A Water Extract from Chlorella sorokiniana Cell Walls Stimulates Growth of Bone Marrow Cells and Splenocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CWE
2.2. Analysis of Chlorella CWE for Lipid A and Lipid X by Mass Spectrometry
2.3. Electron Microscopy
2.4. Cell Culture
2.5. Animals
2.6. Effect of CWE on the Growth of Bone Marrow Cells and Splenocytes in Cell Culture
2.7. Flow Cytometry Analysis of CWE-Induced Proliferation of BMCs In Vitro
2.8. Effect of CWE on the Growth of Jurkat Cells
2.9. Cytokine Expression in CWE-Treated Jurkat Cells
2.10. Morphological Observation of CWE-Treated Jurkat Cells
2.11. Statistical Analysis
3. Results
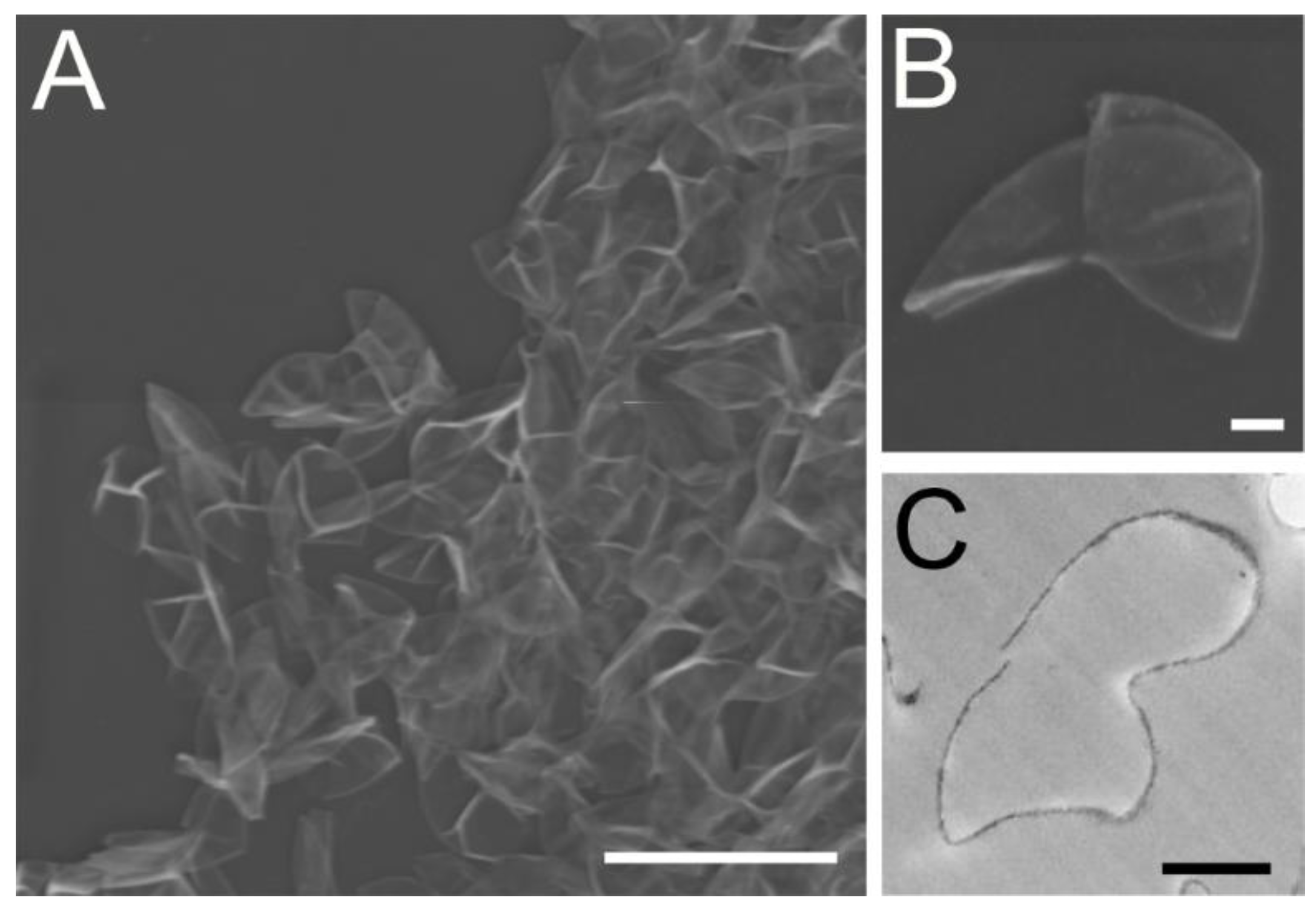
3.1. Morphological Analysis Revealed the Chlorella Cell Wall Fraction Is Composed of Only Cell Walls and Membranes
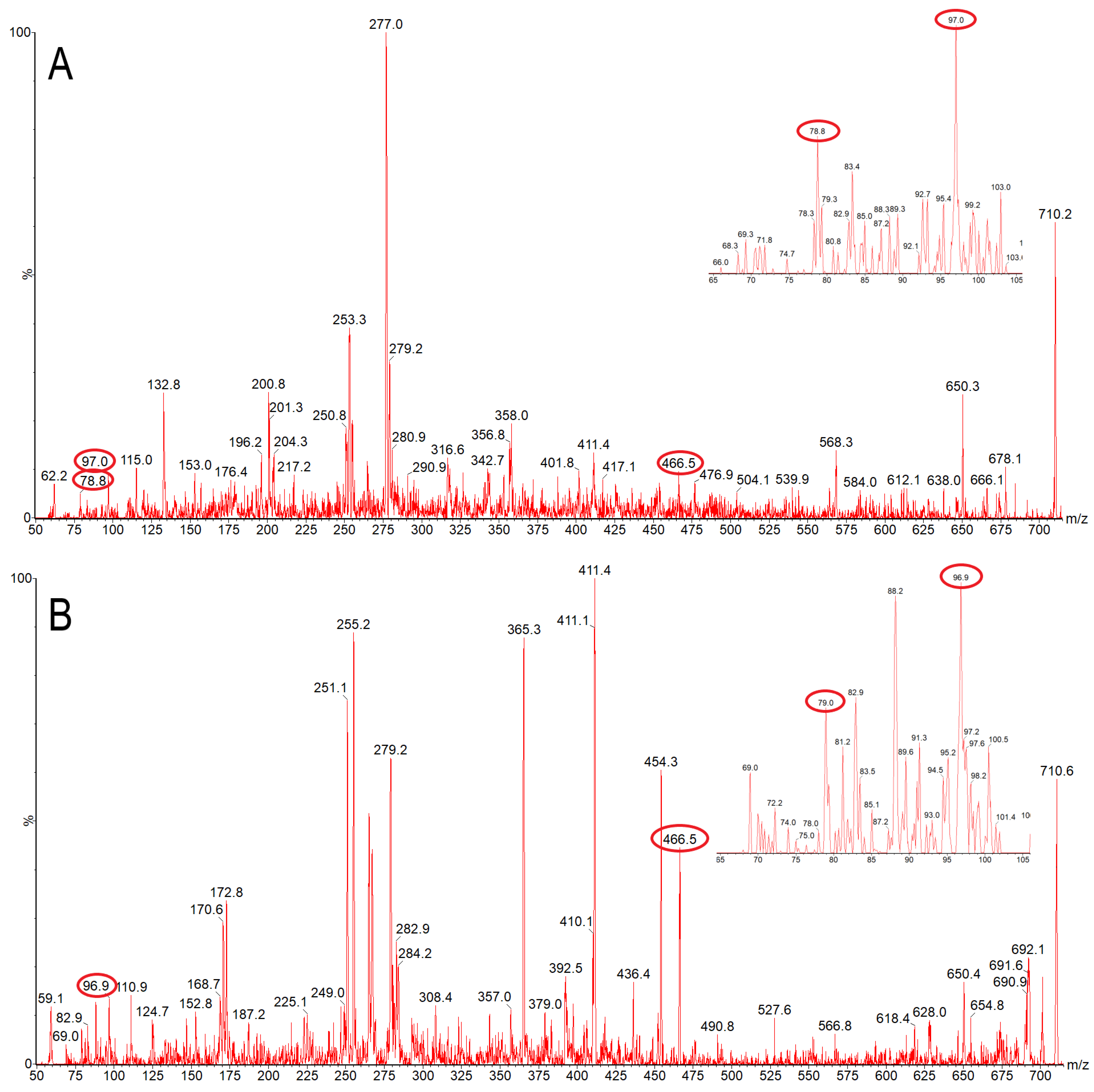
3.2. Mass Spectrometry Suggests the Presence of Lipid X, but Not Lipid A, in Chlorella Cell Wall Fraction
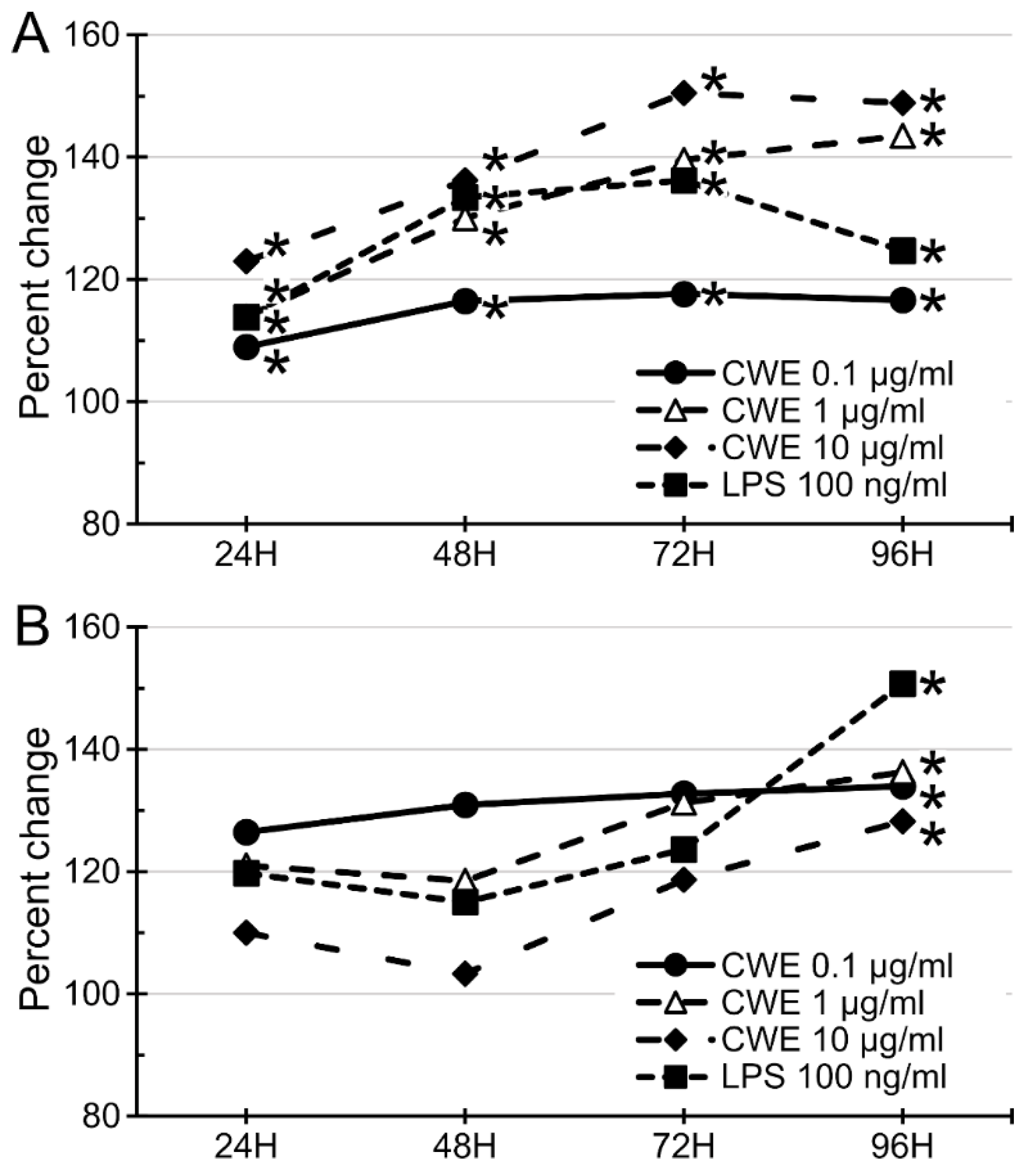
3.3. CWE Treatment Stimulated the Growth of Bone Marrow Cells and Splenocytes
3.4. CWE Treatment Increased Lymphocyte and Antigen-Presenting Cell Populations but Decreased Neutrophil Population in Bone Marrow Cells
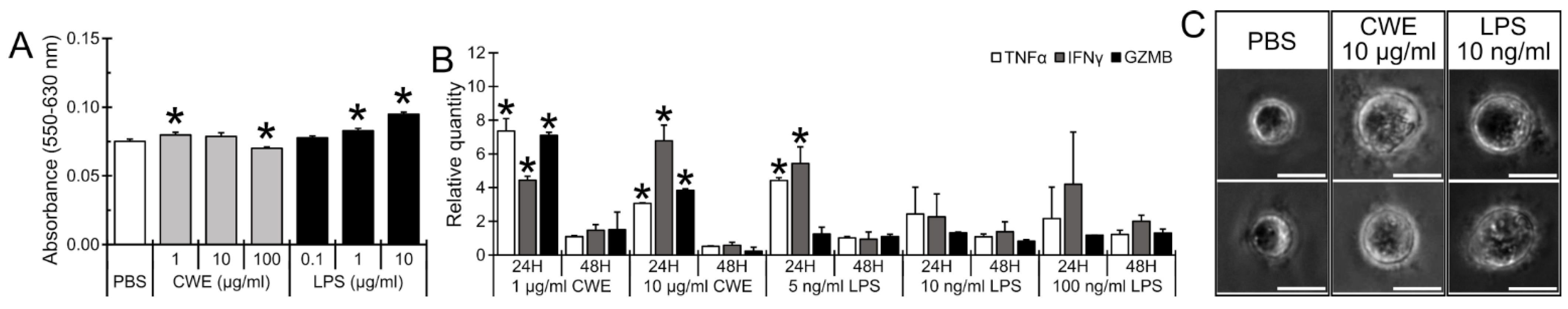
3.5. CWE Treatment Induced Expression of T Lymphocyte Activation-Associated Cytokines and Caused Morphological Differentiation of Lymphoblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darienko, T.; Rad-Menéndez, C.; Campbell, C.; Pröschold, T. Are there any true marine Chlorella species? Molecular phylogenetic assessment and ecology of marine Chlorella-like organisms, including a description of Droopiella gen. nov. Syst. Biodivers. 2019, 17, 811–829. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.G.; Vaz, B.d.S.; de Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, S.; Nikolov, Z. Process for selective extraction of pigments and functional proteins from Chlorella vulgaris. Algal Res. 2018, 35, 185–193. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the Photoautotrophic Growth Regimens of Chlorella sorokiniana in a Photobioreactor for Enhanced Biomass Productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef]
- Chai, S.; Shi, J.; Huang, T.; Guo, Y.; Wei, J.; Guo, M.; Li, L.; Dou, S.; Liu, L.; Liu, G. Characterization of Chlorella sorokiniana growth properties in monosaccharide-supplemented batch culture. PLoS ONE 2018, 13, e0199873. [Google Scholar] [CrossRef] [Green Version]
- Cecchin, M.; Benfatto, S.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular basis of autotrophic vs mixotrophic growth in Chlorella sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Justo, G.Z.; Silva, M.R.; Queiroz, M.L. Effects of the green algae Chlorella vulgaris on the response of the host hematopoietic system to intraperitoneal ehrlich ascites tumor transplantation in mice. Immunopharmacol. Immunotoxicol. 2001, 23, 119–132. [Google Scholar] [CrossRef]
- Konishi, F.; Tanaka, K.; Himeno, K.; Taniguchi, K.; Nomoto, K. Antitumor effect induced by a hot water extract of Chlorella vulgaris (CE): Resistance to Meth-A tumor growth mediated by CE-induced polymorphonuclear leukocytes. Cancer Immunol. Immunother. CII 1985, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, Y.; Murayama, T.; Ooya, N.; Wang, L.F.; Tung, Y.C.; Yamaguchi, N. Immunomodulation by a unicellular green algae (Chlorella pyrenoidosa) in tumor-bearing mice. J. Ethnopharmacol. 1988, 24, 135–146. [Google Scholar] [CrossRef]
- Noda, K.; Ohno, N.; Tanaka, K.; Kamiya, N.; Okuda, M.; Yadomae, T.; Nomoto, K.; Shoyama, Y. A water-soluble antitumor glycoprotein from Chlorella vulgaris. Planta Med. 1996, 62, 423–426. [Google Scholar] [CrossRef]
- Ramos, A.L.; Torello, C.O.; Queiroz, M.L. Chlorella vulgaris modulates immunomyelopoietic activity and enhances the resistance of tumor-bearing mice. Nutr. Cancer 2010, 62, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Konishi, F.; Himeno, K.; Taniguchi, K.; Nomoto, K. Augmentation of antitumor resistance by a strain of unicellular green algae, Chlorella vulgaris. Cancer Immunol. Immunother. CII 1984, 17, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tomita, Y.; Tsuruta, M.; Konishi, F.; Okuda, M.; Himeno, K.; Nomoto, K. Oral administration of Chlorella vulgaris augments concomitant antitumor immunity. Immunopharmacol. Immunotoxicol. 1990, 12, 277–291. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamada, A.; Noda, K.; Hasegawa, T.; Okuda, M.; Shoyama, Y.; Nomoto, K. A novel glycoprotein obtained from Chlorella vulgaris strain CK22 shows antimetastatic immunopotentiation. Cancer Immunol. Immunother. CII 1998, 45, 313–320. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Konovalova, A.; Kahne, D.E.; Silhavy, T.J. Outer Membrane Biogenesis. Annu. Rev. Microbiol. 2017, 71, 539–556. [Google Scholar] [CrossRef]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Durai, P.; Batool, M.; Choi, S. Structure and Effects of Cyanobacterial Lipopolysaccharides. Mar. Drugs 2015, 13, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Slutzky, G.M.; Londner, M.V.; Greenblatt, C.L. Lipid and lipopolysaccharide-like antigens of Leishmania promastigotes. J. Protozool. 1985, 32, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Bedick, J.C.; Shnyra, A.; Stanley, D.W.; Pardy, R.L. Innate immune reactions stimulated by a lipopolysaccharide-like component of the alga Prototheca (strain 289). Die Nat. 2001, 88, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guan, Z.; Liu, D.; Raetz, C.R. Pathway for lipid A biosynthesis in Arabidopsis thaliana resembling that of Escherichia coli. Proc. Natl. Acad. Sci. USA 2011, 108, 11387–11392. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, E.T.; Ali, A.A.; El-Shaarawy, F.; Mesbah, N.M.; Abo-Elmatty, D.M.; Aborehab, N.M. Anti-Oxidant and Anti-Inflammatory Effects of Lipopolysaccharide from Rhodobacter sphaeroides against Ethanol-Induced Liver and Kidney Toxicity in Experimental Rats. Molecules 2021, 26, 7437. [Google Scholar] [CrossRef]
- Murakami, K.; Kamimura, D.; Hasebe, R.; Uchida, M.; Abe, N.; Yamamoto, R.; Jiang, J.J.; Hidaka, Y.; Nakanishi, Y.; Fujita, S.; et al. Rhodobacter azotoformans LPS (RAP99-LPS) Is a TLR4 Agonist That Inhibits Lung Metastasis and Enhances TLR3-Mediated Chemokine Expression. Front. Immunol. 2021, 12, 675909. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef] [Green Version]
- Baldridge, J.R.; Crane, R.T. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods 1999, 19, 103–107. [Google Scholar] [CrossRef]
- Loos, E.; Meindl, D. Composition of the cell wall of Chlorella fusca. Planta 1982, 156, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Northcote, D.H.; Goulding, K.J.; Horne, R.W. The chemical composition and structure of the cell wall of Chlorella pyrenoidosa. Biochem. J. 1958, 70, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, P.B.; Armstrong, M.T.; Pardy, R.L.; Child, A.; Wainwright, N. Immunohistochemical demonstration of a lipopolysaccharide in the cell wall of a eukaryote, the green alga, Chlorella. Biol. Bull. 2002, 203, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Robben, N.; Burghart, R.; Cote, P.; Greenway, S.; Thakkar, R.; Upreti, D.; Nakashima, A.; Suzuki, K.; Comer, J.; et al. Cell Wall Membrane Fraction of Chlorella sorokiniana Enhances Host Antitumor Immunity and Inhibits Colon Carcinoma Growth in Mice. Integr. Cancer Ther. 2020, 19, 1534735419900555. [Google Scholar] [CrossRef] [Green Version]
- Hannan, P.J.; Patouillet, C. Gas Exchange with Mass Cultures of Algae. I. Effects of Light Intensity and Rate of Carbon Dioxide Input on Oxygen Production. Appl. Microbiol. 1963, 11, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.C.; O’Brien, J.P.; Brodbelt, J.S.; Trent, M.S. Isolation and chemical characterization of lipid A from gram-negative bacteria. J. Vis. Exp. JoVE 2013, 79, e50623. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, J.E.; Li, J.; Cahoon, E.B.; Jaworski, J.G. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 2006, 281, 22684–22694. [Google Scholar] [CrossRef] [Green Version]
- Toledo, M.S.; Suzuki, E.; Straus, A.H.; Takahashi, H.K. Glycolipids from Paracoccidioides brasiliensis. Isolation of a galactofuranose-containing glycolipid reactive with sera of patients with paracoccidioidomycosis. J. Med. Vet. Mycol. Bi-Mon. Publ. Int. Soc. Hum. Anim. Mycol. 1995, 33, 247–251. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar]
- Ishiguro, S.; Uppalapati, D.; Goldsmith, Z.; Robertson, D.; Hodge, J.; Holt, H.; Nakashima, A.; Turner, K.; Tamura, M. Exopolysaccharides extracted from Parachlorella kessleri inhibit colon carcinoma growth in mice via stimulation of host antitumor immune responses. PLoS ONE 2017, 12, e0175064. [Google Scholar] [CrossRef] [Green Version]
- Proctor, R.A.; Textor, J.A. Activation and inhibition of Limulus amebocyte lysate coagulation by chemically defined substructures of lipid A. Infect. Immun. 1985, 49, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Takayama, K.; Qureshi, N.; Raetz, C.R.; Ribi, E.; Peterson, J.; Cantrell, J.L.; Pearson, F.C.; Wiggins, J.; Johnson, A.G. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect. Immun. 1984, 45, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.W.; Shaffer, S.A.; Ernst, R.K.; Goodlett, D.R.; Turecek, F. Determination of pyrophosphorylated forms of lipid A in Gram-negative bacteria using a multivaried mass spectrometric approach. Proc. Natl. Acad. Sci. USA 2008, 105, 12742–12747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibusuki, K.; Minamishima, Y. Effect of Chlorella vulgaris extracts on murine cytomegalovirus infections. Nat. Immun. Cell Growth Regul. 1990, 9, 121–128. [Google Scholar] [PubMed]
- Merchant, R.E.; Andre, C.A.; Sica, D.A. Nutritional supplementation with Chlorella pyrenoidosa for mild to moderate hypertension. J. Med. Food 2002, 5, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Iizuka, Y.; Murakami, T.; Miyake, H.; Suzuki, T. Effects of chlorella alkali extract on blood pressure in SHR. Jpn. Heart J. 1978, 19, 622–623. [Google Scholar]
- Sano, T.; Tanaka, Y. Effect of dried, powdered Chlorella vulgaris on experimental atherosclerosis and alimentary hypercholesterolemia in cholesterol-fed rabbits. Artery 1987, 14, 76–84. [Google Scholar]
- Chou, N.T.; Cheng, C.F.; Wu, H.C.; Lai, C.P.; Lin, L.T.; Pan, I.H.; Ko, C.H. Chlorella sorokiniana-Induced Activation and Maturation of Human Monocyte-Derived Dendritic Cells through NF-kappaB and PI3K/MAPK Pathways. Evid. Based Complement. Altern. Med. Ecam 2012, 2012, 735396. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.Y.; Jeyashoke, N.; Yeh, C.H.; Song, Y.J.; Hua, K.F.; Chao, L.K. Immunostimulatory bioactivity of algal polysaccharides from Chlorella pyrenoidosa activates macrophages via Toll-like receptor 4. J. Agric. Food Chem. 2010, 58, 927–936. [Google Scholar] [CrossRef]
- Hasegawa, T.; Matsuguchi, T.; Noda, K.; Tanaka, K.; Kumamoto, S.; Shoyama, Y.; Yoshikai, Y. Toll-like receptor 2 is at least partly involved in the antitumor activity of glycoprotein from Chlorella vulgaris. Int. Immunopharmacol. 2002, 2, 579–589. [Google Scholar]
- Armstrong, M.T.; Theg, S.M.; Braun, N.; Wainwright, N.; Pardy, R.L.; Armstrong, P.B. Histochemical evidence for lipid A (endotoxin) in eukaryote chloroplasts. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 2145–2146. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Bi, E.; Hu, Y.; Deng, W.; Tian, Z.; Dong, C.; Hu, Y.; Sun, B. A novel NF-kappaB binding site controls human granzyme B gene transcription. J. Immunol. 2006, 176, 4173–4181. [Google Scholar] [CrossRef] [Green Version]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M.W.; Wick, M.J.; Rhen, M.; Normark, S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 2002, 3, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, A.J.; Flad, H.; Rietschel, T.; Mattern, T. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology 2000, 152, 37–45. [Google Scholar] [CrossRef]

| Primer | Sequence | Size | |
|---|---|---|---|
| Human | Forward (5′-3′) | GCCAGAATGCTGCAGGACTT | 63 bp |
| TNFα | Reverse (5′-3′) | GGCCTAAGGTCCACTTGTGTCA | |
| Human | Forward (5′-3′) | AGGGAAGCGAAAAAGGAGTCA | 64 bp |
| IFNγ | Reverse (5′-3′) | GGACAACCATTACTGGGATGCT | |
| Human | Forward (5′-3′) | ATGAGACAGCAACCATTGTAGAATTT | 87 bp |
| IL-2 | Reverse (5′-3′) | CACTTAATTATCAAGTCAGTGTTGAGATGA | |
| Human | Forward (5′-3′) | TGCAGGAAGATCGAAAGTGCG | 180 bp |
| GZMB | Reverse (5′-3′) | GAGGCATGCCATTGTTTCGTC | |
| 18S | Forward (5′-3′) | GAGGTTCGAAGACGATCAGA | 315 bp |
| Reverse (5′-3′) | TCGCTCCACCAACTAAGAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiguro, S.; Roth, M.; Welti, R.; Loyd, M.; Thakkar, R.; Phillips, M.; Robben, N.; Upreti, D.; Nakashima, A.; Suzuki, K.; et al. A Water Extract from Chlorella sorokiniana Cell Walls Stimulates Growth of Bone Marrow Cells and Splenocytes. Nutrients 2022, 14, 2901. https://doi.org/10.3390/nu14142901
Ishiguro S, Roth M, Welti R, Loyd M, Thakkar R, Phillips M, Robben N, Upreti D, Nakashima A, Suzuki K, et al. A Water Extract from Chlorella sorokiniana Cell Walls Stimulates Growth of Bone Marrow Cells and Splenocytes. Nutrients. 2022; 14(14):2901. https://doi.org/10.3390/nu14142901
Chicago/Turabian StyleIshiguro, Susumu, Mary Roth, Ruth Welti, Mayme Loyd, Ravindra Thakkar, Morgan Phillips, Nicole Robben, Deepa Upreti, Ayaka Nakashima, Kengo Suzuki, and et al. 2022. "A Water Extract from Chlorella sorokiniana Cell Walls Stimulates Growth of Bone Marrow Cells and Splenocytes" Nutrients 14, no. 14: 2901. https://doi.org/10.3390/nu14142901
APA StyleIshiguro, S., Roth, M., Welti, R., Loyd, M., Thakkar, R., Phillips, M., Robben, N., Upreti, D., Nakashima, A., Suzuki, K., Comer, J., & Tamura, M. (2022). A Water Extract from Chlorella sorokiniana Cell Walls Stimulates Growth of Bone Marrow Cells and Splenocytes. Nutrients, 14(14), 2901. https://doi.org/10.3390/nu14142901







