Maternal High-Fat Diet Reduces Type-2 Neural Stem Cells and Promotes Premature Neuronal Differentiation during Early Postnatal Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model of HFD
2.2. Measurement of Offspring Brain Structure and Neocortical Thickness
2.3. Tissue Collection
2.4. Immunohistochemical Staining and Cell Counting
2.5. Western Blotting Analysis
2.6. Statistical Analysis
3. Results
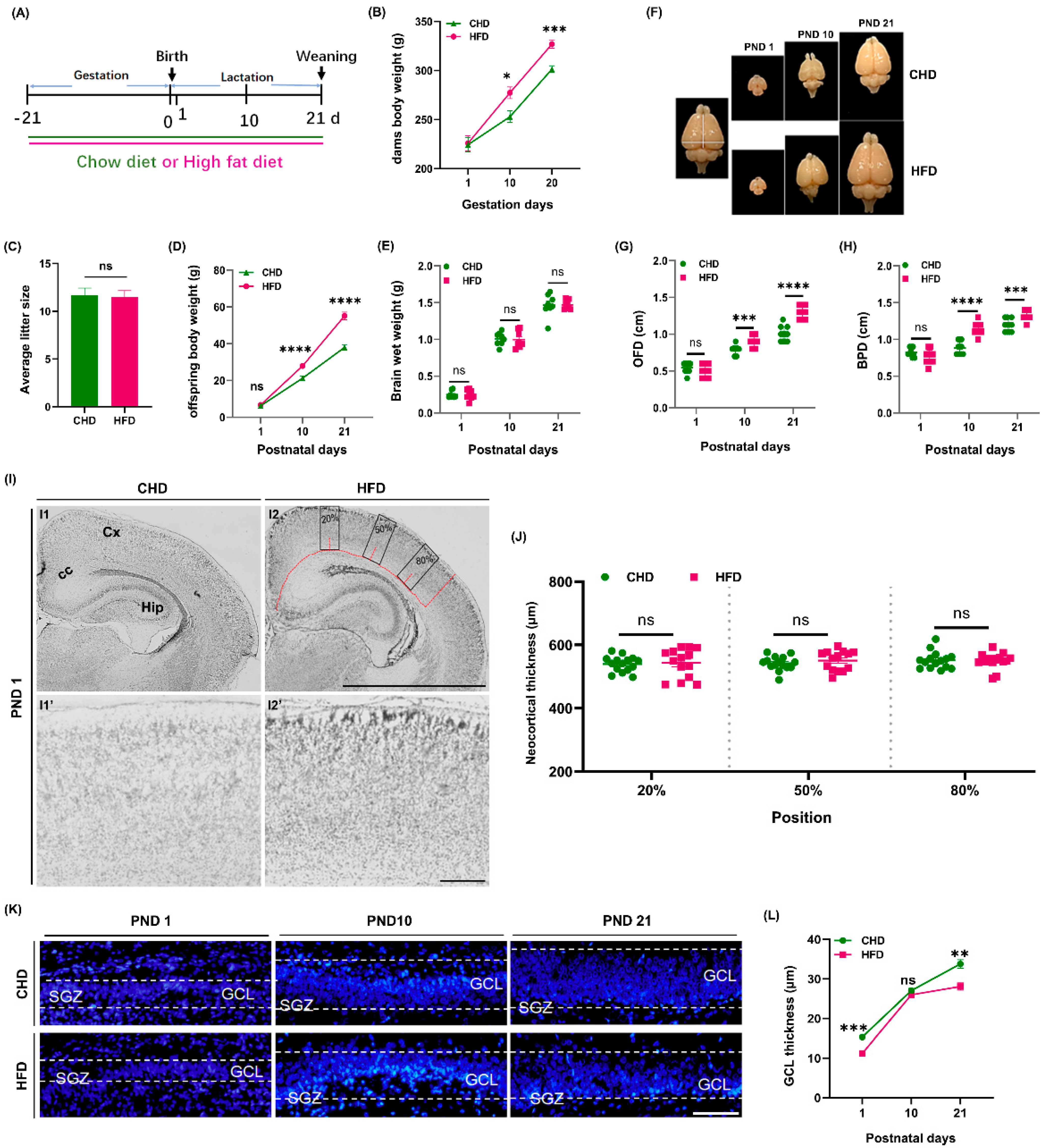
3.1. Maternal HFD Alters Offspring Brain Structural Development
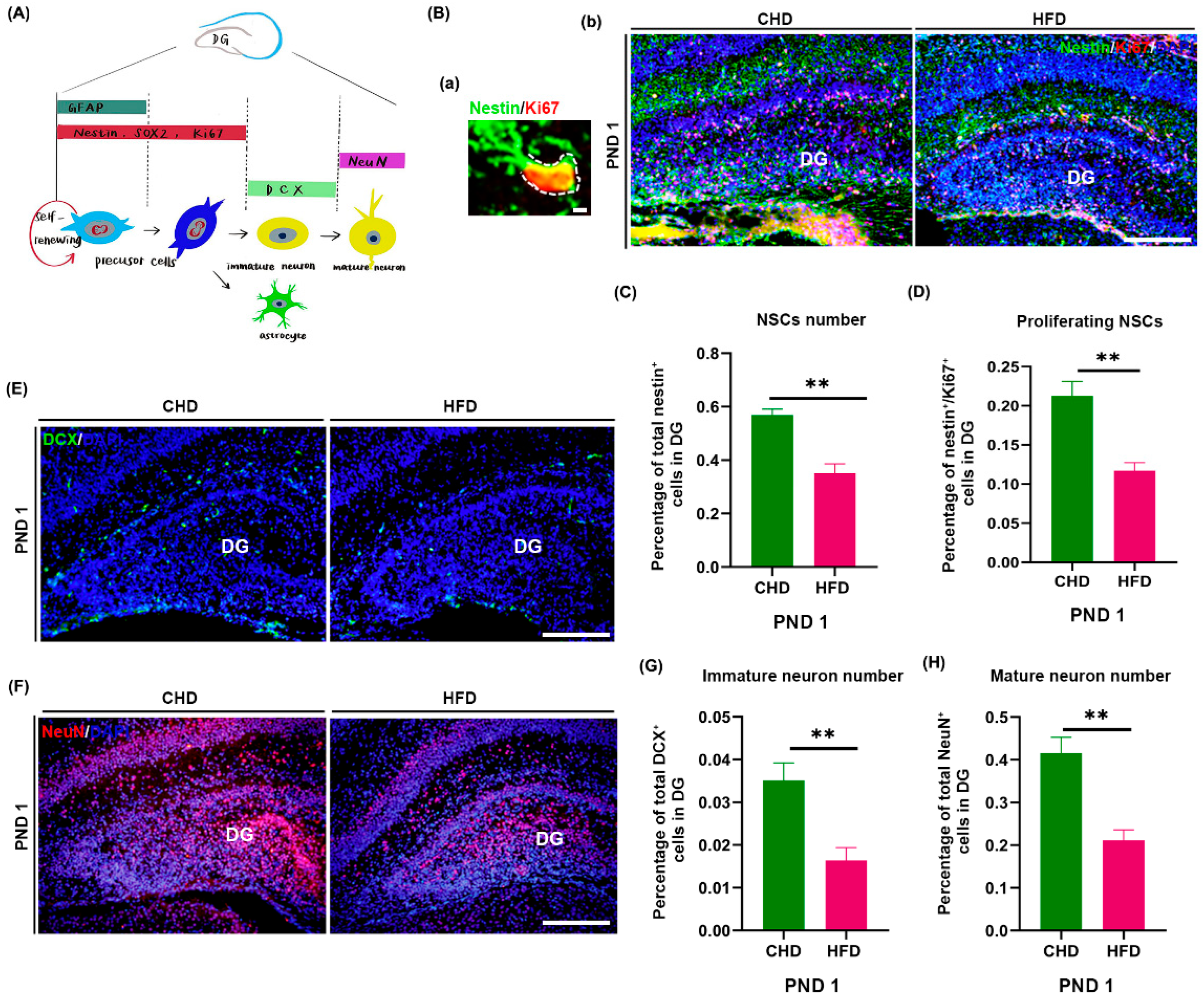
3.2. Maternal HFD during Pregnancy Attenuates Neurogenesis of Offspring DG at Birth
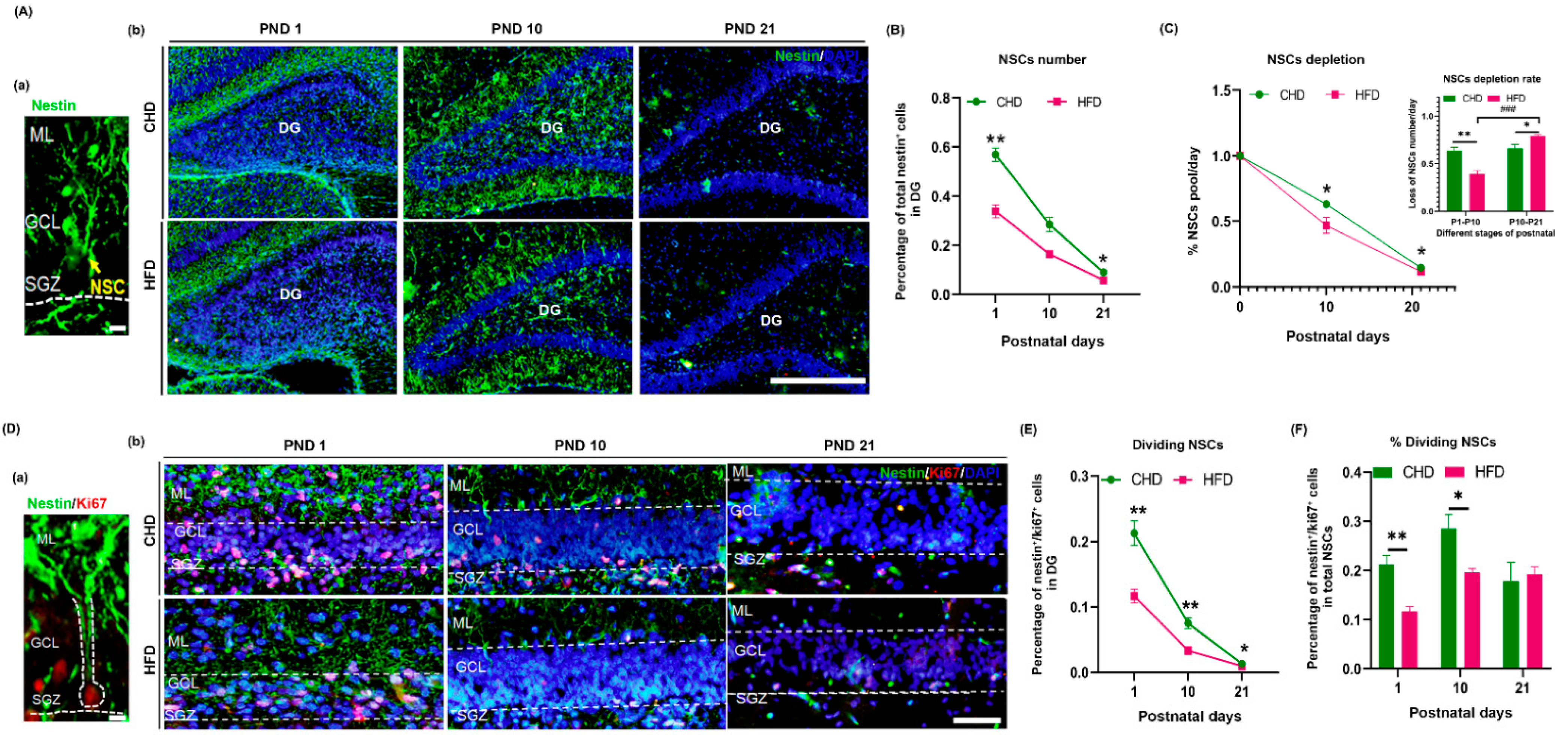
3.3. Maternal HFD Accelerates Depletion of Offspring NSCs in DG from Birth to Weaning
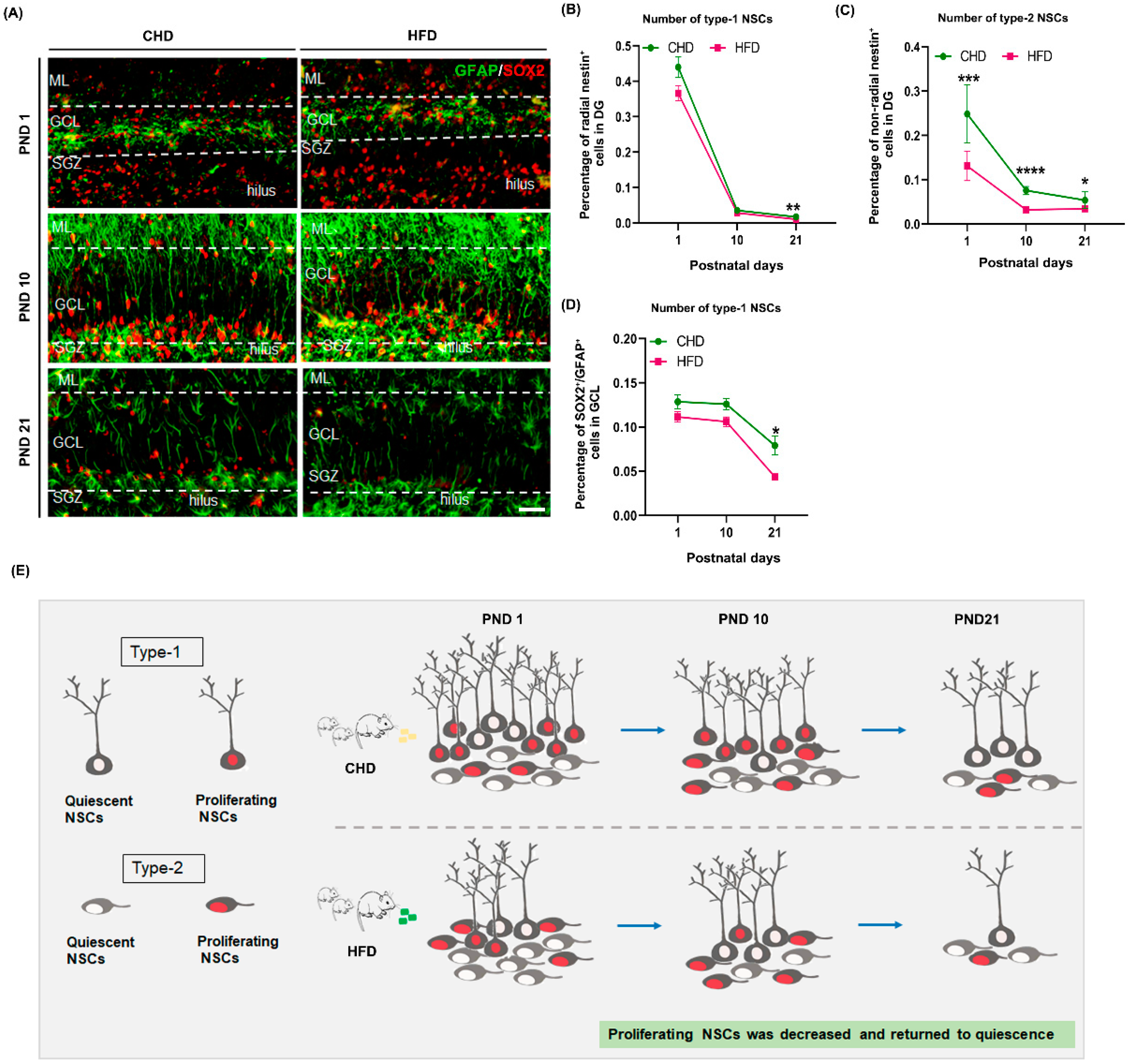
3.4. Maternal HFD Induces a Decrease in Type-2 NSCs
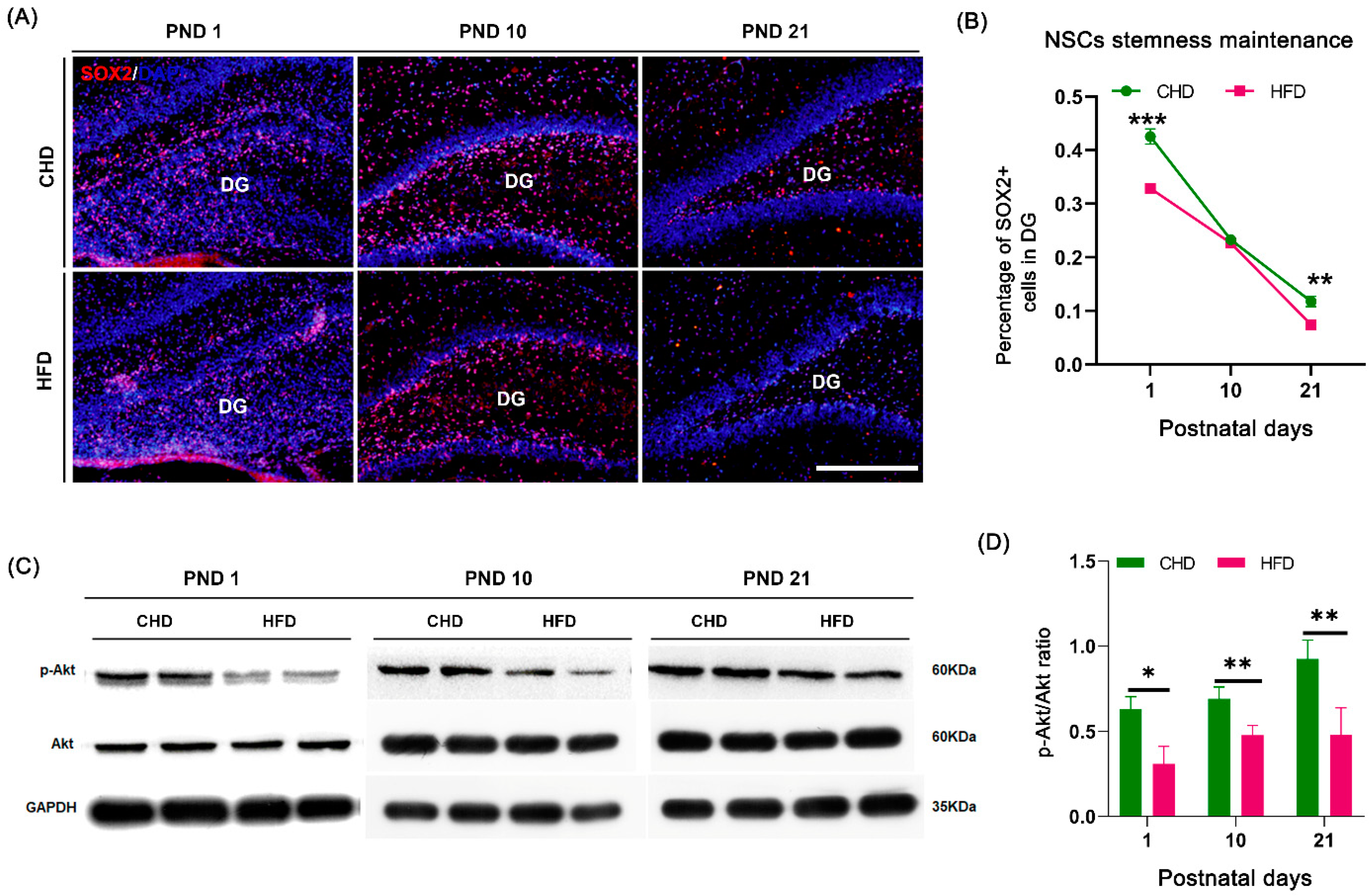
3.5. Maternal HFD Reduces Offspring NSCs Stemness Maintenance in DG
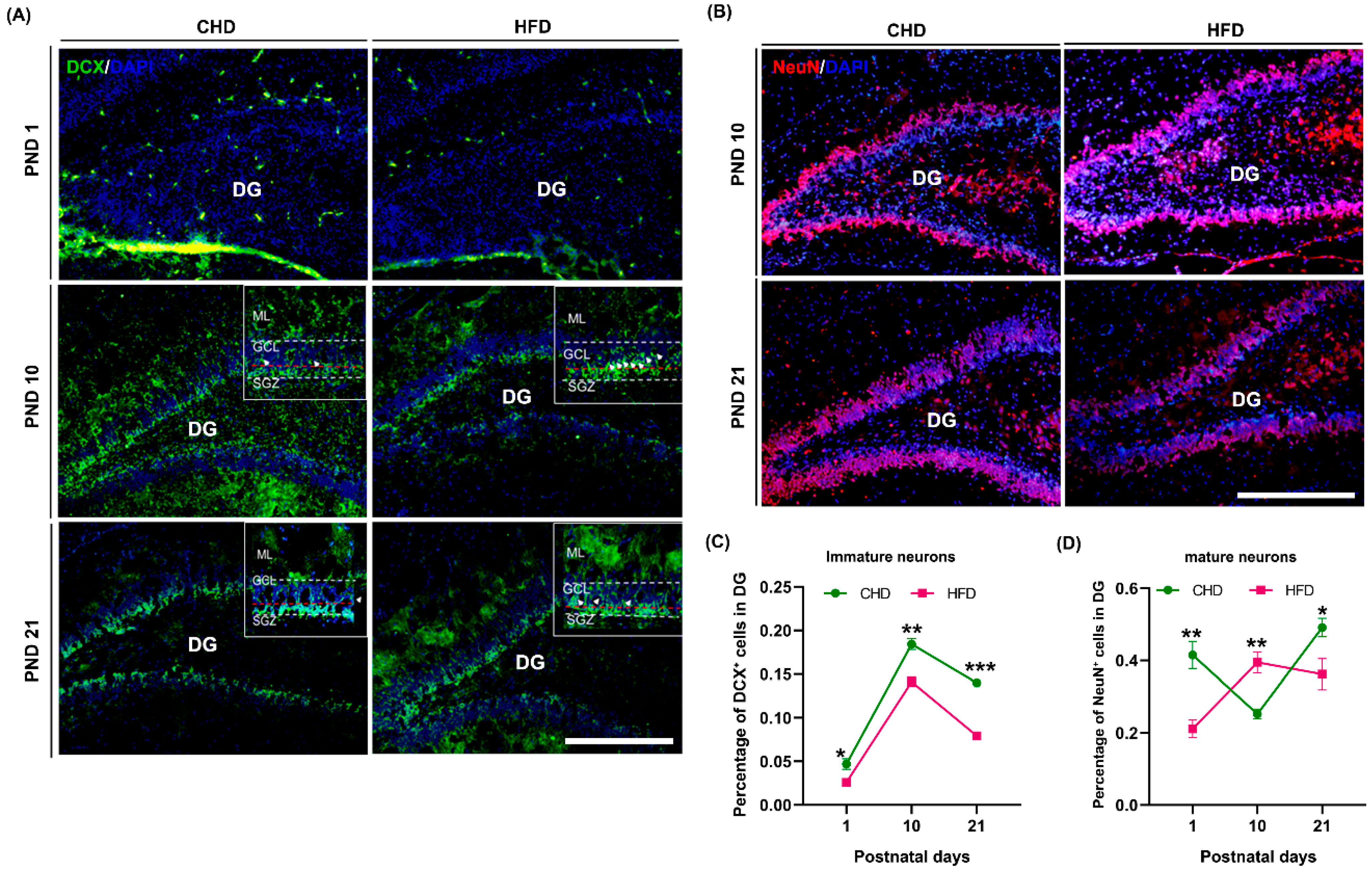
3.6. Maternal HFD Speeds Up Neuronal Differentiation in DG
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerf, M.E. High fat programming of beta cell compensation, exhaustion, death and dysfunction. Pediatr. Diabetes 2015, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Riper, K.M.; Lockard, R.; Valleau, J.C. Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm. Behav. 2015, 76, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, C.A.; Boulet, S.; Schieve, L.A.; Cohen, R.A.; Blumberg, S.J.; Yeargin-Allsopp, M.; Visser, S.; Kogan, M.D. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011, 127, 1034–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcin, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Chen, X.; Gissler, M.; Lavebratt, C. Relationship of prenatal maternal obesity and diabetes to offspring neurodevelopmental and psychiatric disorders: A narrative review. Int. J. Obes. 2020, 44, 1981–2000. [Google Scholar] [CrossRef]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Radic, T.; Friess, L.; Vijikumar, A.; Jungenitz, T.; Deller, T.; Schwarzacher, S.W. Differential postnatal expression of neuronal maturation markers in the dentate gyrus of mice and rats. Front. Neuroanat. 2017, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Tang, X.T.; Wang, Y.; Xu, H.W.; Fan, X.T. Radial Glia, the keystone of the development of the hippocampal dentate gyrus. Mol. Neurobiol. 2015, 51, 131–141. [Google Scholar] [CrossRef]
- Drew, L.J.; Fusi, S.; Hen, R. Adult neurogenesis in the mammalian hippocampus: Why the dentate gyrus? Learn. Mem. 2013, 20, 710–729. [Google Scholar] [CrossRef] [Green Version]
- Abbott, L.C.; Nigussie, F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 2020, 49, 3–16. [Google Scholar] [CrossRef]
- Lazutkin, A.; Podgorny, O.; Enikolopov, G. Modes of division and differentiation of neural stem cells. Behav. Brain Res. 2019, 374, 112118. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.A.; Su, Y.J.; Jimenez-Cyrus, D.; Patel, A.; Huang, N.; Morizet, D.; Lee, S.; Shah, R.; Ringeling, F.R.; Jain, R.; et al. A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell 2019, 177, 654–668.e15. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.; Rigo, P.; Stiehl, T.; Gaber, Z.B.; Austin, S.H.L.; Masdeu, M.D.; Edwards, A.; Urban, N.; Marciniak-Czochra, A.; Guillemot, F. Coordinated changes in cellular behavior ensure the lifelong maintenance of the hippocampal stem cell population. Cell Stem Cell 2021, 28, 863–876.e6. [Google Scholar] [CrossRef] [PubMed]
- Imayoshi, I.; Kageyama, R. The role of Notch signaling in adult neurogenesis. Mol. Neurobiol. 2011, 44, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Daynac, M.; Petritsch, C.K. Regulation of asymmetric cell division in mammalian neural stem and cancer precursor cells. Asymmetric Cell Div. Dev. Differ. Cancer 2017, 61, 375–399. [Google Scholar] [CrossRef]
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of adult neurogenesis in mammalian brain. Int. J. Mol. Sci. 2020, 21, 4869. [Google Scholar] [CrossRef]
- Chia-Hsuan, F.; Daniel Maxim, I.; Iraklis, P.; Anupam, H.; Xiaohong, Z.; Mark, S.P.; Umberto, T.; Brian, F.C.; Jingli, C.; Jason, L.; et al. Early seizure activity accelerates depletion of hippocampal neural stem cells and impairs spatial discrimination in an alzheimer’s disease model. Cell Rep. 2019, 27, 3741–3751.e4. [Google Scholar] [CrossRef]
- Speder, P.; Liu, J.; Brand, A.H. Nutrient control of neural stem cells. Curr. Opin. Cell Biol. 2011, 23, 724–729. [Google Scholar] [CrossRef]
- Tozuka, Y.; Wada, E.; Wada, K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009, 23, 1920–1934. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S. High fat diet-induced maternal obesity alters fetal hippocampal development. Int. J. Dev. Neurosci. 2009, 27, 627–633. [Google Scholar] [CrossRef] [Green Version]
- Page, K.C.; Jones, E.K.; Anday, E.K. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R527–R537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes-da-Silva, C.; Lemes, S.F.; Baliani, T.D.; Versutti, M.D.; Torsoni, M.A. Increased expression of Hes5 protein in Notch signaling pathway in the hippocampus of mice offspring of dams fed a high-fat diet during pregnancy and suckling. Int. J. Dev. Neurosci. 2015, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jiang, M.Y.; Yang, C.B.; Wu, Y.X.; Liu, Y.Z.; Cui, Y.J.; Huang, G.W. Maternal high-fat diet affects Msi/Notch/Hes signaling in neural stem cells of offspring mice. J. Nutr. Biochem. 2014, 25, 227–231. [Google Scholar] [CrossRef]
- Cortes-Alvarez, N.Y.; Vuelvas-Olmos, C.R.; Pinto-Gonzalez, M.F.; Guzman-Muniz, J.; Gonzalez-Perez, O.; Moy-Lopez, N.A. A high-fat diet during pregnancy impairs memory acquisition and increases leptin receptor expression in the hippocampus of rat offspring. Nutr. Neurosci. 2020, 25, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, Y.; Luo, J.; Yu, J.; Cheng, Y.; Wu, X.; Lin, L.; Lin, Y. Maternal high-fat diet multigenerationally impairs hippocampal synaptic plasticity and memory in male rat offspring. Endocrinology 2021, 162, bqaa214. [Google Scholar] [CrossRef]
- Sun, B.; Purcell, R.H.; Terrillion, C.E.; Yan, J.Q.; Moran, T.H.; Tamashiro, K.L.K. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 2012, 61, 2833–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or excess folic acid supply during pregnancy alter cortical neurodevelopment in mouse offspring. Cereb. Cortex 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Ge, Q.; Hu, X.; Ma, N.; Sun, M.; Zhang, L.; Cai, Z.; Tan, R.; Lu, H. Maternal high-salt diet during pregnancy impairs synaptic plasticity and memory in offspring. FASEB J. 2021, 35, e21244. [Google Scholar] [CrossRef]
- Cui, J.; Song, L.; Wang, R.; Hu, S.; Yang, Z.; Zhang, Z.; Sun, B.; Cui, W. Maternal metformin treatment during gestation and lactation improves skeletal muscle development in offspring of rat dams fed high-fat diet. Nutrients 2021, 13, 3417. [Google Scholar] [CrossRef]
- Danglot, L.; Triller, A.; Marty, S. The development of hippocampal interneurons in rodents. Hippocampus 2006, 16, 1032–1060. [Google Scholar] [CrossRef]
- Fukuda, S.; Kato, F.; Tozuka, Y.; Yamaguchi, M.; Miyamoto, Y.; Hisatsune, T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J. Neurosci. 2003, 23, 9357–9366. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, S.; Alberti, C.; Serra, L.; Meneghini, S.; Berico, P.; Bertolini, J.; Becchetti, A.; Nicolis, S.K. An early Sox2-dependent gene expression programme required for hippocampal dentate gyrus development. Open Biol. 2021, 11, 200339. [Google Scholar] [CrossRef] [PubMed]
- Favaro, R.; Valotta, M.; Ferri, A.L.; Latorre, E.; Mariani, J.; Giachino, C.; Lancini, C.; Tosetti, V.; Ottolenghi, S.; Taylor, V.; et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 2009, 12, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Piatti, V.C.; Davies-Sala, M.G.; Esposito, M.S.; Mongiat, L.A.; Trinchero, M.F.; Schinder, A.F. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J. Neurosci. 2011, 31, 7715–7728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinkle, S.N.; Schieve, L.A.; Stein, A.D.; Swan, D.W.; Ramakrishnan, U.; Sharma, A.J. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int. J. Obes. 2012, 36, 1312–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, B.R.; Hodge, R.D.; Daza, R.A.; Tripathi, P.P.; Arnold, S.J.; Millen, K.J. Intermediate progenitors support migration of neural stem cells into dentate gyrus outer neurogenic niches. Elife 2020, 9, e53777. [Google Scholar] [CrossRef]
- Youssef, M.; Krish, V.S.; Kirshenbaum, G.S.; Atsak, P.; Lass, T.J.; Lieberman, S.R.; Leonardo, E.D.; Dranovsky, A. Ablation of proliferating neural stem cells during early life is sufficient to reduce adult hippocampal neurogenesis. Hippocampus 2018, 28, 586–601. [Google Scholar] [CrossRef]
- Liu, M.; Guan, Z.; Shen, Q.; Flinter, F.; Dominguez, L.; Ahn, J.W.; Collier, D.A.; O’Brien, T.; Shen, S. Ulk4 regulates neural stem cell pool. Stem Cells 2016, 34, 2318–2331. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.L.; Song, H. Ontogeny of adult neural stem cells in the mammalian brain. Curr. Top. Dev. Biol. 2021, 142, 67–98. [Google Scholar] [CrossRef]
- Obernier, K.; Cebrian-Silla, A.; Thomson, M.; Parraguez, J.I.; Anderson, R.; Guinto, C.; Rodas Rodriguez, J.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Adult neurogenesis is sustained by symmetric self-renewal and differentiation. Cell Stem Cell 2018, 22, 221–234.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bast, L.; Calzolari, F.; Strasser, M.K.; Hasenauer, J.; Theis, F.J.; Ninkovic, J.; Marr, C. Increasing Neural Stem Cell Division Asymmetry and quiescence are predicted to contribute to the age-related decline in neurogenesis. Cell Rep. 2018, 25, 3231–3240.e8. [Google Scholar] [CrossRef] [Green Version]
- Ashton, R.S.; Conway, A.; Pangarkar, C.; Bergen, J.; Lim, K.I.; Shah, P.; Bissell, M.; Schaffer, D.V. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 2012, 15, 1399–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallucci, V.; Fidaleo, M.; Pani, G. Neural stem cells and nutrients: Poised between quiescence and exhaustion. Trends Endocrinol. Metab. 2016, 27, 756–769. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [Green Version]
- Paling, N.R.; Wheadon, H.; Bone, H.K.; Welham, M.J. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 2004, 279, 48063–48070. [Google Scholar] [CrossRef] [Green Version]
- Gurska, L.M.; Ames, K.; Gritsman, K. Signaling pathways in leukemic stem cells. Adv. Exp. Med. Biol. 2019, 1143, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Peltier, J.; Conway, A.; Keung, A.J.; Schaffer, D.V. Akt increases sox2 expression in adult hippocampal neural progenitor cells, but increased sox2 does not promote proliferation. Stem Cells Dev. 2011, 20, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 2007, 1, 515–528. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, F.; Lent, R.; Herculano-Houzel, S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc. Natl. Acad. Sci. USA 2009, 106, 14108–14113. [Google Scholar] [CrossRef] [Green Version]
- Stein-Behrens, B.; Mattson, M.P.; Chang, I.; Yeh, M.; Sapolsky, R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J. Neurosci. 1994, 14, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Vaahtokari, A.; Enikolopov, G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 2006, 103, 8233–8238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robb, J.L.; Messa, I.; Lui, E.; Yeung, D.; Thacker, J.; Satvat, E.; Mielke, J.G. A maternal diet high in saturated fat impairs offspring hippocampal function in a sex-specific manner. Behav. Brain Res. 2017, 326, 187–199. [Google Scholar] [CrossRef]
- Glendining, K.A.; Higgins, M.B.A.; Fisher, L.C.; Jasoni, C.L. Maternal obesity modulates sexually dimorphic epigenetic regulation and expression of leptin receptor in offspring hippocampus. Brain Behav. Immun. 2020, 88, 151–160. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; An, J.; Ge, Q.; Sun, M.; Zhang, Z.; Cai, Z.; Tan, R.; Ma, T.; Lu, H. Maternal High-Fat Diet Reduces Type-2 Neural Stem Cells and Promotes Premature Neuronal Differentiation during Early Postnatal Development. Nutrients 2022, 14, 2813. https://doi.org/10.3390/nu14142813
Hu X, An J, Ge Q, Sun M, Zhang Z, Cai Z, Tan R, Ma T, Lu H. Maternal High-Fat Diet Reduces Type-2 Neural Stem Cells and Promotes Premature Neuronal Differentiation during Early Postnatal Development. Nutrients. 2022; 14(14):2813. https://doi.org/10.3390/nu14142813
Chicago/Turabian StyleHu, Xiaoxuan, Jing An, Qian Ge, Meiqi Sun, Zixuan Zhang, Zhenlu Cai, Ruolan Tan, Tianyou Ma, and Haixia Lu. 2022. "Maternal High-Fat Diet Reduces Type-2 Neural Stem Cells and Promotes Premature Neuronal Differentiation during Early Postnatal Development" Nutrients 14, no. 14: 2813. https://doi.org/10.3390/nu14142813
APA StyleHu, X., An, J., Ge, Q., Sun, M., Zhang, Z., Cai, Z., Tan, R., Ma, T., & Lu, H. (2022). Maternal High-Fat Diet Reduces Type-2 Neural Stem Cells and Promotes Premature Neuronal Differentiation during Early Postnatal Development. Nutrients, 14(14), 2813. https://doi.org/10.3390/nu14142813





