Effects of Five Amino Acids (Serine, Alanine, Glutamate, Aspartate, and Tyrosine) on Mental Health in Healthy Office Workers: A Randomized, Double-Blind, Placebo-Controlled Exploratory Trial
Abstract
1. Introduction
2. Materials and Methods
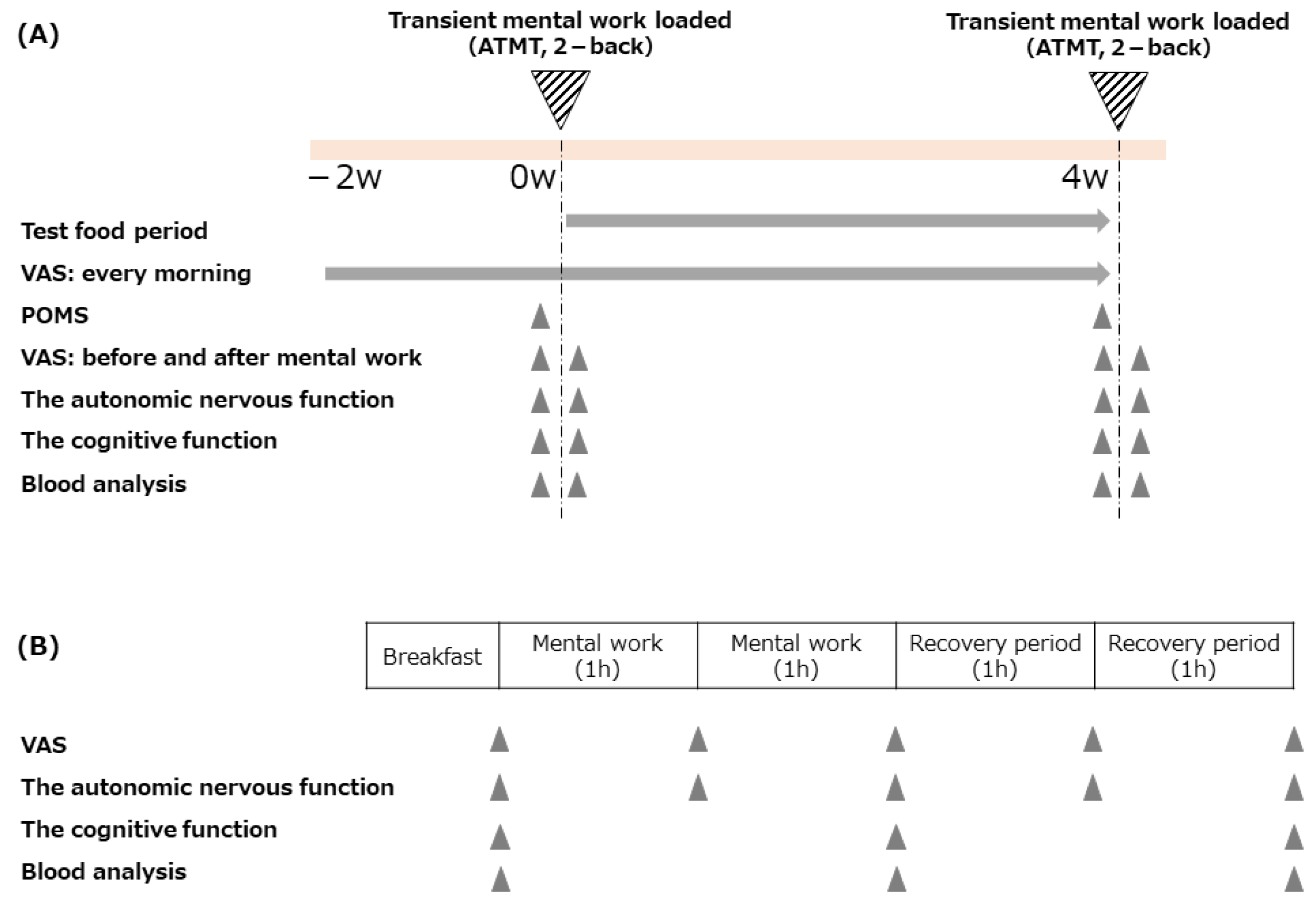
2.1. Study Design
2.2. Participants
2.3. Test Food
2.4. Outcomes
2.4.1. Primary Outcomes
Fatigue Sensation
Autonomic Nervous Function
2.4.2. Secondary Outcomes
Mood Status
Cognitive Function
Blood Marker
Work Efficiency
2.5. Statistics Analysis
2.5.1. Analysis of Daily VAS
2.5.2. Analysis of Transient Work Loaded-VAS, Autonomic Nervous Function, Cognitive Function, Blood Markers
2.5.3. Analysis of Questionnaires (Chalder Fatigue Scale and POMS2)
2.5.4. Analysis of Work Efficiency (ATMT and 2-Back Task)
3. Results
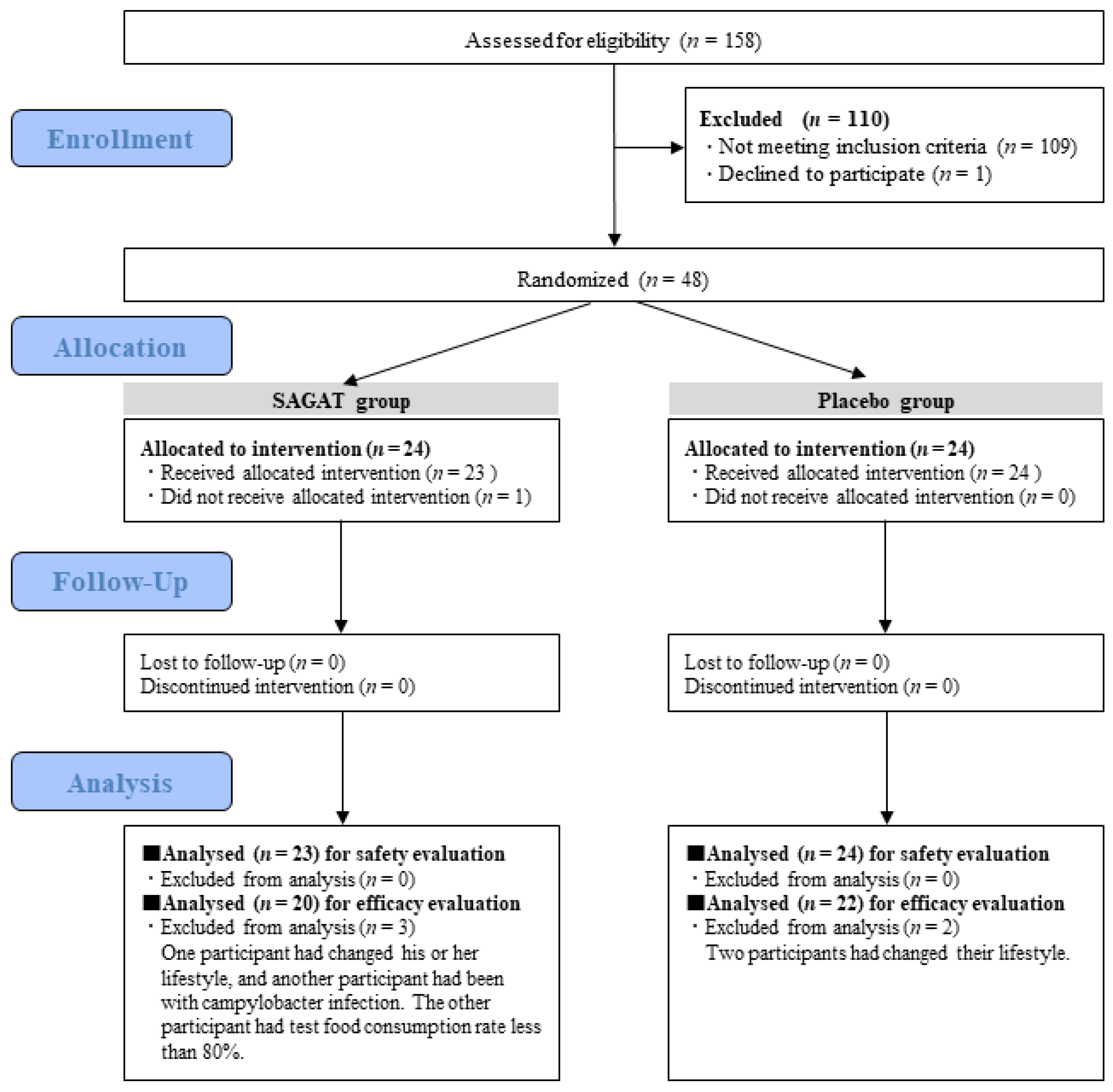
3.1. Participants
3.2. Primary Outcomes
3.2.1. Fatigue Sensation
3.2.2. Autonomic Nervous Function
3.3. Secondary Outcomes
3.3.1. Mood Status
3.3.2. Cognitive Function
3.3.3. Blood Marker
3.3.4. Work Efficiency
3.3.5. Evaluation of Test Food Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/stf/houdou/2r9852000002coxc.html (accessed on 11 April 2022).
- Nochaiwong, S.; Ruengorn, C.; Thavorn, K.; Hutton, B.; Awiphan, R.; Phosuya, C.; Ruanta, Y.; Wongpakaran, N.; Wongpakaran, T. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10173. [Google Scholar] [CrossRef]
- Aknin, L.B.; De Neve, J.E.; Dunn, E.W.; Fancourt, D.; Goldberg, E.; Helliwell, J.; Amor, Y.B. Mental health during the first year of the COVID-19 pandemic: A review and recommendations for moving forward. PsyArXiv 2021, preprint. Available online: https://psyarxiv.com/zw93g/ (accessed on 14 April 2022).
- Walsh, R. Lifestyle and mental health. Am. Psychol. 2011, 66, 579–592. [Google Scholar] [CrossRef]
- Bhakta, A.; Gavini, K.; Yang, E.; Lymanhenley, L.; Parameshwaran, K. Chronic traumatic stress impairs memory in mice: Potential roles of acetylcholine, neuroinflammation and corticotropin releasing factor expression in the hippocampus. Behav. Brain Res. 2017, 335, 32–40. [Google Scholar] [CrossRef]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An 11C-(R)-PK11195 PET study. J. Nucl. Med. 2014, 55, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Feenstra, K.A.; Heringa, J.; Huang, Z. Infuence of gut microbiota on mental health via neurotransmitters: A review. J. Artif. Intell. Med. Sci. 2020, 1, 1–14. [Google Scholar]
- Hufner, K.; Galffy, M.; Egeter, J.; Giesinger, J.M.; Arnhard, K.; Oberacher, H.; Gostner, J.M.; Guchs, D.; Sperner-Unterweger, B. Acute and chronic mental stress both influence levels of neurotransmitter precursor amino acids and derived biogenic amines. Brain Sci. 2020, 10, 322. [Google Scholar] [CrossRef]
- Tartaglia, M.C.; Narayanan, S.; Francis, S.J.; Santos, A.C.; Stefano, N.D.; Lapierre, T.; Arnold, D.L. The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch. Neurol. 2004, 61, 201–207. [Google Scholar] [CrossRef][Green Version]
- Yamano, E.; Tanaka, M.; Ishii, A.; Tsuruoka, N.; Abe, K.; Watanabe, Y. Effects of chicken essence on recovery from mental fatigue in healthy males. Med. Sci. Monit. 2013, 19, 540–547. [Google Scholar]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Nakamura, H.; Takishima, T.; Yokogoshi, H. Psychological stress-reducing effect of chocolate enriched with gamma-aminobutyric acid (GABA) in humans: Assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S5), 106–113. [Google Scholar] [CrossRef]
- Fujibayashi, M.; Kamiya, T.; Takagaki, K.; Moritani, T. Activation of autonomic nervous system activity by the oral ingestion of GABAJ. Jpn. Soc. Nutr. Food Sci. 2008, 61, 129–133. [Google Scholar] [CrossRef]
- Kanehira, T.; Nakamura, Y.; Nakamura, K.; Horie, K.; Horie, N.; Furugori, K.; Sauchi, Y.; Yokogoshi, H. Relieving occupational fatigue by consumption of a beverage containing γ-amino butyric acid. J. Nutr. Sci. Vitaminol. 2011, 57, 9–15. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented milk containing lactobacillus casei strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kato-Kataoka, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Vieira, K.F. Nutritional therapies for mental disorders. Nutr. J. 2018, 7, 2. [Google Scholar] [CrossRef]
- Banderet, L.E.; Lieberman, H.R. Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans. Brain Res. Bull. 1989, 22, 759–762. [Google Scholar] [CrossRef]
- Borg, P.C.; Fekkes, D.; Vrolijk, J.M.; van Buuren, H.R. The relation between plasma tyrosine concentration and fatigue in primary biliary cirrhosis and primary sclerosing cholangitis. BMC Gastroenterol. 2005, 5, 11. [Google Scholar]
- Hase, A.; Jung, S.E.; aan het Rot, M. Behavioral and cognitive effects of tyrosine intake in healthy human adults. Pharmacol. Biochem. Behav. 2015, 133, 1–6. [Google Scholar] [CrossRef]
- Peyy, D.K.; Hannun, Y.A. The role of ceramide in cell signaling. Biochim. Biophys. Acta 1998, 1436, 233–243. [Google Scholar]
- Sasahara, I.; Yamamoto, A.; Takeshita, M.; Suga, Y.; Suzuki, K.; Nishikata, N.; Takada, M.; Hashimoto, M.; Mine, T.; Kobuna, Y.; et al. l-serine and EPA relieve chronic low-back and knee pain in adults: A randomized, double-blind, placebo-controlled trial. J. Nutr. 2020, 150, 2278–2286. [Google Scholar] [CrossRef]
- Nishigaki, R.; Yokoyama, Y.; Shimizu, Y.; Marumoto, R.; Misumi, S.; Ueda, Y.; Ishida, A.; Shibuya, Y.; Hida, H. Monosodium glutamate ingestion during the development period reduces aggression mediated by the vagus nerve in a rat model of attention deficit-hyperactivity disorder. Brain Res. 2018, 1690, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Francisco, E.S.; Guedes, R.C.A. Neonatal taurine and alanine modulate anxiety like behavior and decelerate cortical spreading depression in rats previously suckled under different litter sizes. Amino Acids 2015, 47, 2437–2445. [Google Scholar] [CrossRef]
- Marquezi, M.L.; Roschel, H.A.; Costa, A.D.S.; Sawada, L.A.; Lancha, A.H., Jr. Effect of aspartate and asparagine supplementation on fatigue determinants in intense exercise. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 65–75. [Google Scholar] [CrossRef]
- Japanese Society of Fatigue Science. Available online: https://www.hirougakkai.com/VAS.pdf (accessed on 11 April 2022).
- Lorish, C.D.; Maisiak, R. The face scale: A brief, nonverbal method for assessing patient mood. Arthritis Rheum. 1986, 29, 906–909. [Google Scholar] [CrossRef]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Tanaka, M.; Mizuno, K.; Tajima, S.; Sasabe, T.; Watanabe, Y. Central nervous system fatigue alters autonomic nerve activity. Life Sci. 2009, 84, 235–239. [Google Scholar] [CrossRef]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef]
- Mizuno, K.; Watanabe, Y. Utility of an advanced trail making test as a neuropsychological tool for an objective evaluation of work efficiency during mental fatigue. In Fatigue Science for Human Health; Springer: New York, NY, USA, 2008; pp. 47–54. [Google Scholar]
- Fukuda, S.; Nojima, J.; Kajimoto, O.; Yamaguti, K.; Nakatomi, Y.; Kuratsune, H.; Watanabe, Y. Ubiquinol-10 supplementation improves autonomic nervous function and cognitive function in chronic fatigue syndrome. Biofactors 2016, 42, 431–440. [Google Scholar] [CrossRef]
- Miyazaki, H.; Ohsawa, K.; Maruya, R.; Koikeda, T.; Ohki, K. Effect of tablets containing lactotripeptides (VPP, IPP) on lassitude in young healthy subjects: A randomized, double-blind, placebo-controlled, parallel-group comparison study. Jpn. Pharmacol. Ther. 2018, 46, 375–381. [Google Scholar]
- Hirayama, Y. Development of guideline of clinical evaluation of anti-fatigue. J. Clin. Exp. Med. 2009, 228, 733–736. [Google Scholar]
- Suenaga, H.; Murakami, K.; Murata, N.; Nishikawa, S.; Tsutsumi, M.; Nogaki, H. The effects of an artificial garden on heart rate variability among healthy young Japanese adults. Int. J. Environ. Res. Public Health 2020, 17, 9465. [Google Scholar] [CrossRef]
- Lin, S.; Hsiao, Y.-Y.; Wang, M. Test review: The profile of mood states 2nd edition. J. Psychoeduc. Assess. 2014, 32, 273–277. [Google Scholar] [CrossRef]
- Ichii, S.; Nakamura, T.; Kawarabayashi, T.; Takatama, M.; Ohgami, T.; Ihara, K.; Shoji, M. CogEvo, a cognitive function balancer, is a sensitive and easy psychiatric test battery for agerelated cognitive decline. Geriatr. Gerontol. Int. 2020, 20, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Karakawa, S.; Nishimoto, R.; Harada, M.; Arashida, N.; Nakayama, A. Simultaneous analysis of tryptophan and its metabolites in human plasma using liquid chromatography—Electrospray ionization tandem mass spectrometry. Chromatography 2019, 40, 127–133. [Google Scholar] [CrossRef]
- Najima M and Miyata, A. Taheebo syokuhin ni yoru hiroukan no kanwa koka. Jpn. Pharmacol. Ther. 2018, 55, 410–416. (In Japanese) [Google Scholar]
- Tanaka, M.; Mizuno, K.; Yamaguti, K.; Kuratsune, H.; Fujii, A.; Baba, H.; Matsuda, K.; Nishimae, A.; Takesaka, T.; Watanabe, Y. Autonomic nervous alterations associated with daily level of fatigue. Behav. Brain Funct. 2011, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Deijen, J.B.; Orlebeke, J.F. Effect of tyrosine on cognitive function and blood pressure under stress. Brain Res. Bull. 1994, 33, 319–323. [Google Scholar] [CrossRef]
- Lieberman, H.R. Nutrition, brain function and cognitive performance. Appetite 2003, 40, 245–254. [Google Scholar] [CrossRef]
- Kochetkov, A.M.; Shlygin, G.K.; Loranskaya, T.I.; Vasilevskaia, L.S.; Kondrashev, S.Y.U. Utilization of monosodium glutamate in combined therapy of atrophic gastritis. Vopr. Pitan 1992, 5–6, 19–22. (In Russian) [Google Scholar]
- Hodson, N.A.; Linden, R.W. The effect of monosodium glutamate on parotid salivary flow in comparison to the response to representatives of the other four basic tastes. Physiol. Behav. 2006, 89, 711–717. [Google Scholar] [CrossRef]
- Kume, S.; Yamato, M.; Tamura, Y.; Jin, G.; Nakano, M.; Miyashige, Y.; Eguchi, A.; Ogata, Y.; Goda, N.; Iwai, K.; et al. Potential biomarkers of fatigue identified by plasma metabolome analysis in rats. PLoS ONE 2015, 10, e0120106. [Google Scholar] [CrossRef]
- Yamano, E.; Sugimoto, M.; Hirayama, A.; Kume, S.; Yamato, M.; Jin, G.; Tajima, S.; Goda, N.; Iwai, K.; Fukuda, S.; et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci. Rep. 2016, 6, 34990. [Google Scholar] [CrossRef]
- Felig, P.; Pozefsk, T.; Marlis, E.; Cahill, G.F. Alanine: Key role in gluconeogenesis. Science 1970, 167, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef]
- Barch, D.M.; Yodkovik, N.; Sypher-Locke, H.; Hanewinkel, M. Intrinsic motivation in schizophrenia: Relationships to cognitive function, depression, anxiety, and personality. J. Abnorm. Psychol. 2008, 117, 776–787. [Google Scholar] [CrossRef]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef]
- Park, H.Y.; Jeon, H.J.; Bang, Y.R.; Yoon, I.Y. Multidimensional comparison of cancer-related fatigue and chronic fatigue syndrome: The role of psychophysiological markers. Psychiatry Investig. 2019, 16, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Snieder, H.; Penninx, B.W. Uric acid in major depressive and anxiety disorders. J. Affect. Disord. 2018, 225, 684–690. [Google Scholar] [CrossRef]
- Dessoki, H.H.; Emadeldin, M.; Ezzat, A.A.; Salah, H.; Hakim, S.M. Serum uric acid level and its association with severity of manic and depressive symptoms. Egypt. J. Psychiatry 2019, 40, 35–40. [Google Scholar]
| (A) FAS | |||||||
| Group (n) | M | F | age (years) | BMI (kg/m2) | VAS (fatigue) | Autonomic nervous function (LF/HF) | High-sensitivity CRP (mg/dL) |
| SAGAT (23) | 9 | 14 | 46.0 ± 11.5 | 22.7 ±2.6 | 58.6 ± 10.2 | 2.1 ± 2.5 | 0.05 ± 0.05 |
| Placebo (24) | 11 | 13 | 45.5 ± 10.2 | 22.7 ± 1.9 | 58.4 ± 9.8 | 2.1 ± 1.8 | 0.06 ± 0.07 |
| (B) PPS | |||||||
| Group (n) | M | F | age (years) | BMI (kg/m2) | VAS (fatigue) | Autonomic nervous function (LF/HF) | High-sensitivity CRP (mg/dL) |
| SAGAT (20) | 8 | 12 | 48.0 ± 10.4 | 22.9 ± 2.6 | 57.9 ± 10.6 | 2.3 ± 2.6 | 0.05 ± 0.05 |
| Placebo (22) | 10 | 12 | 46.2 ± 10.0 | 22.6 ± 1.9 | 59.3 ± 9.4 | 1.9 ± 1.7 | 0.05 ± 0.07 |
| (A) | |||||
| fatigue | Before | 1 week | 2 weeks | 3 weeks | 4 weeks |
| SAGAT | 55.7 ± 11.9 | 55.4 ± 17.5 | 47.1 ± 14.3 §§ | 45.9 ± 16.5 §§ | 45.2 ± 16.5 §§ |
| Placebo | 52.9 ± 12.8 | 48.7 ± 14.2 | 45.6 ± 15.1 §§ | 42.3 ± 17.1 §§ | 40.9 ± 17.4 §§ |
| (B) | |||||
| Δfatigue | 1 week | 2 weeks | 3 weeks | 4 weeks | |
| SAGAT | −0.3 ± 12.2 | −8.6 ± 10.2 | −9.7 ± 11.4 | −10.5 ± 11.4 | |
| Placebo | −4.3 ± 8.5 | −7.3 ± 9.9 | −10.6 ± 13.2 | −12.0 ± 16.3 | |
| (C) | |||||
| fatigue | Before Load | 1 h after Load | 2 h after Load | 1 h after Recovery | 2 h after Recovery |
| SAGAT | 41.1 ± 17.8 | 56.5 ± 17.6 §§ | 59.8 ± 17.1 §§ | 44.2 ± 14.9 | 38.5 ± 18.5 |
| Placebo | 37.1 ± 16.0 | 50.9 ± 12.0 §§ | 56.1 ± 11.7 §§ | 42.1 ± 16.1 | 38.2 ± 14.0 |
| (D) | |||||
| Δfatigue | 1 h after Load | 2 h after Load | 1 h after Recovery | 2 h after Recovery | |
| SAGAT | 15.4 ± 13.3 | 18.7 ± 13.3 | 3.1 ± 10.9 | −2.7 ± 10.3 | |
| Placebo | 13.8 ± 10.7 | 19.0 ± 10.2 | 5.0 ± 8.2 | 1.1 ± 8.7 |
| (A) | |||||||
| Before | 4 weeks | Changes before and after intervention | |||||
| LF (ms2) | SAGT | 439.9 ± 490.7 | 442.7 ± 402.6 | 2.8 ± 277.5 | |||
| Placebo | 278.6 ± 221.7 | 510.8 ± 1057.6 | 253.0 ± 942.7 | ||||
| HF (ms2) | SAGT | 414.3 ± 354.9 | 353.6 ± 326.5 | −60.7 ± 260.4 | |||
| Placebo | 340.8 ± 377.4 | 387.9 ± 674.9 | 37.0 ± 519.8 | ||||
| LF/HF | SAGT | 1.4 ± 1.0 | 2.1 ± 1.7 | 0.6 ± 1.8 | |||
| Placebo | 1.3 ± 1.3 | 2.0 ± 2.7 | 0.8 ± 1.9 | ||||
| (B) | |||||||
| Before Load | 1 h after Load | 2 h after Load | 1 h after Recovery | 2 h after Recovery | |||
| LF (ms2) | SAGAT | 442.7 ± 402.6 | 289.3 ± 200.2 | 259.9 ± 173.7 § | 280.3 ± 213.7 | 307.6 ± 275.5 | |
| Placebo | 510.8 ± 1057.6 | 687.5 ± 1554.0 | 405.1 ± 426.2 | 602.7 ± 915.7 | 401.6 ± 423.3 | ||
| HF (ms2) | SAGAT | 353.6 ± 326.5 | 296.2 ± 265.1 | 337.1 ± 345.8 | 325.5 ± 265.1 | 481.7 ± 482.2 | |
| Placebo | 387.9 ± 674.9 | 527.8 ± 1172.6 | 391.5 ± 425.6 | 562.8 ± 641.8 | 485.7 ± 508.0 | ||
| LF/HF | SAGAT | 2.1 ± 1.7 | 2.1 ± 2.6 | 1.7 ± 1.9 | 1.8 ± 2.2 | 1.4 ± 1.9 | |
| Placebo | 2.0 ± 2.7 | 2.8 ± 4.7 | 2.2 ± 3.7 | 2.3 ± 2.9 | 1.4 ± 1.5 | ||
| (C) | |||||||
| 1 h after Load | 2 h after Load | 1 h after Recovery | 2 h after Recovery | ||||
| LF (ms2) | SAGAT | −153.4 ± 417.6 | −182.8 ± 304.4 | −162.4 ± 319.7 | ] * | −135.1 ± 450.7 | |
| Placebo | 176.7 ± 638.7 | −105.7 ± 719.1 | 91.9 ± 339.3 | −109.3 ± 729.5 | |||
| HF (ms2) | SAGAT | −57.4 ± 219.0 | −16.5 ± 148.4 | −28.1 ± 181.8 | 128.1 ± 353.2 | ||
| Placebo | 139.9 ± 563.4 | 3.5 ± 423.3 | 174.9 ± 452.9 | 97.7 ± 403.4 | |||
| LF/HF | SAGAT | 0.0 ± 3.0 | −0.3 ± 2.0 | −0.3 ± 2.3 | −0.7 ± 2.1 | ||
| Placebo | 0.8 ± 4.9 | 0.2 ± 1.2 | 0.3 ± 2.0 | −0.5 ± 2.5 | |||
| (A) | ||||||||
| Before | 1 week | 2 weeks | 3 weeks | 4 weeks | ||||
| Sleepiness | SAGAT | 57.4 ± 14.3 | 55.5 ± 19.2 | 48.6 ± 15.5 § | 50.0 ± 17.0 | 46.6 ± 18.1 §§ | ||
| Placebo | 52.9 ± 13.6 | 51.1 ± 13.8 | 47.9 ± 14.5 | 44.5 ± 16.9 §§ | 42.9 ± 18.0 § | |||
| Motivation | SAGAT | 45.1 ± 11.5 | 45.8 ± 17.8 | 52.7 ± 14.5 §§ | 52.4 ± 16.6 § | 54.6 ± 17.3 §§ | ||
| Placebo | 47.7 ± 12.4 | 50.0 ± 12.5 | 54.3 ± 14.6 § | 57.1 ± 17.0 § | 59.8 ± 16.7 §§ | |||
| Exhilaration | SAGAT | 41.2 ± 14.0 | 44.0 ± 18.9 | 50.2 ± 16.0 §§ | 50.6 ± 17.9 § | 53.5 ± 18.1 §§ | ||
| Placebo | 44.3 ± 12.9 | 48.8 ± 12.2 | 53.4 ± 15.5 § | 57.2 ± 16.9 §§ | 58.4 ± 18.1 §§ | |||
| (B) | ||||||||
| 1 week | 2 weeks | 3 weeks | 4 weeks | |||||
| ΔSleepiness | SAGAT | −1.9 ± 14.7 | −8.8 ± 11.8 | −7.4 ± 12.9 | −10.8 ± 14.1 | |||
| Placebo | −1.8 ± 9.2 | −5.0 ± 10.0 | −8.4 ± 10.7 | −10.0 ± 14.4 | ||||
| ΔMotivation | SAGAT | 0.7 ± 10.9 | 7.6 ± 8.1 | 7.3 ± 10.9 | 9.5 ± 11.6 | |||
| Placebo | 2.3 ± 5.3 | 6.5 ± 10.5 | 9.4 ± 14.1 | 12.1 ± 15.8 | ||||
| ΔExhilaration | SAGAT | 2.8 ± 12.6 | 9.0 ± 10.6 | 9.4 ± 12.9 | 12.3 ± 12.2 | |||
| Placebo | 4.6 ± 8.3 | 9.1 ± 13.2 | 13.0 ± 14.9 | 14.2 ± 18.1 | ||||
| (C) | ||||||||
| Before Load | 1 h after Load | 2 h after Load | 1 h after Recovery | 2 h after Recovery | ||||
| Tension | SAGAT | 40.5 ± 18.7 | 47.4 ± 17.3 §§ | 47.1 ± 19.3 | 40.0 ± 15.0 | 35.0 ± 16.3 | ||
| Placebo | 36.7 ± 14.9 | 42.6 ± 14.5 | 46.2 ± 12.9 §§ | 36.9 ± 16.9 | 33.1 ± 15.5 | |||
| Sleepiness | SAGAT | 44.6 ± 20.7 | 59.6 ± 17.6 §§ | 59.4 ± 21.7 §§ | 44.8 ± 17.0 | 37.5 ± 19.2 | ||
| Placebo | 42.8 ± 18.4 | 55.5 ± 15.1 §§ | 56.6 ± 17.9 §§ | 42.0 ± 18.6 | 41.2 ± 18.7 | |||
| Relaxation | SAGAT | 58.9 ± 18.4 | 47.5 ± 17.8 §§ | 50.7 ± 19.7 | 61.8 ± 13.6 | 66.2 ± 15.3 | ||
| Placebo | 62.8 ± 17.3 | 49.3 ± 11.1 §§ | 48.0 ± 12.1 §§ | 62.5 ± 16.6 | 64.8 ± 16.2 | |||
| Motivation | SAGAT | 55.1 ± 17.0 | 43.7 ± 16.9 §§ | 45.0 ± 20.1 § | 58.2 ± 14.4 | 62.7 ± 14.7 § | ||
| Placebo | 62.6 ± 16.8 | 48.7 ± 13.5 §§ | 47.4 ± 13.5 §§ | 57.1 ± 15.6 | 59.0 ± 13.9 | |||
| Concentration | SAGAT | 54.7 ± 19.4 | 40.3 ± 17.4 §§ | 43.8 ± 21.9 | 55.0 ± 15.3 | 59.6 ± 16.1 | ||
| Placebo | 61.4 ± 17.4 | 46.6 ± 12.8 §§ | 45.2 ± 13.9 §§ | 58.9 ± 16.7 | 61.7 ± 16.2 | |||
| (D) | ||||||||
| 1 h after Load | 2 h after Load | 1 h after Recovery | 2 h after Recovery | |||||
| ΔTension | SAGAT | 6.9 ± 8.9 | 6.6 ± 13.4 | −0.5 ± 10.0 | −5.5 ± 10.5 | |||
| Placebo | 5.9 ± 10.4 | 9.5 ± 11.1 | 0.2 ± 11.6 | −3.6 ± 11.3 | ||||
| ΔSleepiness | SAGAT | 15.1 ± 14.8 | 14.9 ± 16.8 | 0.2 ± 15.6 | −7.1 ± 19.8 | |||
| Placebo | 12.7 ± 11.0 | 13.8 ± 13.0 | −0.9 ± 13.4 | −1.6 ± 15.0 | ||||
| ΔRelaxation | SAGAT | −11.4 ± 15.5 | −8.2 ± 17.4 | 2.9 ± 11.2 | 7.3 ± 14.6 | |||
| Placebo | −13.4 ± 15.6 | −14.7 ± 14.8 | −0.3 ± 12.7 | 2.0 ± 12.6 | ||||
| ΔMotivation | SAGAT | −11.4 ± 11.7 | −10.1 ± 15.1 | 3.1 ± 10.4 | ] ** | 7.6 ± 11.1 | ] ** | |
| Placebo | −13.9 ± 13.7 | −15.2 ± 14.6 | −5.5 ± 9.5 | −3.6 ± 9.9 | ||||
| ΔConcentration | SAGAT | −14.4 ± 13.0 | −10.9 ± 18.5 | 0.3 ± 11.5 | 4.9 ± 12.0 | |||
| Placebo | −14.8 ± 13.2 | −16.1 ± 13.3 | −2.5 ± 8.9 | ± 12.6 | ||||
| (A) | |||||||
| Before | 4 weeks | Changes before and after intervention | |||||
| Orientation, Time management | SAGAT | 306 ± 53 | 328 ± 42 | 21 ± 51 | |||
| Placebo | 304 ± 54 | 321 ± 47 | 18 ± 77 | ||||
| Attention, Follow the order | SAGAT | 313 ± 40 | 326 ± 19 | 13 ± 36 | |||
| Placebo | 326 ± 16 | 313 ± 51 | −13 ± 49 | ||||
| Attention, Stroop test | SAGAT | 647 ± 74 | ] * | 699 ± 65 § | 51 ± 86 | ||
| Placebo | 701 ± 75 | 735 ± 58 | 25 ± 54 | ||||
| Memory, Recall | SAGAT | 224 ± 21 | 226 ± 19 | 3 ± 29 | |||
| Placebo | 223 ± 20 | 230 ± 9 | 8 ± 21 | ||||
| Memory, Delayed recall | SAGAT | 205 ± 40 | 224 ± 21 | 19 ± 46 | |||
| Placebo | 212 ± 32 | 221 ± 30 | 14 ± 46 | ||||
| Executive function, Route 99 | SAGAT | 98 ± 70 | 138 ± 53 § | 40 ± 70 | |||
| Placebo | 102 ± 66 | 125 ± 64 | 25 ± 87 | ||||
| Spatial cognition, Just Fit | SAGAT | 103 ± 60 | 95 ± 65 | ] * | −8 ± 76 | ||
| Placebo | 118 ± 70 | 134 ± 55 | 18 ± 94 | ||||
| Total score | SAGAT | 1897 ± 160 | 2036 ± 138 §§ | 139 ± 157 | |||
| Placebo | 1986 ± 125 | 2079 ± 157 §§ | 95 ± 147 | ||||
| (B) | |||||||
| Before Load | 2 h after Load | 2 h after Recovery | |||||
| Orientation, Time management | SAGAT | 328 ± 42 | 297 ± 51 | 339 ± 19 | ] * | ||
| Placebo | 321 ± 47 | 295 ± 73 | 308 ± 49 | ||||
| Attention, Follow the order | SAGAT | 326 ± 19 | 325 ± 20 | 330 ± 19 | |||
| Placebo | 313 ± 51 | 322 ± 42 | 325 ± 39 | ||||
| Attention, Stroop test | SAGAT | 699 ± 65 | 694 ± 90 | 720 ± 52 | |||
| Placebo | 735 ± 58 | 720 ± 52 | 733 ± 58 | ||||
| Memory, Recall | SAGAT | 226 ± 19 | 215 ± 32 | 218 ± 32 | |||
| Placebo | 230 ± 9 | 216 ± 32 | 215 ± 29 | ||||
| Memory, Delayed recall | SAGAT | 224 ± 21 | 200 ± 43 | 188 ± 46 §§ | |||
| Placebo | 221 ± 30 | 201 ± 52 | 193 ± 46 | ||||
| Executive function, Route99 | SAGAT | 138 ± 53 | 142 ± 56 | 160 ± 42 | |||
| Placebo | 125 ± 64 | 141 ± 42 | 140 ± 57 | ||||
| Spatial cognition, Just Fit | SAGAT | 95 ± 65 | ] * | 116 ± 58 | 139 ± 54 § | ||
| Placebo | 134 ± 55 | 138 ± 57 | 122 ± 54 | ||||
| Total score | SAGAT | 2036 ± 138 | 1989 ± 162 | 2094 ± 158 | |||
| Placebo | 2079 ± 157 | 2034 ± 162 | 2036 ± 145 | ||||
| (C) | |||||||
| 2 h after Load | 2 h after Recovery | ||||||
| ΔOrientation, Time management | SAGAT | −30 ± 68 | 12 ± 45 | ||||
| Placebo | −26 ± 77 | −12 ± 65 | |||||
| ΔAttention, Follow the order | SAGAT | −1 ± 16 | 4 ± 16 | ||||
| Placebo | 9 ± 61 | 12 ± 64 | |||||
| ΔAttention, Stroop test | SAGAT | −5 ± 77 | 21 ± 62 | ||||
| Placebo | −15 ± 60 | −2 ± 65 | |||||
| ΔMemory, Recall | SAGAT | −11 ± 37 | −8 ± 26 | ||||
| Placebo | −14 ± 31 | −15 ± 31 | |||||
| ΔMemory, Delayed recall | SAGAT | −25 ± 50 | −37 ± 50 | ||||
| Placebo | −20 ± 62 | −29 ± 56 | |||||
| ΔExecutive function, Route99 | SAGAT | 4 ± 68 | 22 ± 57 | ||||
| Placebo | 17 ± 66 | 15 ± 81 | |||||
| ΔSpatial cognition, Just Fit | SAGAT | 22 ± 78 | 44 ± 81 | ] * | |||
| Placebo | 4 ± 73 | −12 ± 87 | |||||
| ΔTotal score | SAGAT | −47 ± 141 | 58 ± 177 | ||||
| Placebo | −45 ± 164 | −43 ± 170 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umeda, K.; Shindo, D.; Somekawa, S.; Nishitani, S.; Sato, W.; Toyoda, S.; Karakawa, S.; Kawasaki, M.; Mine, T.; Suzuki, K. Effects of Five Amino Acids (Serine, Alanine, Glutamate, Aspartate, and Tyrosine) on Mental Health in Healthy Office Workers: A Randomized, Double-Blind, Placebo-Controlled Exploratory Trial. Nutrients 2022, 14, 2357. https://doi.org/10.3390/nu14112357
Umeda K, Shindo D, Somekawa S, Nishitani S, Sato W, Toyoda S, Karakawa S, Kawasaki M, Mine T, Suzuki K. Effects of Five Amino Acids (Serine, Alanine, Glutamate, Aspartate, and Tyrosine) on Mental Health in Healthy Office Workers: A Randomized, Double-Blind, Placebo-Controlled Exploratory Trial. Nutrients. 2022; 14(11):2357. https://doi.org/10.3390/nu14112357
Chicago/Turabian StyleUmeda, Kentaro, Daichi Shindo, Shinji Somekawa, Shinobu Nishitani, Wataru Sato, Sakiko Toyoda, Sachise Karakawa, Mika Kawasaki, Tomoyuki Mine, and Katsuya Suzuki. 2022. "Effects of Five Amino Acids (Serine, Alanine, Glutamate, Aspartate, and Tyrosine) on Mental Health in Healthy Office Workers: A Randomized, Double-Blind, Placebo-Controlled Exploratory Trial" Nutrients 14, no. 11: 2357. https://doi.org/10.3390/nu14112357
APA StyleUmeda, K., Shindo, D., Somekawa, S., Nishitani, S., Sato, W., Toyoda, S., Karakawa, S., Kawasaki, M., Mine, T., & Suzuki, K. (2022). Effects of Five Amino Acids (Serine, Alanine, Glutamate, Aspartate, and Tyrosine) on Mental Health in Healthy Office Workers: A Randomized, Double-Blind, Placebo-Controlled Exploratory Trial. Nutrients, 14(11), 2357. https://doi.org/10.3390/nu14112357






