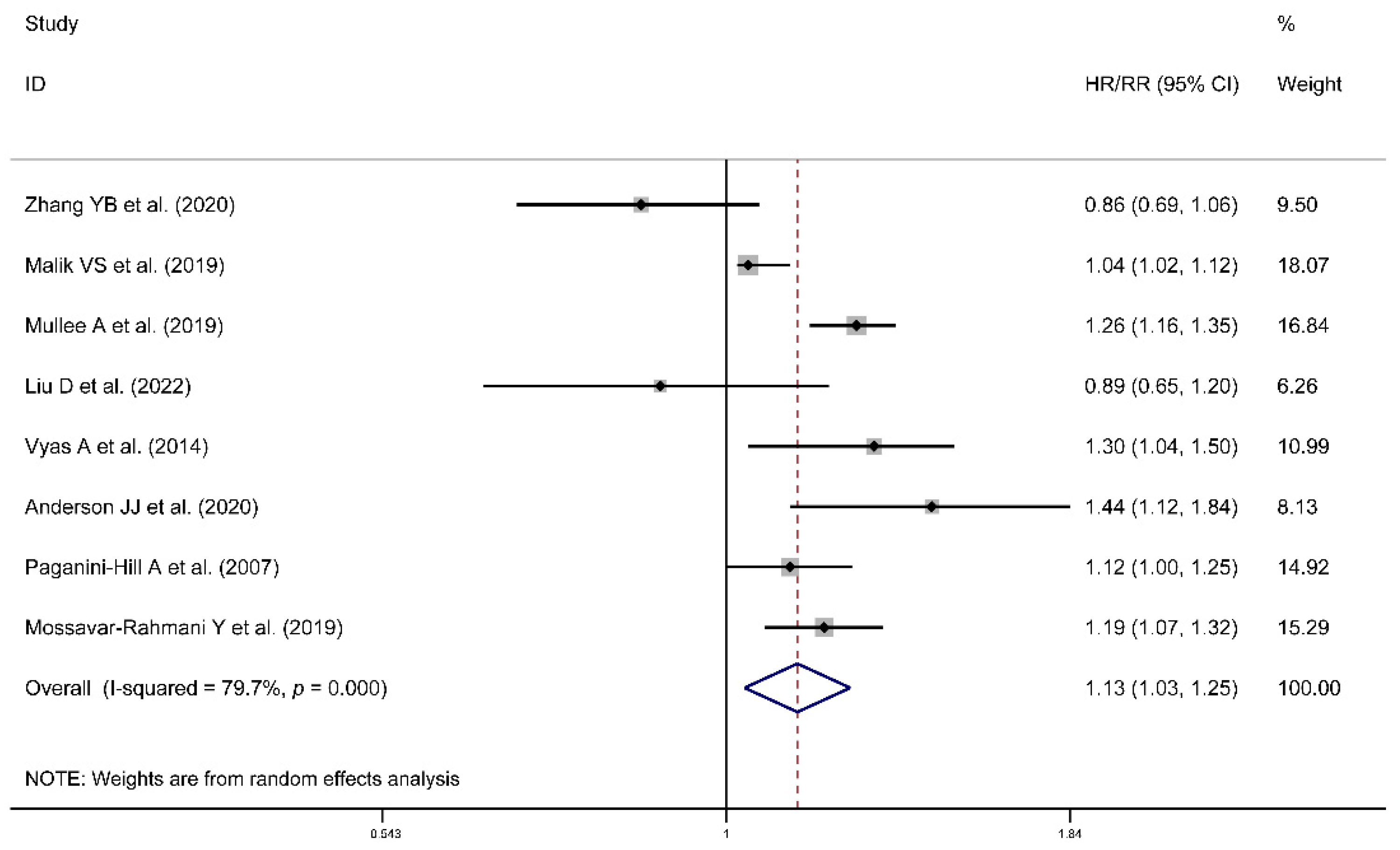
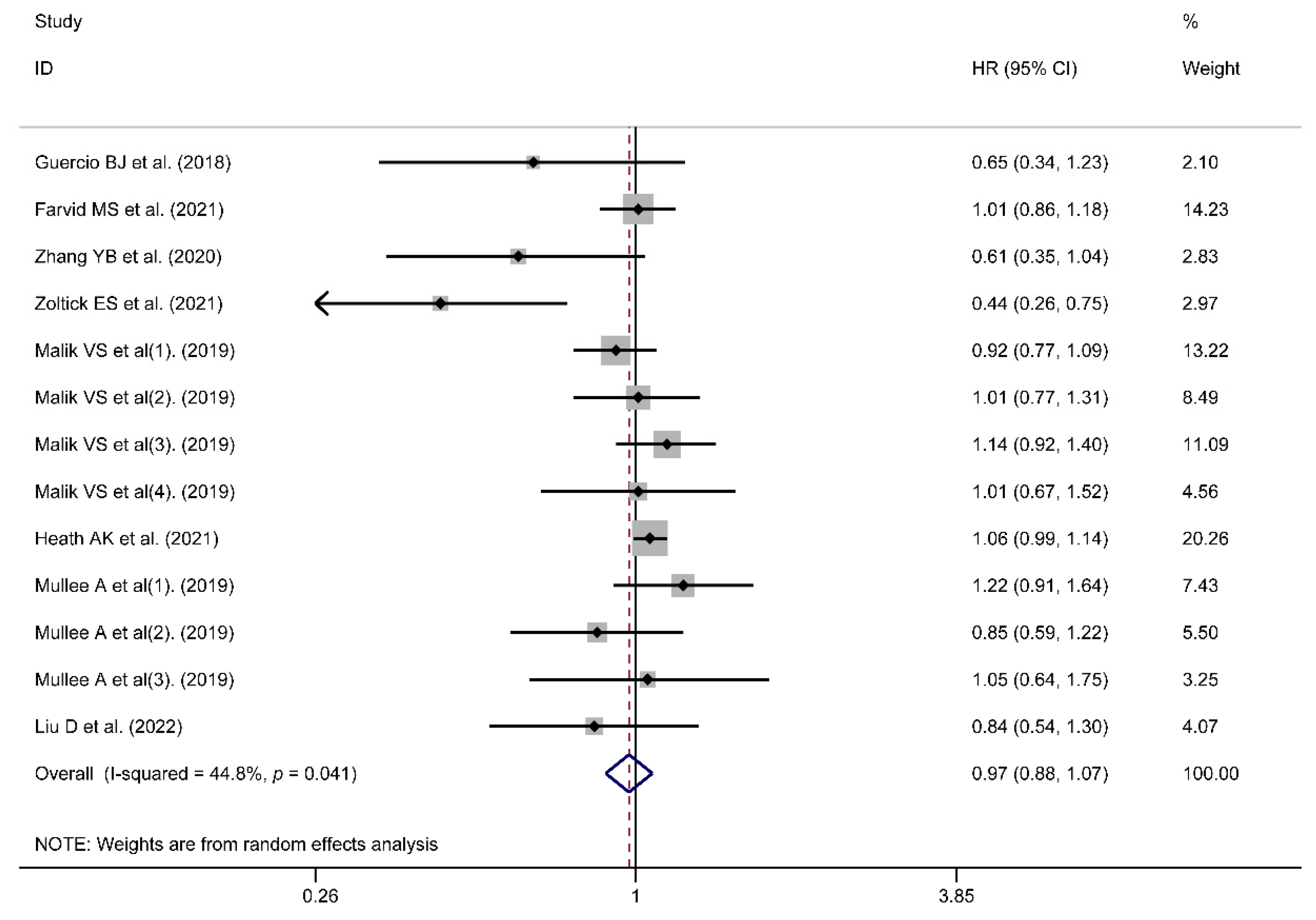
3.2. Risk of All-Cause or Cancer Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Moorman, P.G.; Alberg, A.J.; Barnholtz-Sloan, J.S.; Bondy, M.; Cote, M.L.; Funkhouser, E.; Peters, E.S.; Schwartz, A.G.; Terry, P.; et al. Dietary carbohydrate intake, glycaemic load, glycaemic index and ovarian cancer risk in African-American women. Br. J. Nutr. 2016, 115, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Ferrari, P.; Rinaldi, S.; Slimani, N.; Jenab, M.; Olsen, A.; Tjonneland, A.; Overvad, K.; Boutron-Ruault, M.C.; Lajous, M.; et al. Dietary glycemic index and glycemic load and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2012, 96, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Sieri, S.; Krogh, V.; Agnoli, C.; Ricceri, F.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; Giurdanella, M.C.; et al. Dietary glycemic index and glycemic load and risk of colorectal cancer: Results from the EPIC-Italy study. Int. J. Cancer 2015, 136, 2923–2931. [Google Scholar] [CrossRef]
- Weihrauch, M.R.; Diehl, V. Artificial sweeteners—Do they bear a carcinogenic risk? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2004, 15, 1460–1465. [Google Scholar] [CrossRef]
- Muenprasitivej, N.; Tao, R.; Nardone, S.J.; Cho, S. The Effect of Steviol Glycosides on Sensory Properties and Acceptability of Ice Cream. Foods 2022, 11, 1745. [Google Scholar] [CrossRef]
- Chadha, D.; Hamid, N.; Kantono, K.; Marsan, M. Changes in temporal sensory profile, liking, satiety, and postconsumption attributes of yogurt with natural sweeteners. J. Food Sci. 2022, 87, 3190–3206. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. TEM 2013, 24, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, L.A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chang, J.; Checklin, H.L.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br. J. Nutr. 2010, 104, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Frey, F.; Töpfer, A.; Drewe, J.; Beglinger, C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br. J. Nutr. 2011, 105, 1320–1328. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016, 164 Pt B, 446–450. [Google Scholar] [CrossRef]
- Johnson, R.K.; Lichtenstein, A.H.; Anderson, C.A.M.; Carson, J.A.; Després, J.P.; Hu, F.B.; Kris-Etherton, P.M.; Otten, J.J.; Towfighi, A.; Wylie-Rosett, J. Low-Calorie Sweetened Beverages and Cardiometabolic Health: A Science Advisory From the American Heart Association. Circulation 2018, 138, e126–e140. [Google Scholar] [CrossRef]
- Pearlman, M.; Obert, J.; Casey, L. The Association Between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ge, L.; Lai, H.; Wang, Q.; Wang, Q.; Zhang, Q.; Yin, M.; Li, S.; Tian, J.; Yang, K.; et al. Association of soft drink and 100% fruit juice consumption with all-cause mortality, cardiovascular diseases mortality, and cancer mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tepler, A.; Hoffman, G.; Jindal, S.; Narula, N.; Shah, S.C. Intake of artificial sweeteners among adults is associated with reduced odds of gastrointestinal luminal cancers: A meta-analysis of cohort and case-control studies. Nutr. Res. 2021, 93, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 August 2022).
- Egger, M. Bias in meta-analysis detected by a simple, graphical test. BMJ Br. Med. J. 1997, 315, 629. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Andrea, R.N.; Collins, L.C.; Hu, F.B.; Willett, W.C.; Rosner, B.A.; Estefania, T.; Heather, E.A. Sugar-Sweetened Beverages, Artificially Sweetened Beverages, and Breast Cancer Risk: Results From 2 Prospective US Cohorts. J. Nutr. 2021, 151, 2768–2779. [Google Scholar]
- Bassett, J.K.; Milne, R.L.; English, D.R.; Giles, G.G.; Hodge, A.M. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of cancers not related to obesity. Int. J. Cancer 2020, 146, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Chazelas, E.; Srour, B.; Desmetz, E.; Kesse-Guyot, E.; Julia, C.; Deschamps, V.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Latino-Martel, P.; et al. Sugary drink consumption and risk of cancer: Results from NutriNet-Santé prospective cohort. BMJ 2019, 366, l2408. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Clasen, J.L.; Jayanth, N.P.; Jenab, M.; Tjønneland, A.; Petersen, K.E.N.; Overvad, K.; Srour, B.; Katzke, V.; Bergmann, M.M.; et al. Soft Drink and Juice Consumption and Renal Cell Carcinoma Incidence and Mortality in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1270–1274. [Google Scholar] [CrossRef]
- Hodge, A.M.; Bassett, J.K.; Milne, R.L.; English, D.R.; Giles, G.G. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr. 2018, 21, 1618–1626. [Google Scholar] [CrossRef]
- Hur, J.; Otegbeye, E.; Joh, H.-K.; Nimptsch, K.; Ng, K.; Ogino, S.; Meyerhardt, J.A.; Chan, A.T.; Willett, W.C.; Wu, K.; et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021, 70, 2222–2223. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Robien, K.; Mariani, A.; Cerhan, J.R.; Anderson, K.E. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2384–2394. [Google Scholar] [CrossRef]
- Lim, U.; Subar, A.F.; Mouw, T.; Hartge, P.; Morton, L.M.; Stolzenberg-Solomon, R.; Campbell, D.; Hollenbeck, A.R.; Schatzkin, A. Consumption of aspartame-containing beverages and incidence of hematopoietic and brain malignancies. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1654–1659. [Google Scholar] [CrossRef]
- McCullough, M.L.; Teras, L.R.; Shah, R.; Diver, W.R.; Gaudet, M.M.; Gapstur, S.M. Artificially and sugar-sweetened carbonated beverage consumption is not associated with risk of lymphoid neoplasms in older men and women. J. Nutr. 2014, 144, 2041–2049. [Google Scholar] [CrossRef]
- Navarrete-Muñoz, E.M.; Wark, P.A.; Romaguera, D.; Bhoo-Pathy, N.; Michaud, D.; Molina-Montes, E.; Tjønneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.C.; et al. Sweet-beverage consumption and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2016, 104, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Bertrand, K.A.; Birmann, B.M.; Sampson, L.; Willett, W.C.; Feskanich, D. Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am. J. Clin. Nutr. 2012, 96, 1419–1428. [Google Scholar] [CrossRef] [Green Version]
- Stepien, M.; Duarte-Salles, T.; Fedirko, V.; Trichopoulou, A.; Lagiou, P.; Bamia, C.; Overvad, K.; Tjønneland, A.; Hansen, L.; Boutron-Ruault, M.C.; et al. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a European cohort. Eur. J. Nutr. 2016, 55, 7–20. [Google Scholar] [CrossRef]
- Farvid, M.S.; Spence, N.D.; Rosner, B.A.; Chen, W.Y.; Eliassen, A.H.; Willett, W.C.; Holmes, M.D. Consumption of sugar-sweetened and artificially sweetened beverages and breast cancer survival. Cancer 2021, 127, 2762–2773. [Google Scholar] [CrossRef] [PubMed]
- Guercio, B.J.; Zhang, S.; Niedzwiecki, D.; Li, Y.; Babic, A.; Morales-Oyarvide, V.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). PLoS ONE 2018, 13, e0199244. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef]
- Mullee, A.; Romaguera, D.; Pearson-Stuttard, J.; Viallon, V.; Stepien, M.; Freisling, H.; Fagherazzi, G.; Mancini, F.R.; Boutron-Ruault, M.C.; Kühn, T.; et al. Association Between Soft Drink Consumption and Mortality in 10 European Countries. JAMA Intern. Med. 2019, 179, 1479–1490. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Chen, J.-X.; Jiang, Y.-W.; Xia, P.-F.; Pan, A. Association of sugar-sweetened beverage and artificially sweetened beverage intakes with mortality: An analysis of US National Health and Nutrition Examination Survey. Eur. J. Nutr. 2021, 60, 1945–1955. [Google Scholar] [CrossRef]
- Zoltick, E.S.; Smith-Warner, S.A.; Yuan, C.; Wang, M.; Fuchs, C.S.; Meyerhardt, J.A.; Chan, A.T.; Ng, K.; Ogino, S.; Stampfer, M.J.; et al. Sugar-sweetened beverage, artificially sweetened beverage and sugar intake and colorectal cancer survival. Br. J. Cancer 2021, 125, 1016–1024. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.-H.; Shen, D.; Zhang, P.-D.; Song, W.-Q.; Zhang, W.-T.; Huang, Q.-M.; Chen, P.-L.; Zhang, X.-R.; Mao, C. Association of Sugar-Sweetened, Artificially Sweetened, and Unsweetened Coffee Consumption with All-Cause and Cause-Specific Mortality. Ann. Intern. Med. 2022, 175, 909–917. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Cayssials, V.; Clèries, R.; Torrents, M.; Byrnes, G.; Weiderpass, E.; Sandstrm, M.; Almquist, M.; Boutron-Ruault, M.C.; Tjnneland, A. Sweetened beverages are associated with a higher risk of differentiated thyroid cancer in the EPIC cohort: A dietary pattern approach. Eur. J. Nutr. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mossavar-Rahmani, Y.; Kamensky, V.; Manson, J.E.; Silver, B.; Rapp, S.R.; Haring, B.; Beresford, S.A.A.; Snetselaar, L.; Wassertheil-Smoller, S. Artificially Sweetened Beverages and Stroke, Coronary Heart Disease, and All-Cause Mortality in the Women’s Health Initiative. Stroke 2019, 50, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Rubenstein, L.; Robinson, J.; Seguin, R.A.; Vitolins, M.Z.; Kazlauskaite, R.; Shikany, J.M.; Johnson, K.C.; Snetselaar, L.; Wallace, R. Diet drink consumption and the risk of cardiovascular events: A report from the Women’s Health Initiative. J. Gen. Intern. Med. 2015, 30, 462–468. [Google Scholar] [CrossRef]
- Anderson, J.J.; Gray, S.R.; Welsh, P.; Mackay, D.F.; Celis-Morales, C.A.; Lyall, D.M.; Forbes, J.; Sattar, N.; Gill, J.M.R.; Pell, J.P. The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: A prospective cohort study. BMC Med. 2020, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Paganini-Hill, A.; Kawas, C.H.; Corrada, M.M. Non-alcoholic beverage and caffeine consumption and mortality: The Leisure World Cohort Study. Prev. Med. 2007, 44, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Imamura, F.; Lentjes, M.A.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia 2015, 58, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Malik, V.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption and risk of coronary heart disease in women. Am. J. Clin. Nutr. 2009, 89, 1037–1042. [Google Scholar] [CrossRef]
- Dokova, K.G.; Pancheva, R.Z.; Usheva, N.V.; Haralanova, G.A.; Nikolova, S.P.; Kostadinova, T.I.; Egea Rodrigues, C.; Singh, J.; Illner, A.K.; Aleksandrova, K. Nutrition Transition in Europe: East-West Dimensions in the Last 30 Years—A Narrative Review. Front. Nutr. 2022, 9, 919112. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Jiang, Y.W.; Chen, J.X.; Xia, P.F.; Pan, A. Association of Consumption of Sugar-Sweetened Beverages or Artificially Sweetened Beverages with Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2021, 12, 374–383. [Google Scholar] [CrossRef]
- Wiebe, N.; Padwal, R.; Field, C.; Marks, S.; Jacobs, R.; Tonelli, M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med. 2011, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Rycerz, K.; Jaworska-Adamu, J.E. Effects of aspartame metabolites on astrocytes and neurons. Folia Neuropathol. 2013, 51, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ylmaz, S.; Uar, A. A review of the genotoxic and carcinogenic effects of aspartame: Does it safe or not? Cytotechnology 2014, 66, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, M.; Belpoggi, F.; Manservigi, M.; Tibaldi, E.; Lauriola, M.; Falcioni, L.; Bua, L. Aspartame administered in feed, beginning prenatally through life span, induces cancers of the liver and lung in male Swiss mice. Am. J. Ind. Med. 2010, 53, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, M.; Belpoggi, F.; Degli Esposti, D.; Lambertini, L.; Tibaldi, E.; Rigano, A. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ. Health Perspect. 2006, 114, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. Efsa J. 2013, 11, 3496. [Google Scholar]

| Author | Year | Region | Sample Size | NOS | Cancer Type | Type of Artificial Sweetener Intake | Aspartame Intake Only | Follow-Up Time | Dose of Artificial Sweetener | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Heath A. K. et al [26]. | 2021 | Europe | 281,483 | 8 | Kidney Cancer | ASBs alone | No | Incidence (15 years); Mortality (16 years) | per 100 g/day increment | Incidence/Cancer Mortality |
| Romanos-Nanclares A. et al [22]. | 2021 | US | 175,798 | 9 | Breast Cancer | ASBs alone | No | 36 years | ≥1/day | Incidence |
| Debras C. et al [25]. | 2022 | France | 102,865 | 9 | Cancer (unclassified) | Mixed intake | Yes | 7.8 years | >17.44 mg/day in men and >19.00 mg/day in women | Incidence |
| Chazelas E. et al [24]. (1). | 2019 | France | 101,257 | 9 | Breast Cancer | ASBs alone | No | 5.1 years | >7.9 mL/day in men and >11.6 mL/day in women | Incidence |
| Chazelas E. et al [24]. (2). | Colorectal Cancer | |||||||||
| Chazelas E. et al [24]. (3). | Prostate Cancer | |||||||||
| Navarrete-Munoz E. M. et al [32]. | 2016 | Europe | 477,199 | 9 | Pancreatic Cancer | ASBs alone | No | 11.60 years | >92.2 g/day | Incidence |
| Schernhammer E. S. et al [33]. | 2012 | US | 125,028 | 9 | Hematopoietic Malignancy | Mixed intake | Yes | 22 years | † | Incidence |
| Hodge A. M. et al [27]. (1). | 2018 | Australia | 35,593 | 9 | Prostate Cancer | ASBs alone | No | 9–17 years | ≥1/day | Incidence |
| Hodge A. M. et al [27]. (2). | Ovary Cancer | |||||||||
| Hodge A. M. et al [27]. (3). | Kidney Cancer | |||||||||
| Hodge A. M. et al [27]. (4). | Colorectal Cancer | |||||||||
| Hodge A. M. et al [27]. (5). | Breast Cancer | |||||||||
| Hodge A. M. et al [27]. (6). | Endometrium Cancer | |||||||||
| Hodge A. M. et al [27]. (7). | Gastric Cancer | |||||||||
| Hur J. et al [28]. | 2021 | US | 95,464 | 8 | Colorectal Cancer | ASBs alone | No | 24 years | ≥2 servings/day | Incidence |
| Bassett J. K. et al [23]. | 2019 | Australia | 35,109 | 9 | Cancers not related to obesity | ASBs alone | No | 19 years | >1/day | Incidence |
| Stepien M. et al [34]. | 2016 | Europe | 477,206 | 7 | Liver Cancer | ASBs alone | No | 11.4 years | per 1 serving/day increment | Incidence |
| McCullough M. L. et al [31]. | 2014 | US | 100,442 | 9 | Hematopoietic Malignancy | Mixed intake | Yes | 10 years | ≥1 can/day | Incidence |
| Lim U. et al [30]. (1). | 2006 | US | 473,984 | 8 | Hematopoietic Malignancy | Mixed intake | Yes | 5 years | ≥600 mg/d | Incidence |
| Lim U. et al [30]. (2). | Gliomas | |||||||||
| Inoue-Choi M. et al [29]. | 2013 | US | 23,039 | 8 | Endometrial Cancer | ASBs alone | No | 24 years | 2.8–64.1 servings/week | Incidence |
| Zamora-Ros R. et al [42]. | 2022 | Europe | 450,064 | 8 | Thyroid cancer | ASBs alone | No | 14 years | 43.0–3389.5 mL/d | Incidence |
| Guercio B. J. et al [36]. | 2018 | US | 1018 | 8 | Colorectal Cancer | ASBs alone | No | 10 months | ≥2 servings/day | Cancer Mortality |
| Farvid M. S. et al [35]. | 2021 | US | 8863 | 8 | Breast Cancer | ASBs alone | No | 11.5 years | >3 servings/week | Cancer Mortality |
| Zhang Y. B. et al [39]. | 2020 | US | 31,402 | 8 | Cancer (unclassified) | ASBs alone | No | 7.9 years | ≥2 servings/day | Cancer/All-Cause Mortality |
| Zoltick E. S. et al [40]. | 2021 | US | 1463 | 9 | Colorectal Cancer | ASBs alone | No | 8.0 years | per 1 serving/day increment | Cancer Mortality |
| Mullee A. et al [38]. (1). | 2019 | Europe | 252,357 | 9 | Colorectal Cancer | ASBs alone | No | 16.4 years | ≥1 servings/day | Cancer/All-Cause Mortality |
| Mullee A. et al [38]. (2). | Breast Cancer | |||||||||
| Mullee A. et al [38]. (3). | Prostate Cancer | |||||||||
| Malik V. S. et al [37]. (1). | 2019 | US | 85,030 | 9 | Lung Cancer | ASBs alone | No | 34 years | ≥2 servings/day | Cancer/All-Cause Mortality |
| Malik V. S. et al [37]. (2). | Colorectal Cancer | |||||||||
| Malik V. S. et al [37]. (3). | Breast Cancer | |||||||||
| Malik V. S. et al [37]. (4). | Prostate Cancer | |||||||||
| Liu D. et al [41]. | 2022 | Europe | 51,874 | 9 | Cancer (unclassified) | ASBs alone | No | 7.0 years | >4.5 servings /day | Cancer/All-Cause Mortality |
| Vyas A. et al [44]. | 2014 | Columbia | 59,614 | 9 | - | ASBs alone | No | 8.7 years | ≥2/day | All-Cause Mortality |
| Anderson J. J. et al [45]. | 2020 | Europe | 198,285 | 9 | - | ASBs alone | No | 7 years | >2/day | All-Cause Mortality |
| Paganini-Hill A. et al [46]. | 2007 | US | 13,624 | 8 | - | ASBs alone | No | 23 years | >1 can/week | All-Cause Mortality |
| Mossavar-Rahmani Y. et al [43]. | 2019 | US | 81,714 | 8 | - | ASBs alone | No | 11.9 years | ≥2/day | All-Cause Mortality |
| Outcome | Sub-grouped by | No. of Studies | HR/RR | 95% CI | Heterogeneity I2 (%), p |
|---|---|---|---|---|---|
| Incidence | Region | ||||
| Europe | 6 | 1.07 | (1.02, 1.12) | 25.8%, 0.223 | |
| Americas | 6 | 0.97 | (0.92, 1.02) | 0.0%, 0.476 | |
| Oceania | 2 | 0.99 | (0.85, 1.16) | 25.8%, 0.223 | |
| Cancer type | |||||
| Obesity-related cancers | 7 | 1.01 | (0.94, 1.09) | 51.7%, 0.011 | |
| Cancers not related to obesity | 8 | 1.04 | (0.99, 1.09) | 0.0%, 0.617 | |
| Type of artificial sweetener intake | |||||
| ASBs alone | 10 | 1.02 | (0.97, 1.08) | 42.3%, 0.027 | |
| Mixed intake | 4 | 1.09 | (1.01, 1.18) | 0.0%, 0.513 | |
| Aspartame intake only | |||||
| Yes | 4 | 1.10 | (1.01, 1.19) | 0.0%, 0.467 | |
| No | 10 | 1.02 | (0.97, 1.08) | 42.3%, 0.027 | |
| mortality | Region | ||||
| Europe | 3 | 1.05 | (0.99, 1.13) | 0.0%, 0.502 | |
| Americas | 5 | 0.91 | (0.78, 1.06) | 55.7%, 0.027 | |
| Cancer type | |||||
| Obesity-related cancers | 5 | 0.97 | (0.84, 1.13) | 46.7%, 0.059 | |
| Cancers not related to obesity | 2 | 1.01 | (0.89, 1.15) | 54.4%, 0.139 |
| Variables | I2 (%) | Adj R2 | Exp (b) | Std. Err. | t | p | 95% CI |
|---|---|---|---|---|---|---|---|
| Region (Europe) | 16.22 | 35.98 | 1.11 | 0.06 | 2.09 | 0.048 | (1.01, 1.23) |
| Region (Oceania) | 1.04 | 0.08 | 0.55 | 0.588 | (0.89, 1.22) | ||
| Cancer type | 35.32 | −46.15 | 0.96 | 0.05 | −0.72 | 0.476 | (0.86, 1.08) |
| Type of artificial sweetener intake | 34.14 | 14.61 | 0.95 | 0.05 | −0.93 | 0.360 | (0.84, 1.07) |
| Aspartame intake only | 34.82 | 16.88 | 1.06 | 0.06 | 0.97 | 0.341 | (0.94, 1.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Yan, F.; Liu, L.; Li, B.; Liu, S.; Cui, W. Can Artificial Sweeteners Increase the Risk of Cancer Incidence and Mortality: Evidence from Prospective Studies. Nutrients 2022, 14, 3742. https://doi.org/10.3390/nu14183742
Yan S, Yan F, Liu L, Li B, Liu S, Cui W. Can Artificial Sweeteners Increase the Risk of Cancer Incidence and Mortality: Evidence from Prospective Studies. Nutrients. 2022; 14(18):3742. https://doi.org/10.3390/nu14183742
Chicago/Turabian StyleYan, Shoumeng, Feifei Yan, Liping Liu, Bo Li, Shuxiang Liu, and Weiwei Cui. 2022. "Can Artificial Sweeteners Increase the Risk of Cancer Incidence and Mortality: Evidence from Prospective Studies" Nutrients 14, no. 18: 3742. https://doi.org/10.3390/nu14183742
APA StyleYan, S., Yan, F., Liu, L., Li, B., Liu, S., & Cui, W. (2022). Can Artificial Sweeteners Increase the Risk of Cancer Incidence and Mortality: Evidence from Prospective Studies. Nutrients, 14(18), 3742. https://doi.org/10.3390/nu14183742






