Correlation between Serum 25-Hydroxyvitamin D Level and Depression among Korean Women with Secondary Amenorrhea: A Cross-Sectional Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Statistical Analysis
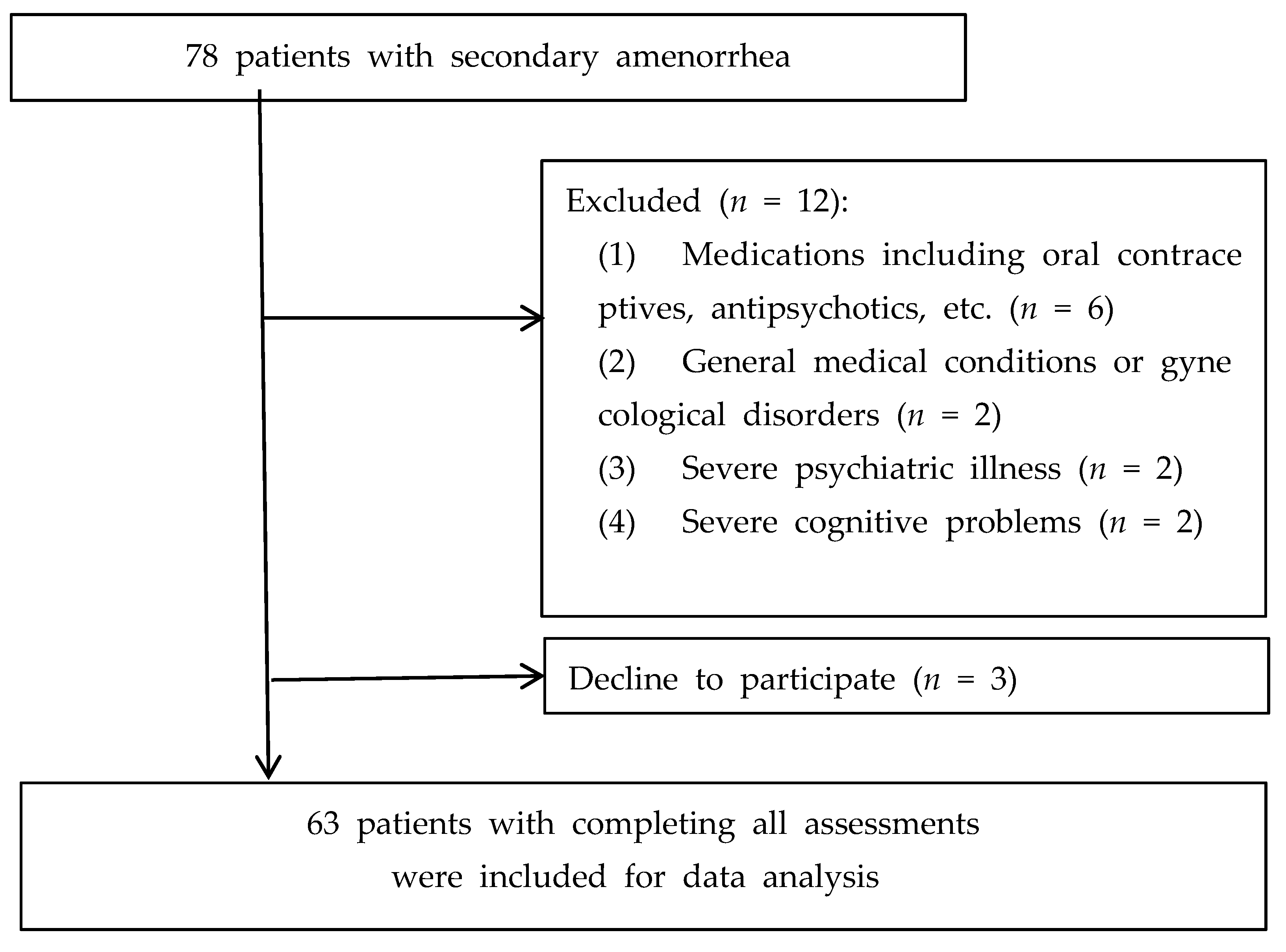
3. Results
3.1. Vitamin D Deficiency and Depressive Symptoms
3.2. Correlations between Depression and Biochemical Variables
3.3. Predictors of Depression in Women with Secondary Amenorrhea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Messa, P.; Curreri, M.; Regalia, A.; Alfieri, C.M. Vitamin D and the cardiovascular system: An overview of the recent literature. Am. J. Cardiovasc. Drugs 2014, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect Disord. 2017, 208, 56–61. [Google Scholar] [CrossRef]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Stewart, R.; Hirani, V. Relationship between vitamin D levels and depressive symptoms in older residents from a national survey population. Psychosom. Med. 2010, 72, 608–612. [Google Scholar] [CrossRef]
- Ronaldson, A.; Arias de la Torre, J.; Gaughran, F.; Bakolis, I.; Hatch, S.L.; Hotopf, M.; Dregan, A. Prospective associations between vitamin D and depression in middle-aged adults: Findings from the UK Biobank cohort. Psychol. Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Ali, A.; Vasileva, S.; Langguth, M.; Alexander, S.; Cui, X.; Whitehouse, A.; McGrath, J.J.; Eyles, D. Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model. Nutrients 2019, 11, 1187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Leung, D.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Wojtusik, J.; Johnson, P.A. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol. Reprod. 2012, 86, 91. [Google Scholar] [CrossRef] [PubMed]
- Cooney, L.G.; Lee, I.; Sammel, M.D.; Dokras, A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2017, 32, 1075–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allshouse, A.A.; Semple, A.L.; Santoro, N.F. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause 2015, 22, 166–174. [Google Scholar] [CrossRef]
- Kim, G.M.; Lee, J.A.; Park, S.W.; Lee, J.G.; Jeon, G.H. Are plasma Brain-Derived Neurotrophic Factor or reproductive hormones related to depression in PCOS patients?: A prospective cohort study. Clin. Exp. Obstet. Gynecol. 2021, 48, 1146–1153. [Google Scholar]
- Klein, D.A.; Poth, M.A. Amenorrhea: An approach to diagnosis and management. Am. Fam. Physician 2013, 87, 781–788. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, C.; van Etten, E.; Decallonne, B.; Guilietti, A.; Gysemans, C.; Bouillon, R.; Overbergh, L. Vitamin D and 1,25-dihydroxyvitamin D3 as modulators in the immune system. J. Steroid Biochem. Mol. Biol. 2004, 89, 449–452. [Google Scholar] [CrossRef]
- Shah, J.; Gurbani, S. Association of Vitamin D Deficiency and Mood Disorders: A Systematic Review. In Vitamin D Deficiency, 1st ed.; Fedotova, J., Ed.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/70606 (accessed on 19 April 2021).
- Wilkins, C.H.; Sheline, Y.I.; Roe, C.M.; Birge, S.J.; Morris, J.C. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am. J. Geriatr. Psychiatry 2006, 14, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Atteritano, M.; Lasco, A.; Mazzaferro, S.; Macrì, I.; Catalano, A.; Santangelo, A.; Bagnato, G.; Bagnato, G.; Frisina, N. Bone mineral density, quantitative ultrasound parameters and bone metabolism in postmenopausal women with depression. Intern. Emerg. Med. 2013, 8, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Łagowska, K. The Relationship between Vitamin D Status and the Menstrual Cycle in Young Women: A Preliminary Study. Nutrients 2018, 10, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jukic, A.M.Z.; Steiner, A.Z.; Baird, D.D. Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study. Reprod. Biol. Endocrinol. 2015, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsamakis, G.; Chrousos, G.; Mastorakos, G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology 2019, 100, 48–57. [Google Scholar] [CrossRef]
- Herrán, A.; Amado, J.A.; García-Unzueta, M.T.; Vázquez-Barquero, J.L.; Perera, L.; González-Macías, J. Increased bone remodeling in first-episode major depressive disorder. Psychosom. Med. 2000, 62, 779–782. [Google Scholar] [CrossRef]
- Rhee, S.J.; Lee, H.; Ahn, Y.M. Serum Vitamin D Concentrations Are Associated With Depressive Symptoms in Men: The Sixth Korea National Health and Nutrition Examination Survey 2014. Front. Psychiatry 2020, 11, 756. [Google Scholar] [CrossRef]
- Sarkar, S. Vitamin D for depression with a seasonal pattern: An effective treatment strategy. Int. Phys. Med. Rehab. J. 2017, 1, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Schaad, K.A.; Bukhari, A.S.; Brooks, D.I.; Kocher, J.D.; Barringer, N.D. The relationship between vitamin D status and depression in a tactical athlete population. J. Int. Soc. Sports Nutr. 2019, 16, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yang, X.; Jia, Y.; Wen, Y.; Cheng, S.; Meng, P.; Li, C.; Zhang, H.; Pan, C.; Zhang, J.; et al. Vitamin D and the Risks of Depression and Anxiety: An Observational Analysis and Genome-Wide Environment Interaction Study. Nutrients 2021, 13, 3343. [Google Scholar] [CrossRef]
- Marshall, I.; Mehta, R.; Ayers, C.; Dhumal, S.; Petrova, A. Prevalence and risk factors for vitamin D insufficiency and deficiency at birth and associated outcome. BMC Pediatr. 2016, 16, 208. [Google Scholar] [CrossRef] [Green Version]
- Ovesen, L.; Andersen, R.; Jakobsen, J. Geographical differences in vitamin D status, with particular reference to European countries. Proc. Nutr. Soc. 2003, 62, 813–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walbert, T.; Jirikowski, G.F.; Prüfer, K. Distribution of 1,25-Dihydroxyvitamin D3 Receptor Immunoreactivity in the Limbic System of the Rat. Horm. Metab. Res. 2001, 33, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Wang, T.T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; MacLeod, N.B.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef] [Green Version]
- Burne, T.H.J.; McGrath, J.J.; Eyles, D.W.; Mackay-Sim, A. Behavioural characterization of vitamin D receptor knockout mice. Behav. Brain Res. 2005, 157, 299–308. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Lou, Y.R.; Laaksi, I.; Tuohimaa, P. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport 2004, 15, 1271–1274. [Google Scholar] [CrossRef]
- Dogan-Sander, E.; Mergl, R.; Willenberg, A.; Baber, R.; Wirkner, K.; Riedel-Heller, S.G.; Röhr, S.; Schmidt, F.M.; Schomerus, G.; Sander, C. Inflammation and the Association of Vitamin D and Depressive Symptomatology. Nutrients 2021, 13, 1972. [Google Scholar] [CrossRef]
- Fernandes de Abreu, D.A.; Eyles, D.; Féron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S265–S277. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D and Depression: Cellular and Regulatory Mechanisms. Pharmacol. Rev. 2017, 69, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Obradovic, D.; Gronemeyer, H.; Lutz, B.; Rein, T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J. Neurochem. 2006, 96, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.F.M.; van Beurden, M.; Gaarenstroom, K.N.; Teunis, T.; Kieffer, J.M.; Aaronson, N.K.; Kenter, G.G.; Korse, C.M. Does anti-Müllerian hormone predict change in menopausal symptoms following risk-reducing salpingo-oophorectomy? A prospective observational study. Climacteric 2018, 21, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golenbock, S.W.; Wise, L.A.; Lambert-Messerlian, G.M.; Eklund, E.E.; Harlow, B.L. Association between a history of depression and anti-müllerian hormone among late-reproductive aged women: The Harvard study of moods and cycles. Women’s Midlife Health 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Kruszyńska, A.; Słowińska-Srzednicka, J. Anti-Müllerian hormone (AMH) as a good predictor of time of menopause. Prz. Menopauzalny 2017, 16, 47–50. [Google Scholar] [CrossRef]
- Martínez-Moreno, C.G.; Calderón-Vallejo, D.; Harvey, S.; Arámburo, C.; Quintanar, J.L. Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy? Int. J. Mol. Sci. 2018, 19, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drakopoulos, P.; van de Vijver, A.; Schutyser, V.; Milatovic, S.; Anckaert, E.; Schiettecatte, J.; Blockeel, C.; Camus, M.; Tournaye, H.; Polyzos, N.P. The effect of serum vitamin D levels on ovarian reserve markers: A prospective cross-sectional study. Hum. Reprod. 2017, 32, 208–214. [Google Scholar] [CrossRef]
- Merhi, Z.O.; Seifer, D.B.; Weedon, J.; Adeyemi, O.; Holman, S.; Anastos, K.; Golub, E.T.; Young, M.; Karim, R.; Greenblatt, R.; et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductiveaged women: Women’s Interagency HIV Study. Fertil. Steril. 2012, 98, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.C.; Huang, Y.C.; Huang, W.L. The effect of vitamin D supplement on negative emotions: A systematic review and meta-analysis. Depress. Anxiety 2020, 37, 549–564. [Google Scholar] [CrossRef]
| Variable | Serum 25-Hydroxyvitamin D(25(OH)D) Levels | p-Value/χ2 | ||
|---|---|---|---|---|
| 25(OH)D < 20 ng/mL (n = 26) | 25(OH)D ≥ 20 ng/mL (n = 37) | |||
| Demographic factors | ||||
| Age (years) | 22.54 ± 5.33 | 27.81 ± 8.65 | 0.014 2 | |
| BMI (kg/m2) | 22.85 ± 5.17 | 22.55 ± 4.39 | 0.823 1 | |
| Menarche (years) | 13.42 ± 1.06 | 13.53 ± 1.33 | 0.909 2 | |
| Parity | 0 | 25 (48.1%) | 27 (51.9%) | 0.069 3 |
| 1 | 1 (14.3%) | 6 (85.7%) | ||
| 2 | 0 (0%) | 4 (100%) | ||
| Hormonal status | ||||
| Estradiol (pg/mL) | 63.18 ± 68.30 (n = 25) | 67.77 ± 63.28 (n = 36) | 0.866 2 | |
| Free testosterone (pg/mL) | 2.19 ± 0.83 | 1.82 ± 0.79 | 0.077 1 | |
| Prolactin (ng/mL) | 17.84 ± 11.54 | 16.79 ± 15.61 | 0.135 2 | |
| AMH (ng/mL) | 10.86 ± 8.94 (n = 25) | 7.24 ± 5.62 (n = 35) | 0.130 2 | |
| LH (U/L) | 18.96 ± 21.04 (n = 25) | 13.81 ± 12.42 (n = 36) | 0.127 2 | |
| FSH (U/L) | 7.78 ± 7.70 | 7.67 ± 9.37 | 0.917 2 | |
| TSH (μmol/L) | 2.05 ± 0.97 (n = 24) | 2.16 ± 1.32 (n = 36) | 0.815 2 | |
| fT4 (ng/dL) | 1.25 ± 0.22 (n = 24) | 1.24 ± 0.19 (n = 36) | 0.988 1 | |
| Metabolic parameters | ||||
| Total cholesterol (mg/dL) | 184.04 ± 38.84 (n = 26) | 192.62 ± 30.42 (n = 36) | 0.334 1 | |
| Fasting glucose (mg/dL) | 99.36 ± 33.69 (n = 25) | 90.86 ± 9.29 (n = 36) | 0.116 2 | |
| Psychiatric assessments | ||||
| Total CES-D score | 18.77 ± 13.56 | 16.54 ± 10.37 | 0.753 2 | |
| Total K-HDRS score | 7.35 ± 5.89 | 5.81 ± 5.69 | 0.317 2 | |
| Variable | CES-D | K-HDRS | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
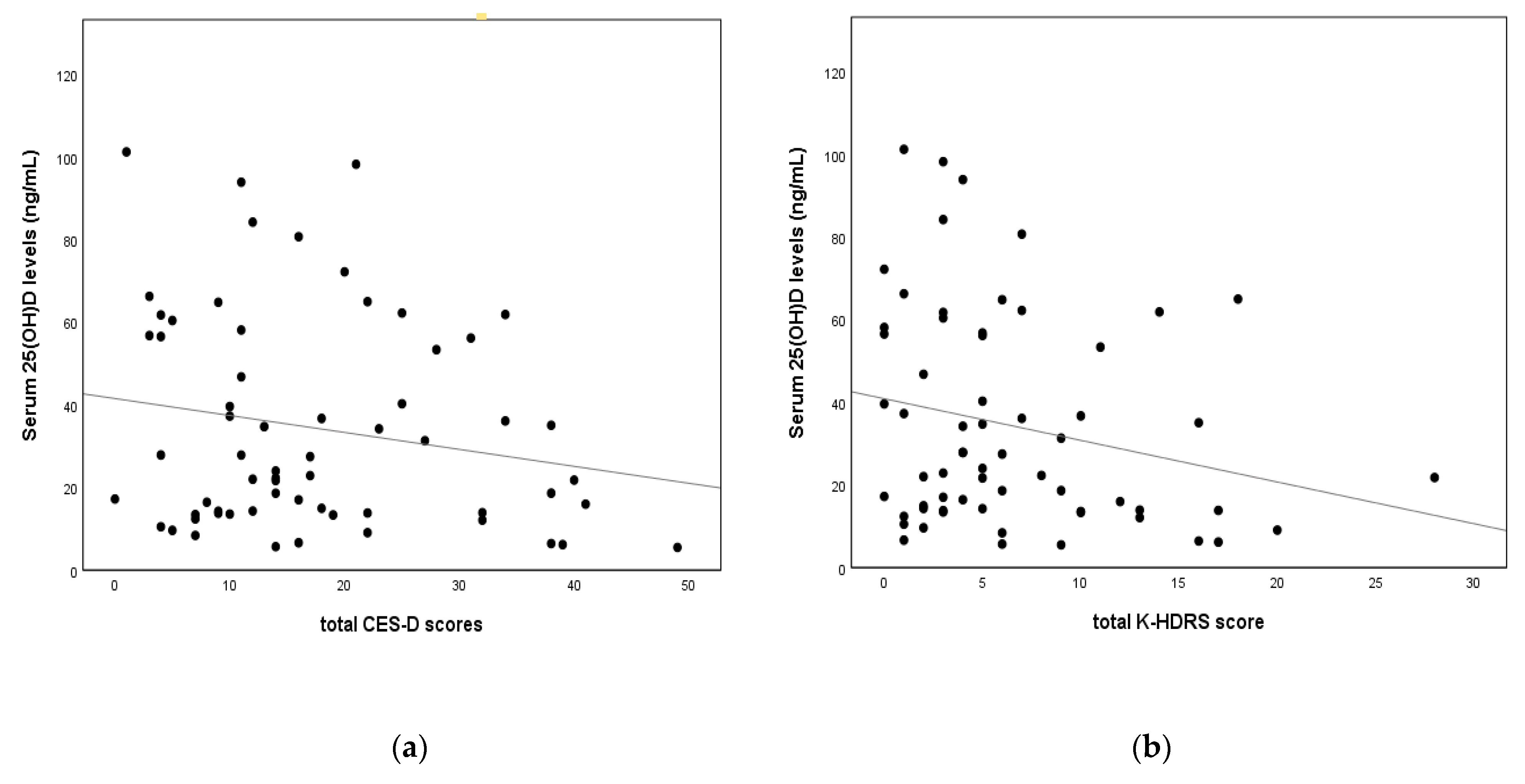
| 25(OH)D(ng/mL) | −0.127 | 0.330 | −0.258 | 0.045 * |
| Estradiol (pg/mL) | −0.077 | 0.552 | 0.049 | 0.705 |
| Free testosterone (pg/mL) | −0.254 | 0.043 * | −0.339 | 0.006 * |
| Prolactin (ng/mL) | 0.026 | 0.836 | 0.174 | 0.168 |
| AMH (ng/mL) | −0.373 | 0.003 ** | −0.450 | <0.001 ** |
| LH (U/L) | −0.104 | 0.420 | −0.216 | 0.092 |
| FSH (U/L) | −0.017 | 0.897 | −0.128 | 0.315 |
| TSH (μmol/L) | 0.002 | 0.986 | 0.136 | 0.297 |
| fT4 (ng/dL) | 0.187 | 0.150 | 0.083 | 0.525 |
| Total cholesterol (mg/dL) | 0.005 | 0.971 | 0.083 | 0.518 |
| Fasting glucose (mg/dL) | 0.061 | 0.639 | 0.127 | 0.325 |
| CES-D | K-HDRS | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | B | SE | β | R2 | B | SE | β | R2 |
| Step 1 | 0.01 | 0.02 | ||||||
| Age | −0.15 | 0.21 | −0.10 | 0.04 | 0.10 | 0.06 | ||
| BMI | 0.18 | 0.38 | 0.06 | −0.21 | 0.18 | −0.15 | ||
| Step 2 | 0.08 * | 0.12 * | ||||||
| Age | −0.21 | 0.20 | −0.14 | 0.00 | 0.10 | 0.01 | ||
| BMI | 0.22 | 0.37 | 0.08 | −0.19 | 0.18 | −0.14 | ||
| Free testosterone | −3.76 | 1.84 | −0.27 * | −2.24 | 0.89 | −0.32 * | ||
| Step 3 | 0.25 ** | 0.21 ** | ||||||
| Age | −0.19 | 0.21 | −0.06 | 0.11 | 0.10 | 0.15 | ||
| BMI | 0.17 | 0.34 | 0.06 | −0.22 | 0.16 | −0.16 | ||
| Free testosterone | −0.56 | 2.02 | −0.04 | −0.87 | 0.94 | −0.12 | ||
| AMH | −0.72 | 0.23 | 0.46 ** | −0.33 | 0.11 | −0.42 ** | ||
| 25(OH)D (ng/mL) | −0.12 | 0.06 | −0.26 | −0.09 | 0.03 | −0.39 ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-M.; Jeon, G.-H. Correlation between Serum 25-Hydroxyvitamin D Level and Depression among Korean Women with Secondary Amenorrhea: A Cross-Sectional Observational Study. Nutrients 2022, 14, 2835. https://doi.org/10.3390/nu14142835
Kim G-M, Jeon G-H. Correlation between Serum 25-Hydroxyvitamin D Level and Depression among Korean Women with Secondary Amenorrhea: A Cross-Sectional Observational Study. Nutrients. 2022; 14(14):2835. https://doi.org/10.3390/nu14142835
Chicago/Turabian StyleKim, Gyung-Mee, and Gyun-Ho Jeon. 2022. "Correlation between Serum 25-Hydroxyvitamin D Level and Depression among Korean Women with Secondary Amenorrhea: A Cross-Sectional Observational Study" Nutrients 14, no. 14: 2835. https://doi.org/10.3390/nu14142835







