Assessing the Health Risk and the Metal Content of Thirty-Four Plant Essential Oils Using the ICP-MS Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials and Preparation
2.2. Chemical and Reagents
2.3. Analytical Performance of the Method
2.4. Characterization of Possible Health Risks Associated with Essential Oil Ingestion
CDI = (C × IR × EF × ED)/(BW × AT)
2.5. Statistical Analysis
3. Results
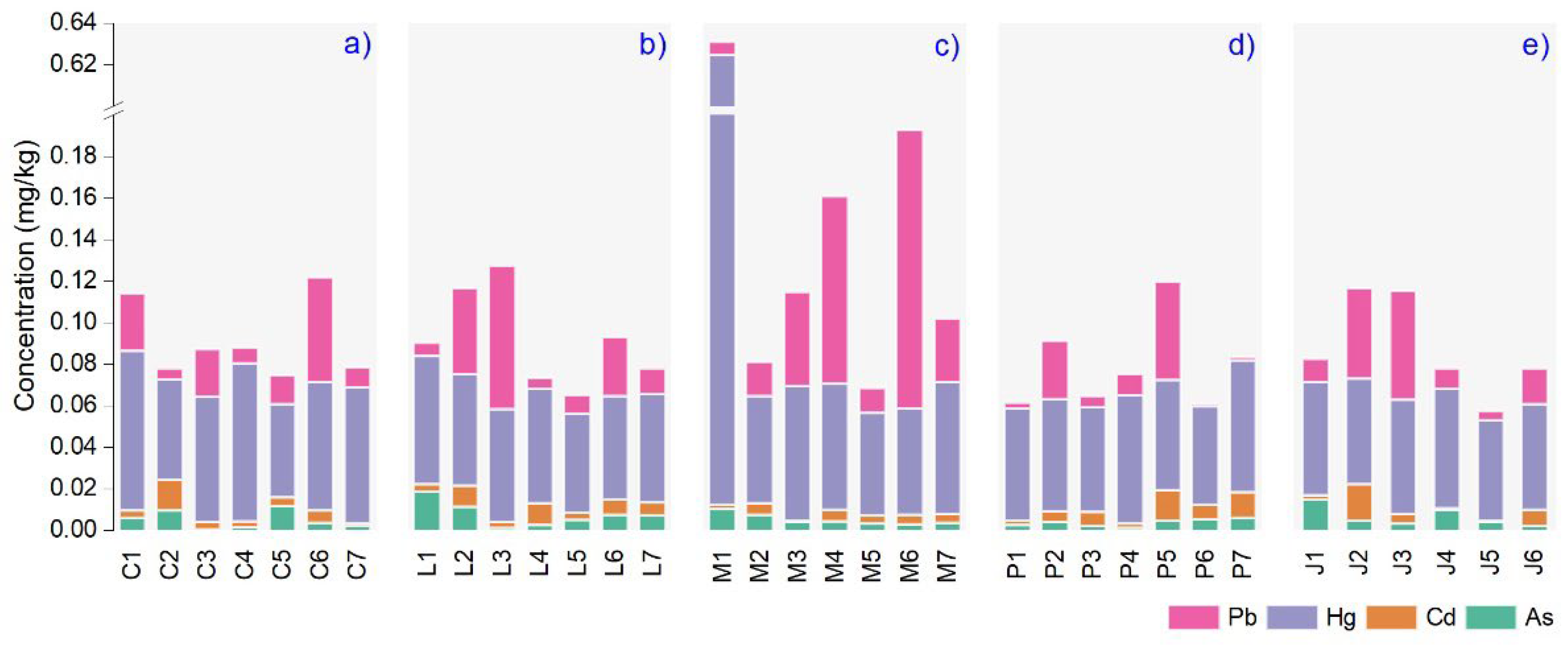
3.1. Toxic Metal Content in Plant Essential Oils
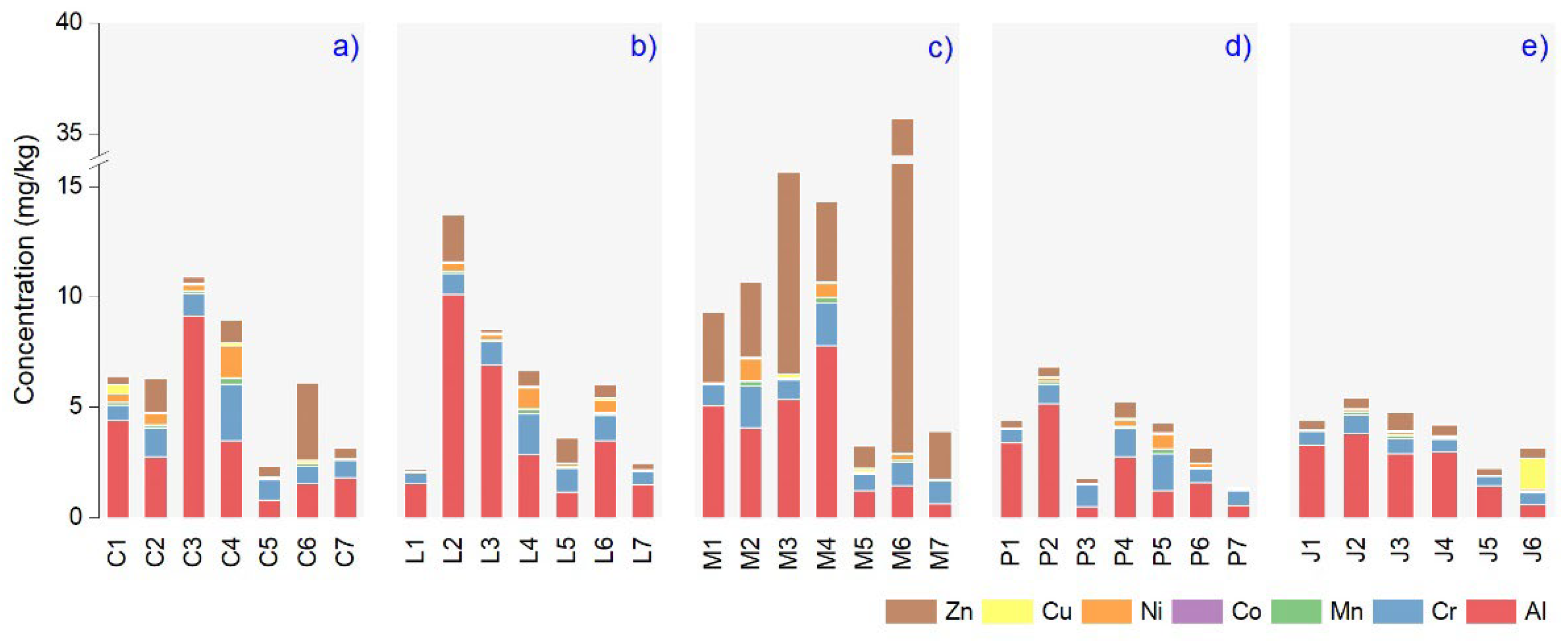
3.2. Microelements Content in Plant Essential Oils
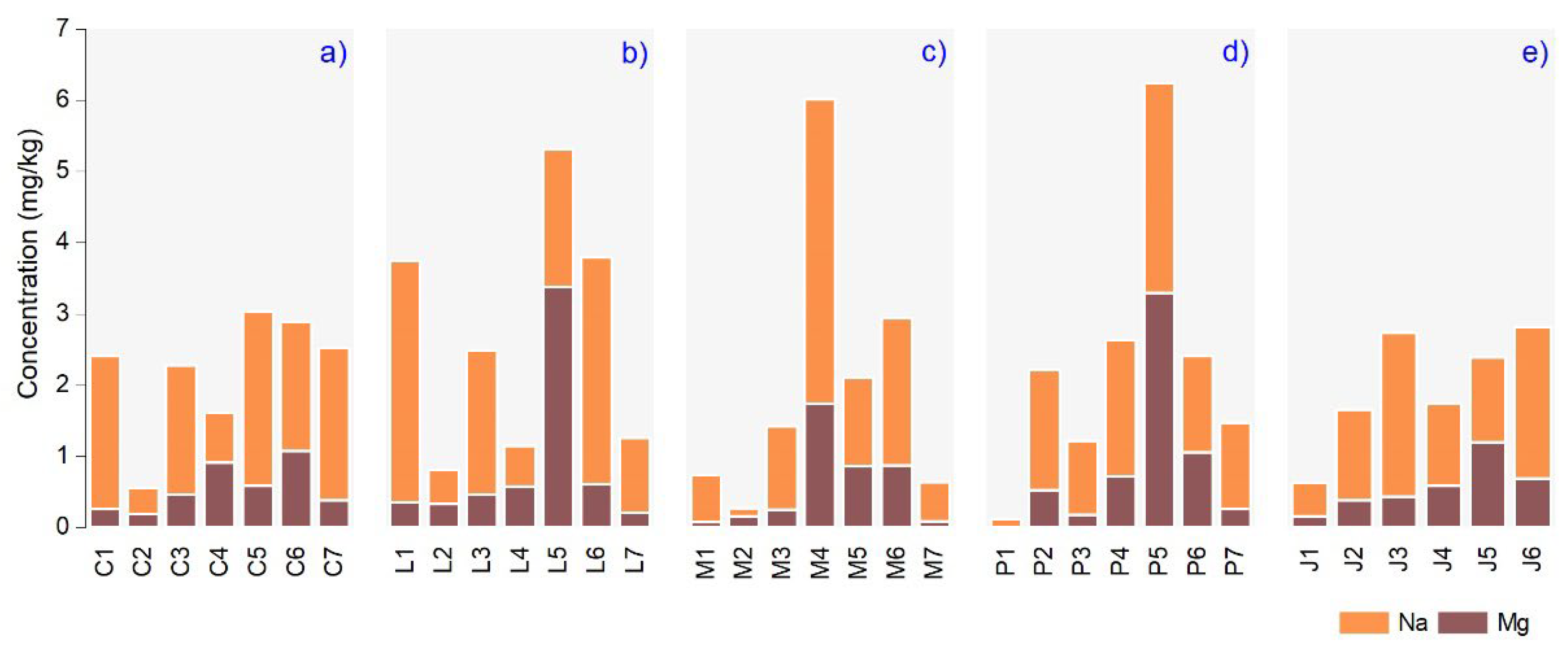
3.3. Macroelements Content in Plant Essential Oils
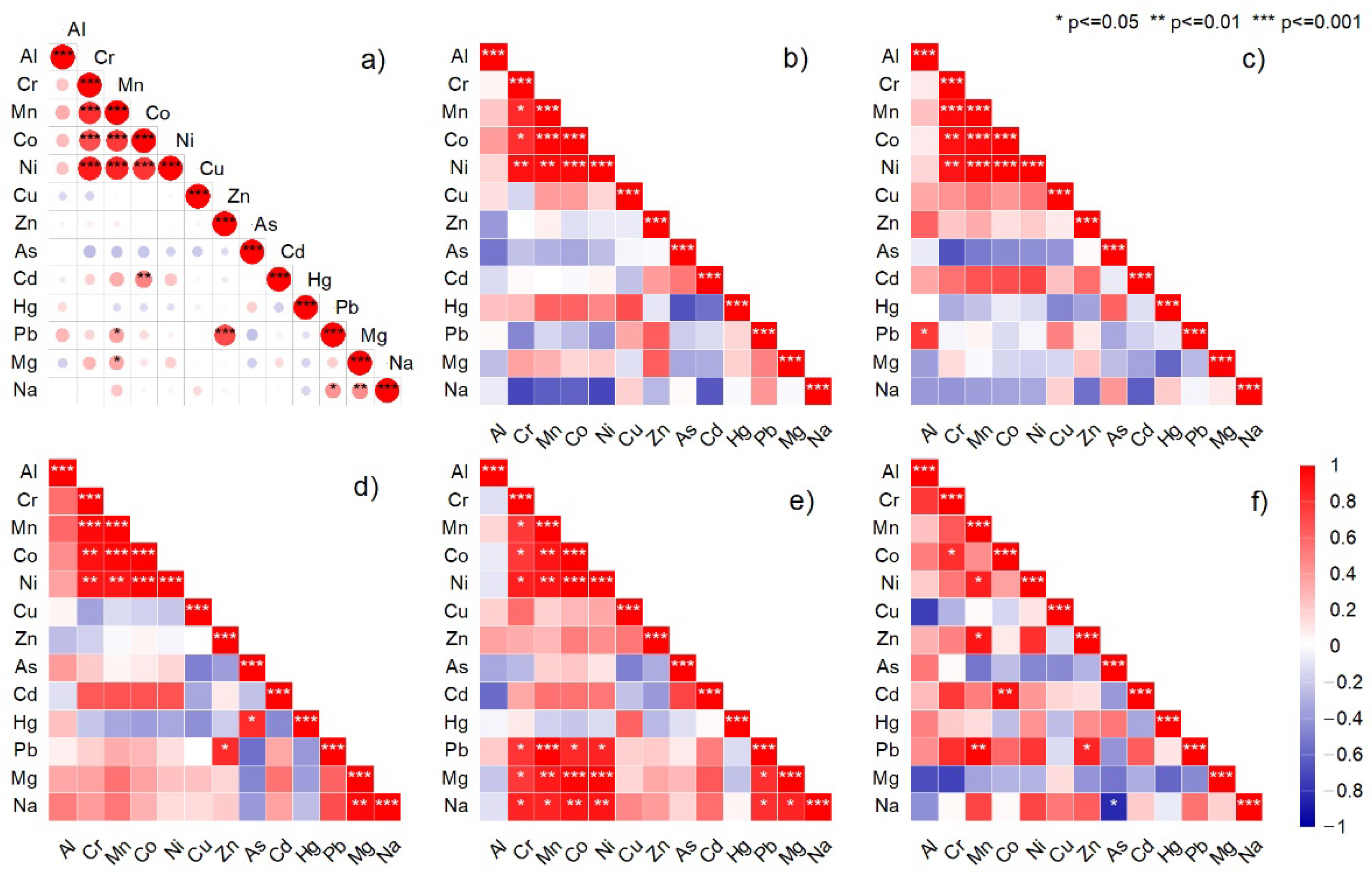
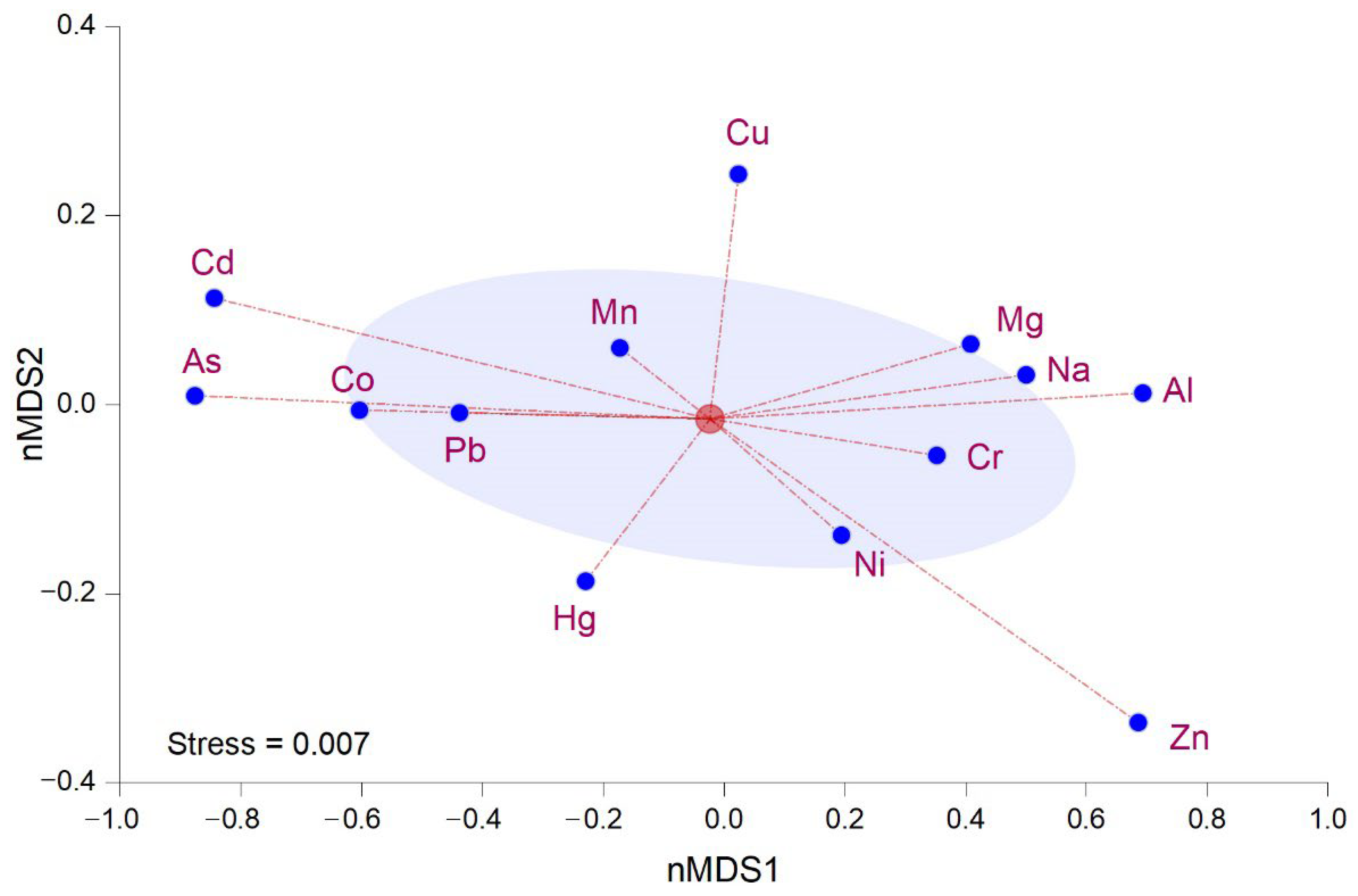
3.4. Multivariate Analysis
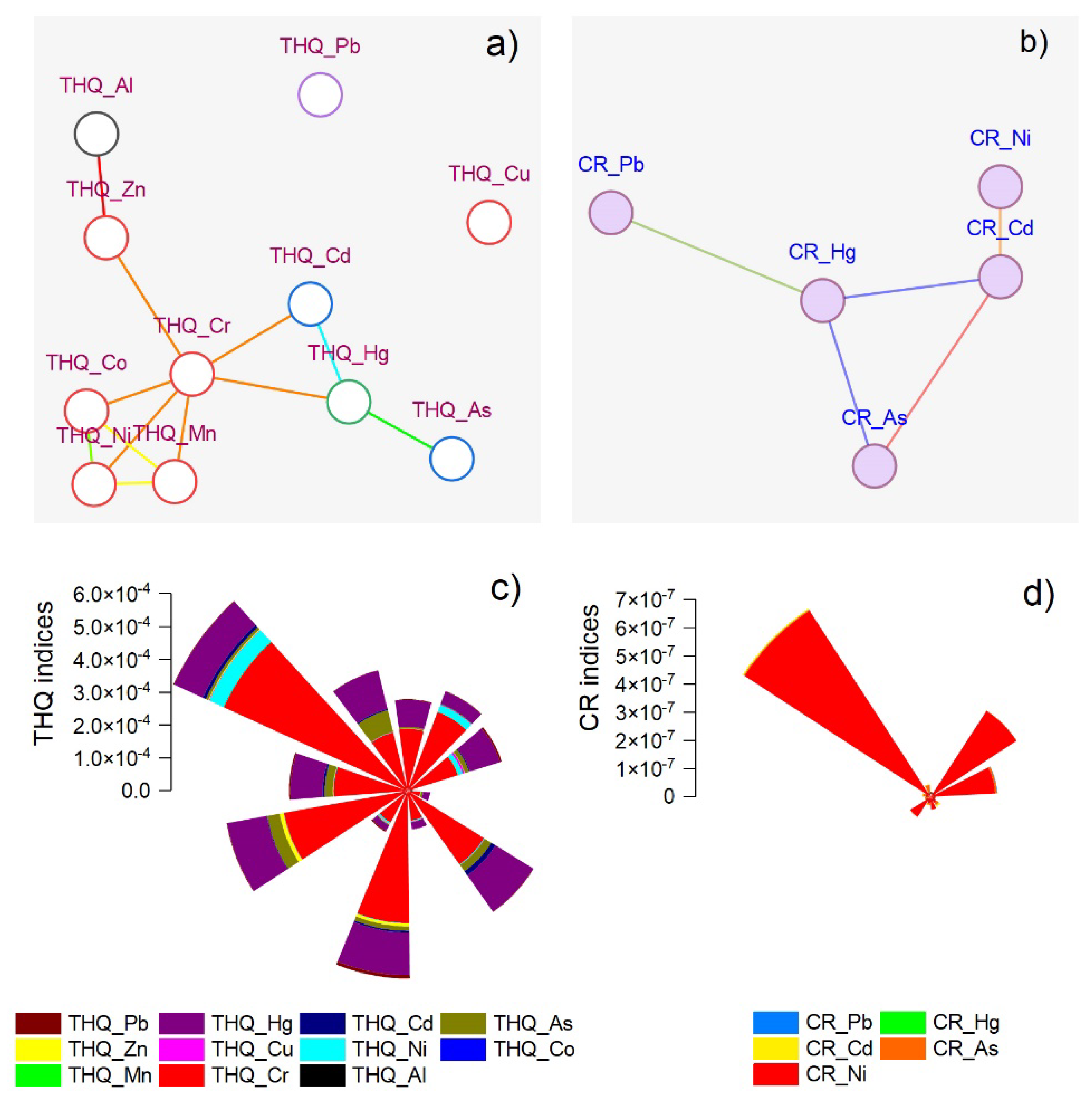
3.5. Health Risk Assessment
4. Discussions
5. Final Consideration and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.; Horhat, F. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Patiño-Bayona, W.R.; Nagles Galeano, L.J.; Bustos Cortes, J.J.; Ávila, W.A.D.; Herrera Daza, E.; Suárez, L.E.C.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Effects of essential oils from 24 plant species on Sitophilus zeamais Motsch (Coleoptera, Curculionidae). Insects 2021, 12, 532. [Google Scholar] [CrossRef]
- Prins, C.L.; Vieira, I.J.C.; Freitas, S.P. Growth regulators and essential oil production. Braz. J. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Camilo, C.J.; Alves Nonato, C.D.F.; Galvão-Rodrigues, F.F.; Costa, W.D.; Clemente, G.G.; Sobreira Macedo, M.A.C.; Galvão Rodrigues, F.F.; Da Costa, J.G.M. Acaricidal activity of essential oils: A review. Trends Phytochem. Res. 2017, 1, 183–198. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2013, 54, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of dietary supplemen-tation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2018, 229, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chong, C.H.; Chua, B.L.; Figiel, A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019, 12, 450–476. [Google Scholar] [CrossRef]
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation. Potential Essent. Oils 2018, 107–127. [Google Scholar] [CrossRef] [Green Version]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal drugs: Standards and regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef]
- Sana, S.S.; Li, H.; Zhang, Z.; Sharma, M.; Usmani, Z.; Hou, T.; Netala, V.R.; Wang, X.; Gupta, V.K. Recent advances in essential oils-based metal nanoparticles: A review on recent developments and biopharmaceutical applications. J. Mol. Liq. 2021, 333, 115951. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Singh, R.; Ahirwar, N.K.; Tiwari, J.; Pathak, J. Review on sources and effect of heavy metal in soil: Its bioremediation. Int. J. Res. Appl. Nat. Soc. Sci. 2018, 1–22. [Google Scholar]
- El-Boshy, M.E.; Refaat, B.; Qasem, A.H.; Khan, A.; Ghaith, M.; Almasmoum, H.; Mahbub, A.; Almaimani, R.A. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res. 2019, 26, 22736–22746. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.F.; Hu, F.; Thakur, K.; Li, X.L.; Zhang, Y.S.; Wei, Z.J. Comparison of antibacterial effects and fumigant toxicity of essen-tial oils extracted from different plants. Ind. Crops Prod. 2018, 124, 192–200. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Nazem, H.; Mousavi, S.M. Green synthesis of magnetic nanoparticles using Satureja hortensis essen-tial oil toward superior antibacterial/fungal and anticancer performance. BioMed Res. Int. 2021, 2021, 8822645. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimum basilicum L.). Ind. Crops Prod. 2019, 138, 111584. [Google Scholar] [CrossRef]
- Dinu, C.; Gheorghe, S.; Tenea, A.G.; Stoica, C.; Vasile, G.-G.; Popescu, R.L.; Serban, E.A.; Pascu, L.F. Toxic Metals (As, Cd, Ni, Pb) impact in the most common medicinal plant (Mentha piperita). Int. J. Environ. Res. Public Health 2021, 18, 3904. [Google Scholar] [CrossRef]
- Ni, Z.; Chen, Z.; Yu, Q.; Sun, X.; Tang, F. Microwave-assisted digestion for trace elements analysis of tree nut oil: Evaluation of residual carbon content. Spectrosc. Lett. 2018, 51, 518–523. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-de Córdova, M.L.; Domínguez-Vidal, A.R.M.A.; Ruiz-Medina, A. In-vestigation by ICP-MS of trace element levels in vegetable edible oils produced in Spain. Food Chem. 2011, 127, 1257–1262. [Google Scholar] [CrossRef]
- Ni, Z.; Tang, F.; Liu, Y.; Shen, D.; Mo, R. Multielemental Analysis of Camellia Oil by Microwave Dry Ashing and Inductively Coupled Plasma Mass Spectrometry. Anal. Lett. 2015, 48, 1777–1786. [Google Scholar] [CrossRef]
- Pereira, J.S.; Moraes, D.P.; Antes, F.G.; Diehl, L.O.; Santos, M.F.; Guimarães, R.C.; Fonseca, T.C.; Dressler, V.L.; Flores, É.M. Determination of metals and metalloids in light and heavy crude oil by ICP-MS after digestion by microwave-induced combustion. Microchem. J. 2010, 96, 4–11. [Google Scholar] [CrossRef]
- Kara, D.; Fisher, A.; Hill, S. Detergentless ultrasound-assisted extraction of trace elements from edible oils using lipase as an extractant. Talanta 2015, 144, 219–225. [Google Scholar] [CrossRef]
- Soós, Á.; Bódi, É.; Várallyay, S.; Molnár, S.; Kovács, B. Microwave-assisted sample preparation of Hungarian raw propolis in quartz vessels and element analysis by ICP-OES and ICP-MS for geographical identification. Talanta 2021, 233, 122613. [Google Scholar] [CrossRef]
- US-EPA. Risk Based Concentration Table. 2010. Available online: http://www.epa.gov/eg3hwmd/risk/human/index.html (accessed on 15 December 2020).
- FAO/WHO. Guidelines for the Simple Evaluation of Dietary Exposure to Food Additives CAC/GL3-1989 Adopted 1989. Revi-sion 2014 (Formerly Guidelines for the Simple Evaluation of Food Additive Intake). 2014. Available online: http://www.fao.org/input/download/standards/6/cxg_003e.pdf (accessed on 17 December 2020).
- European Directorate for the Quality of Medicines. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2007. [Google Scholar]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Gupta, U.C.; Gupta, S.C. Sources and deficiency diseases of mineral nutrients in human health and nutrition: A review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Official Journal of the European Union: Commission Regulation (EC) No. 420/2011 of 29 April 2011, Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32011R0420 (accessed on 1 May 2022).
- WHO. Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. 2007. Available online: http://apps.who.int/medicinedocs/documents/s14878e.pdf (accessed on 1 May 2022).
- Dwivedi, S.; Khan, M.; Srivastava, S.K.; Syamasunnder, K.V.; Srivastava, A. Essential oil composition of different accessions of Mentha × piperita L. grown on the northern plains of India. Flavour Fragr. J. 2004, 19, 437–440. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bharat, G.K.; Šebková, K.; Scheringer, M. Implementation of the Minamata Convention to manage mercury pollution in India: Challenges and opportunities. Environ. Sci. Eur. 2019, 31, 96. [Google Scholar] [CrossRef]
- Gharib, F.A.; Mansour, K.H.; Ahmed, E.Z.; Galal, T.M. Heavy metals concentration, and antioxidant activity of the essential oil of the wild mint (Mentha longifolia L.) in the Egyptian watercourses. Int. J. Phytoremediat. 2020, 23, 641–651. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, M.; Tatsuta, N.; Izumo, K.; Phan, P.T.; Vu, L.D.; Yamamoto, M.; Nakamura, M.; Nakai, K.; Murata, K. Health Impacts and Biomarkers of Prenatal Exposure to Methylmercury: Lessons from Minamata, Japan. Toxics 2018, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iordache, A.M.; Nechita, C.; Voica, C.; Pluháček, T.; Schug, K.A. Climate change extreme and seasonal toxic metal occur-rence in Romanian freshwaters in the last two decades—Case study and critical review. NPJ Clean Water 2022, 5, 2. [Google Scholar] [CrossRef]
- Hoaghia, M.-A.; Cadar, O.; Moisa, C.; Roman, C.; Kovacs, E. Heavy metals and health risk assessment in vegetables grown in the vicinity of a former non-metallic facility located in Romania. Environ. Sci. Pollut. Res. 2022, 29, 40079–40093. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Craker, L.E.; Xing, B. Effects of Cd, Pb, and Cu on growth and essential oil contents in dill, peppermint, and basil. Environ. Exp. Bot. 2006, 58, 9–16. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Nielsen, N.E. Effect of heavy metals on peppermint and cornmint. Plant Soil 1996, 178, 59–66. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Bert, V.; Perlein, A.; Tisserant, B.; Ferrant, P.; Sahraoui, A.L.H. In situ cultivation of aromatic plant species for the phytomanagement of an aged-trace element polluted soil: Plant biomass improvement options and techno-economic assessment of the essential oil production channel. Sci. Total Environ. 2021, 789, 147944. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- European Food Safety Authority (EFSA). Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles. EFSA J. 2009, 7, 1015. [Google Scholar]
- Şenkal, B.C.; Uskutoğlu, T.; Cesur, C.; Özavci, V.; Doğan, H. Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. grown in culture conditions. Turk. J. Agric. For. 2019, 43, 395–404. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R. Review on metal packaging: Materials, forms, food applications, safety and recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Kalbouneh, S.R.; Bernstein, N.; Dudai, N. Chemical profile and bioactive properties of the essential oil isolated from Clinopodium serpyllifolium (M.Bieb.) Kuntze growing in Palestine. Ind. Crops Prod. 2018, 124, 617–625. [Google Scholar] [CrossRef]
- Zeiner, M.; Cindrić, I.J.; Kandler, W.; Stingeder, G. Trace determination of skin-irritating metals in tea tree oil by GFAAS. Microchem. J. 2018, 136, 101–105. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef]
- US-EPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites. OSWER 9355/4–24. Office of Emergency and Remedial Response, Washington, DC. 2002. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/91003IJK.txt?ZyActionD (accessed on 17 December 2020).
- Hayyat, M.S.; Adnan, M.; Awais, M.; Bilal, H.M.; Khan, B.; Rahman, H.A. Effect of heavy metal (Ni) on plants and soil: A review. Int. J. Appl. Res. 2020, 6, 313–318. [Google Scholar]
- Mohajer, A.; Baghani, A.N.; Sadighara, P.; Ghanati, K.; Nazmara, S. Determination and health risk assessment of heavy metals in imported rice bran oil in Iran. J. Food Compos. Anal. 2019, 86, 103384. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Arfi, N.; Khatoon, K.; Alim, F. Zinc Malnutrition in Children and Its Consequences on Health. In Microbial Biofertilizers and Micronutrient Availability; Springer: Cham, Switzerland, 2021; pp. 35–67. [Google Scholar] [CrossRef]
- Messaoudi, M.; Rebiai, A.; Sawicka, B.; Atanassova, M.; Ouakouak, H.; Larkem, I.; Egbuna, C.; Awuchi, C.G.; Boubekeur, S.; Ferhat, M.A.; et al. Effect of Extraction Methods on Polyphenols, Flavonoids, Mineral Elements, and Biological Activities of Essential Oil and Extracts of Mentha pulegium L. Molecules 2021, 27, 11. [Google Scholar] [CrossRef]

| Species | COD | Producer | Origin | ∑metals | Species | COD | Producer | Origin | ∑metals |
|---|---|---|---|---|---|---|---|---|---|
| Thymus vulgaris L. | C1 | Adams | India | 8.9075 | Lavandula augustifolia Mill. | L1 | Adams | India | 6.0237 |
| Satureja hortensis L. | C2 | Hypericum | Romania | 6.9294 | Lavandula augustifolia Mill. | L2 | Hypericum | Romania | 14.6322 |
| Satureja hortensis L. | C3 | Steaua Divina | Romania | 13.2498 | Lavandula augustifolia Mill. | L3 | Steaua Divina | Romania | 11.1439 |
| Thymus vulgaris L. | C4 | Doterra | Salt Lake City, UT, USA | 10.6565 | Lavandula augustifolia Mill. | L4 | Doterra | Salt Lake City, UT, USA | 7.8597 |
| Thymus vulgaris L. | C5 | Herbalsana | UE | 5.4272 | Lavandula augustifolia Mill. | L5 | Herbalsana | UE | 8.9778 |
| Thymus vulgaris L. | C6 | Solaris | Romania | 9.0870 | Lavandula hybrida | L6 | Solaris | Romania | 9.9007 |
| Thymus vulgaris L. | C7 | Fares | Romania | 5.7259 | Lavandula augustifolia Mill. | L7 | Fares | NaN | 3.7830 |
| Mentha × pipperita L. | M1 | Adams | India | 10.6569 | Pinus sylvestris L. | P1 | Adams | Austria | 4.5618 |
| Mentha × pipperita L. | M2 | Hypericum | Romania | 10.9964 | Pinus sylvestris L. | P2 | Hypericum | Romania | 9.1167 |
| Mentha × pipperita L. | M3 | Steaua Divina | Romania | 17.1625 | Pinus sylvestris L. | P3 | Steaua Divina | NaN | 3.0284 |
| Mentha × pipperita L. | M4 | Doterra | Salt Lake City, UT, USA | 20.4874 | Pinus sylvestris L. | P4 | Doterra | Salt Lake City, UT, USA | 7.9497 |
| Mentha × pipperita L. | M5 | Herbalsana | UE | 5.4209 | Pinus sylvestris L. | P5 | Herbalsana | UE | 10.6651 |
| Mentha × pipperita L. | M6 | Solaris | Romania | 38.8149 | Pinus sylvestris L. | P6 | Solaris | NaN | 5.5946 |
| Mentha × pipperita L. | M7 | Fares | Romania | 4.6063 | Pinus sylvestris L. | P7 | Fares | Romania | 2.8702 |
| Juniperus communis L. | J1 | Adams | Croatia | 5.0788 | |||||
| Juniperus communis L. | J2 | Hypericum | Romania | 7.1587 | |||||
| Juniperus communis L. | J3 | Steaua Divina | Romania | 7.6018 | |||||
| Juniperus communis L. | J4 | Doterra | Salt Lake City, UT, USA | 5.9878 | |||||
| Juniperus communis L. | J5 | Herbalsana | UE | 4.6808 | |||||
| Juniperus communis L. | J6 | Solaris | NaN | 6.0233 |
| CDI—Chronic Daily Intake (µg/kg bw/day) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Al | Cr | Mn | Co | Ni | Cu | Zn | As | Cd | Hg | Pb |
| Thyme | 0.00313 | 0.00047 | 9.18 × 10−5 | 1.55 × 10−5 | 27.3958 × 10−5 | 0.000268 | 0.000291 | 4.28 × 10−6 | 2.41 × 10−6 | 5.46 × 10−5 | 1.97 × 10−5 |
| Thymus vulgaris | 0.00105 | 0.00077 | 7.07 × 10−5 | 1.17 × 10−5 | 43.3903 × 10−5 | 3.85 × 10−5 | 0.000322 | 4.34 × 10−7 | 7.91 × 10−7 | 2.29 × 10−5 | 2.21 × 10−6 |
| Thymus vulgaris | 0.00128 | 0.00056 | 1.86 × 10−5 | 2.29 × 10−6 | 2.4002 × 10−6 | 2.99 × 10−5 | 0.000336 | 1.35 × 10−6 | 7.33 × 10−7 | 4.66 × 10−5 | 6.9 × 10−6 |
| Lavandula augustifolia | 0.00171 | 0.00055 | 1.94 × 10−5 | 3.53 × 10−6 | 1.42882 × 10−5 | 1.29 × 10−5 | 0.000132 | 2.05 × 10−5 | 3.72 × 10−6 | 6.88 × 10−5 | 6.76 × 10−6 |
| Lavandula augustifolia | 0.00285 | 0.00183 | 0.000178 | 4.64 × 10−5 | 93.8768 × 10−5 | 5.25 × 10−5 | 0.000727 | 2.34 × 10−6 | 1.05 × 10−5 | 5.49 × 10−5 | 5.42 × 10−6 |
| Lavandula augustifolia | 0.00158 | 0.00067 | 1.61 × 10−5 | 3.38 × 10−6 | 1.97719 × 10−5 | 3.03 × 10−5 | 0.000309 | 7.45 × 10−6 | 6.62 × 10−6 | 5.61 × 10−5 | 1.31 × 10−5 |
| Mentha × pipperita | 0.00571 | 0.00113 | 3.72 × 10−5 | 4.43 × 10−6 | 2.61226 × 10−6 | 2.11 × 10−5 | 0.003654 | 1.16 × 10−5 | 2.23 × 10−6 | 6.95 × 10−5 | 7.28 × 10−6 |
| Mentha × pipperita | 0.00129 | 0.00032 | 4.17 × 10−5 | 3.63 × 10−6 | 10.0599 × 10−5 | 1.15 × 10−5 | 0.000609 | 6.75 × 10−7 | 9.54 × 10−7 | 1.01 × 10−5 | 1.5 × 10−5 |
| Mentha × pipperita | 0.00072 | 0.00121 | 2.54 × 10−5 | 4.55 × 10−6 | 2.75431 × 10−5 | 1.66 × 10−5 | 0.002512 | 4.04 × 10−6 | 4.77 × 10−6 | 7.41 × 10−5 | 3.53 × 10−5 |
| Pinus sylvestris | 0.00057 | 0.00027 | 1.3 × 10−5 | 2.09 × 10−6 | 5.38326 × 10−5 | 2.56 × 10−5 | 0.000155 | 1.97 × 10−7 | 4.21 × 10−7 | 1.28 × 10−5 | 2.14 × 10−6 |
| Pinus sylvestris | 0.00063 | 0.00082 | 3.58 × 10−5 | 5.91 × 10−6 | 2.92926 × 10−5 | 3.4 × 10−5 | 5.51 × 10−5 | 7.07 × 10−6 | 1.5 × 10−5 | 7.74 × 10−5 | 1.95 × 10−6 |
| Juniperus communis | 0.00062 | 0.00012 | 9.52 × 10−6 | 1.17 × 10−6 | 1.79451 × 10−5 | 8.47 × 10−6 | 9.72 × 10−5 | 2.05 × 10−6 | 8.31 × 10−8 | 1.2 × 10−5 | 2.07 × 10−6 |
| PTDI (µg/kg bw/day) * | 142.86 | 300.00 | 66.66 | - | 2.80 | 50–500 | 300–1000 | 2.14 | 0.83 | 0.57 | 3.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordache, A.M.; Nechita, C.; Voica, C.; Roba, C.; Botoran, O.R.; Ionete, R.E. Assessing the Health Risk and the Metal Content of Thirty-Four Plant Essential Oils Using the ICP-MS Technique. Nutrients 2022, 14, 2363. https://doi.org/10.3390/nu14122363
Iordache AM, Nechita C, Voica C, Roba C, Botoran OR, Ionete RE. Assessing the Health Risk and the Metal Content of Thirty-Four Plant Essential Oils Using the ICP-MS Technique. Nutrients. 2022; 14(12):2363. https://doi.org/10.3390/nu14122363
Chicago/Turabian StyleIordache, Andreea Maria, Constantin Nechita, Cezara Voica, Carmen Roba, Oana Romina Botoran, and Roxana Elena Ionete. 2022. "Assessing the Health Risk and the Metal Content of Thirty-Four Plant Essential Oils Using the ICP-MS Technique" Nutrients 14, no. 12: 2363. https://doi.org/10.3390/nu14122363
APA StyleIordache, A. M., Nechita, C., Voica, C., Roba, C., Botoran, O. R., & Ionete, R. E. (2022). Assessing the Health Risk and the Metal Content of Thirty-Four Plant Essential Oils Using the ICP-MS Technique. Nutrients, 14(12), 2363. https://doi.org/10.3390/nu14122363








