A Combination of Nicotinamide and D-Ribose (RiaGev) Is Safe and Effective to Increase NAD+ Metabolome in Healthy Middle-Aged Adults: A Randomized, Triple-Blind, Placebo-Controlled, Cross-Over Pilot Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Trial Design and Subject Population
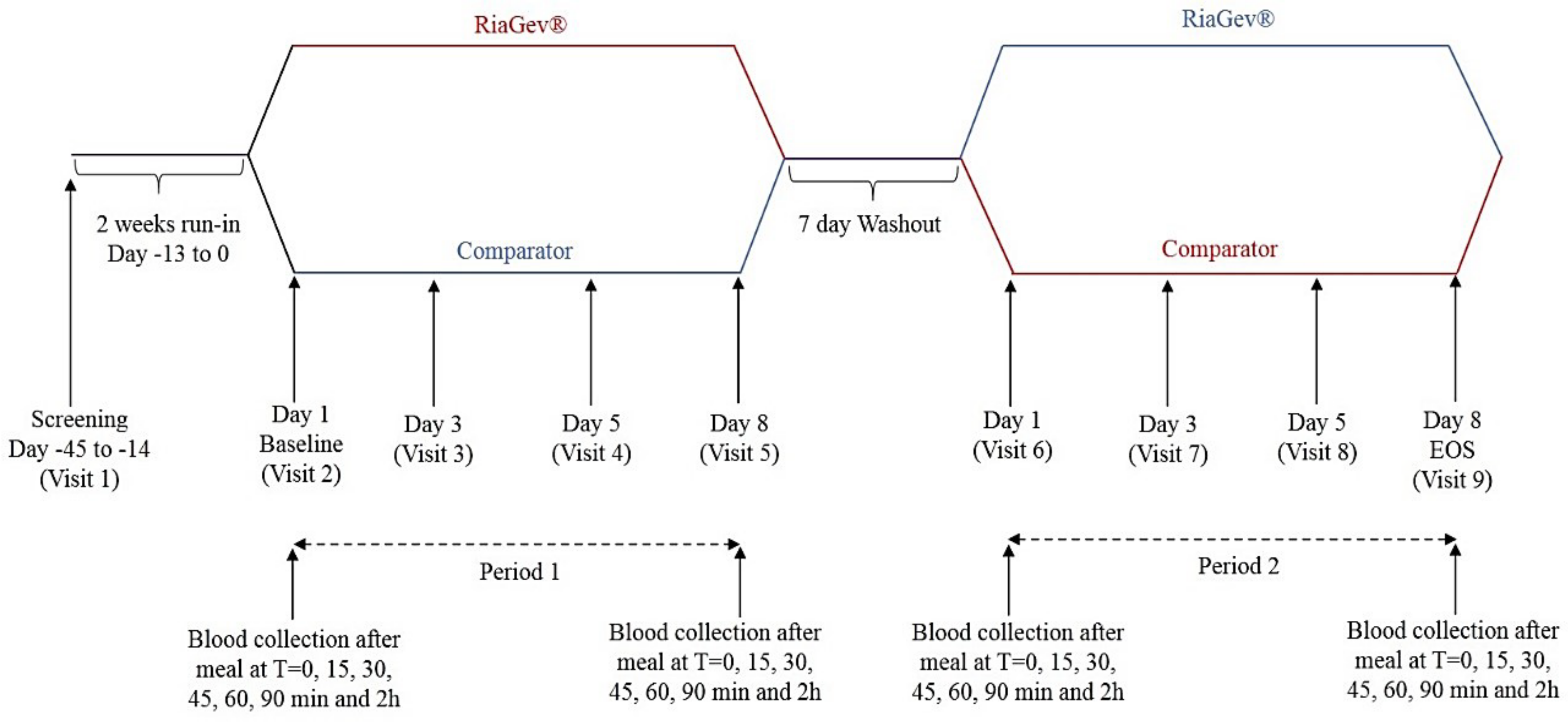
2.1.1. Clinical Trial Design
2.1.2. Subject Population
2.2. Investigational Product and Its Administration
2.2.1. Investigational Product and Placebo
2.2.2. Investigational Product Administration
2.3. Sample Collection
2.3.1. Blood
2.3.2. Saliva Sample Collection
2.4. Clinical Measurements and Additional Assessments
2.4.1. Laboratory Methodologies
2.4.2. Checklist Individual Strength Questionnaire (CIS) Questionnaire
2.4.3. Food Records
2.4.4. Study Compliance
2.4.5. Adverse Event (AEs) Monitoring
2.5. Statistical Analyses
2.5.1. Efficacy Analysis
2.5.2. Safety Analysis
2.5.3. Level of Significance and Statistical Software
3. Results
3.1. Subject Enrollment and Baseline Characteristics
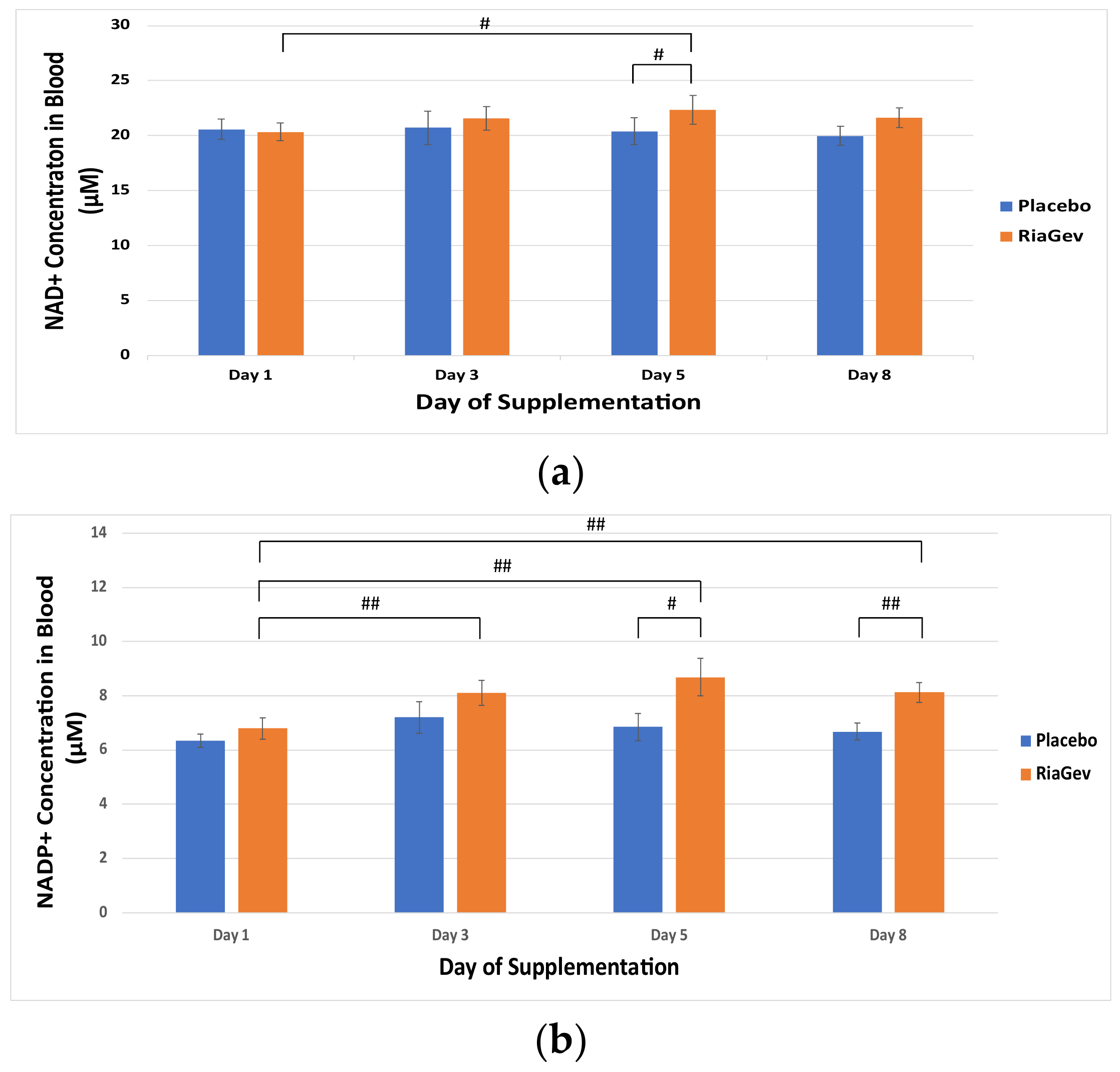
3.2. Primary Outcome-NAD+ Metabolome
3.3. Secondary Outcomes
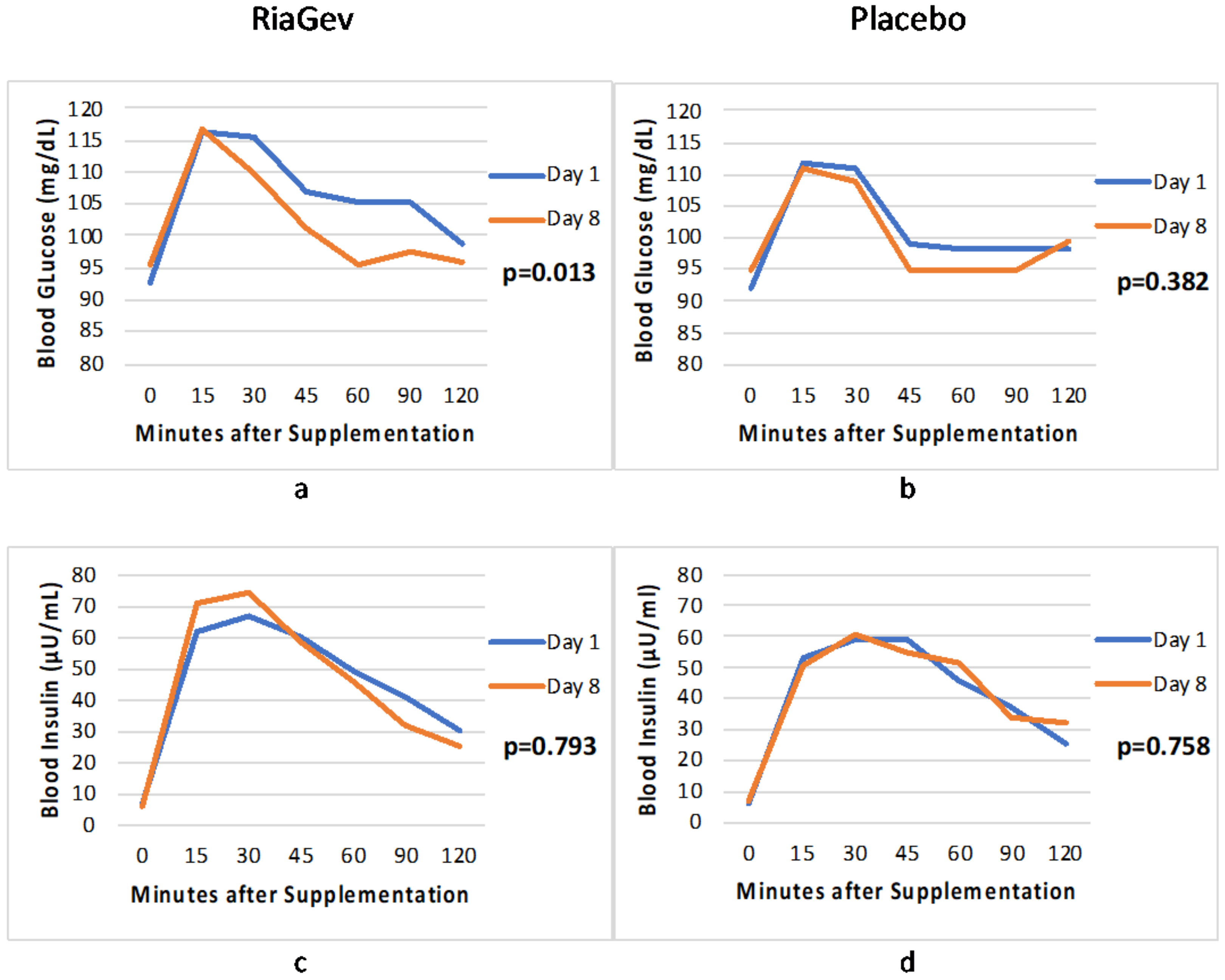
3.3.1. Blood Glucose and Insulin Concentration in OGTT
3.3.2. Total Glutathione
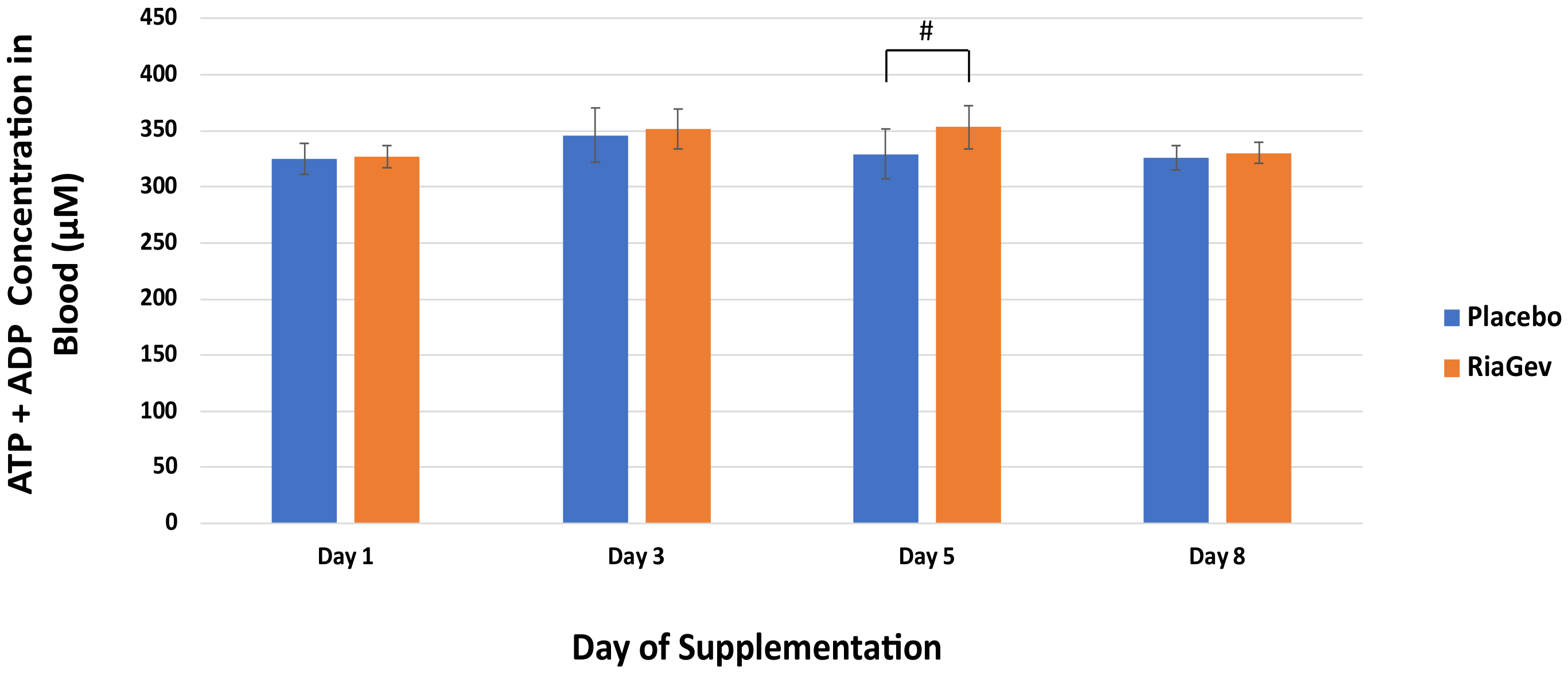
3.3.3. High Energy Phosphates ATP + ADP
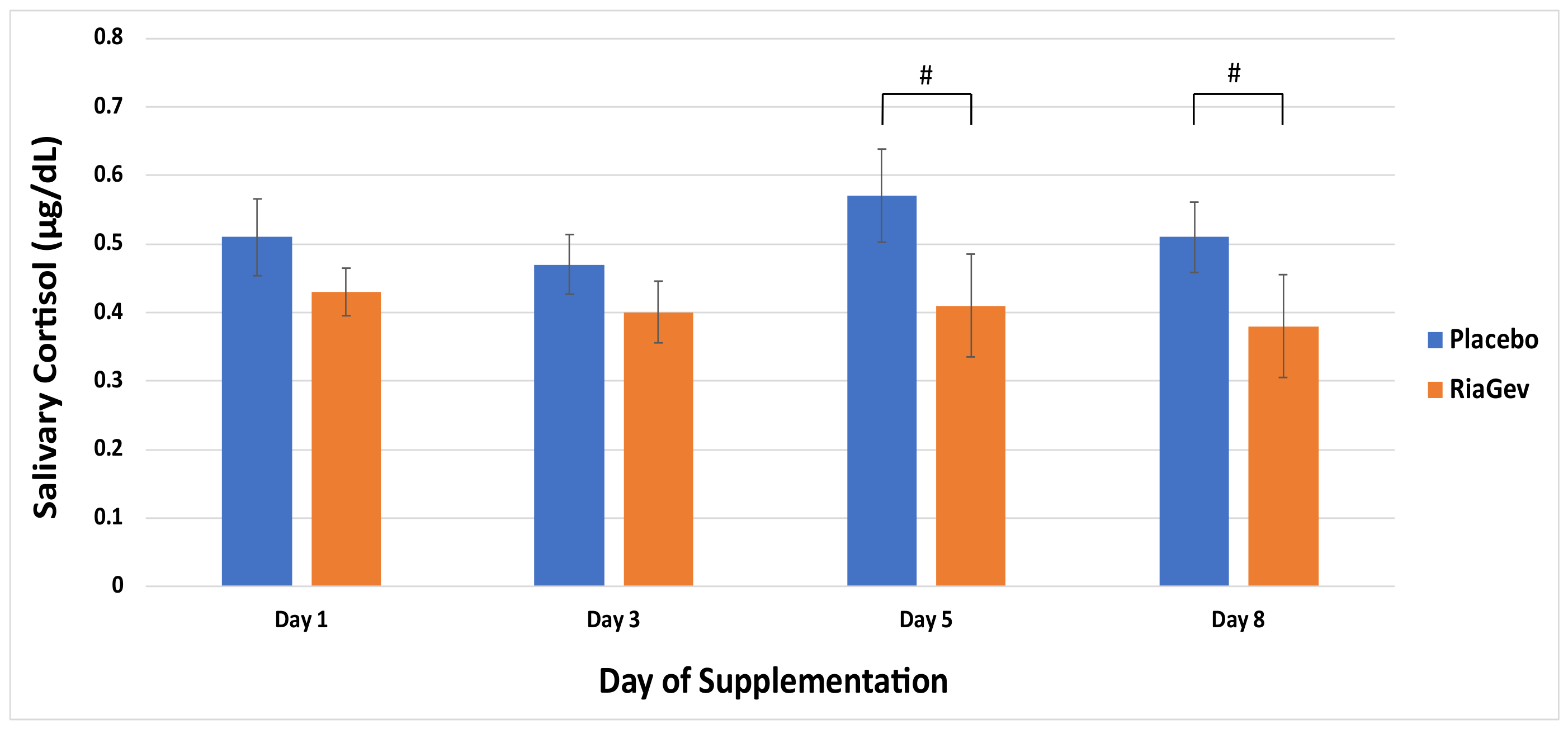
3.3.4. Salivary Cortisol
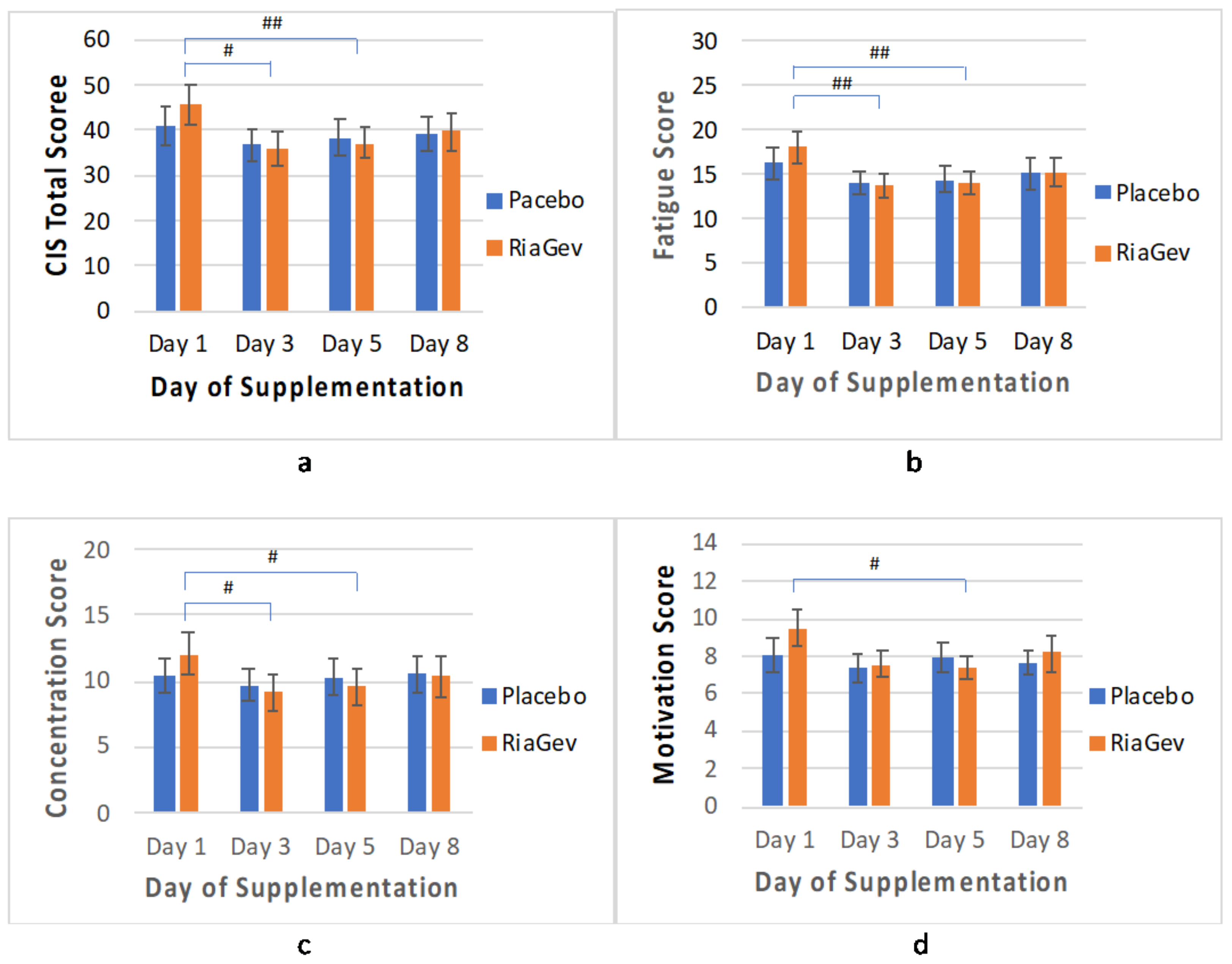
3.3.5. CIS Questionnaire
3.4. Safety Outcomes
3.4.1. Hematology, Clinical Chemistry, Vital Signs, and Anthropometric Measurements
3.4.2. Incidence of Adverse Events (AEs)
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houtkooper, R.H.; Cantoó, C.; Wanders, R.J.; Auwerx, J. The Secret Life of NAD+: An Old Metabolite Controlling New Metabolic Signaling Pathways. Endocr. Rev. 2010, 31, 194–223. [Google Scholar] [CrossRef] [Green Version]
- Satoh, A.; Stein, L.; Imai, S. The Role of Mammalian Sirtuins in the Regulation of Metabolism, Aging, and Longevity. Handb. Exp. Pharmacol. 2011, 206, 125–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, S.-I.; Yoshino, J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes. Metab. 2013, 15, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Bogan, K.L.; Song, P.; Burant, C.F.; Kennedy, R.T.; Brenner, C. NAD+ metabolite levels as a function of vitamins and calorie restriction: Evidence for different mechanisms of longevity. BMC Chem. Biol. 2010, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef]
- Canto, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Mouchiroud, L.; Houtkooper, R.H.; Auwerx, J. NAD+ metabolism: A therapeutic target for age-related metabolic disease. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, A.; Dolle, C.; Niere, M.; Ziegler, M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: From entry of extracellular precursors to mitochondrial nad generation. J. Biol. Chem. 2011, 86, 21767–21778. [Google Scholar] [CrossRef] [Green Version]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD− Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [Green Version]
- Stavrum, A.-K.; Heiland, I.; Schuster, S.; Puntervoll, P.; Ziegler, M. Model of Tryptophan Metabolism, Readily Scalable Using Tissue-specific Gene Expression Data. J. Biol. Chem. 2013, 288, 34555–34566. [Google Scholar] [CrossRef] [Green Version]
- Mackay, D.; Hathcock, J.; Guarneri, E. Niacin: Chemical forms, bioavailability, and health effects. Nutr. Rev. 2012, 70, 357–366. [Google Scholar] [CrossRef]
- Imai, S.-I. Nicotinamide Phosphoribosyltransferase (Nampt): A Link Between NAD Biology, Metabolism, and Diseases. Curr. Pharm. Des. 2009, 15, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Khaidizar, F.D.; Bessho, Y.; Nakahata, Y. Nicotinamide Phosphoribosyltransferase as a Key Molecule of the Aging/Senescence Process. Int. J. Mol. Sci. 2021, 22, 3709. [Google Scholar] [CrossRef]
- Aman, Y.; Qiu, Y.; Tao, J.; Fang, E.F. Therapeutic potential of boosting NAD+ in aging and age-related diseases. Transl. Med. Aging 2018, 2, 30–37. [Google Scholar] [CrossRef]
- De Guia, R.M.; Agerholm, M.; Nielsen, T.S.; Consitt, L.A.; Søgaard, D.; Helge, J.W.; Larsen, S.; Brandauer, J.; Houmard, J.A.; Treebak, J.T. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol. Rep. 2019, 7, e14139. [Google Scholar] [CrossRef] [Green Version]
- Trammell, S.A.J.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Ratajczak, J.; Joffraud, M.; Trammell, S.; Ras, R.; Canela, N.; Boutant, M.; Kulkarni, S.S.; Rodrigues, M.; Redpath, P.; Migaud, M.E.; et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 2016, 7, 13103. [Google Scholar] [CrossRef]
- Liu, L.; Su, X.; Quinn, W.J., III; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E.; et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018, 27, 1067–1080. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.T.; Jones, D.S.; Andrews, G.P.; Li, S. Understanding the physicochemical properties and degradation kinetics of nicotinamide riboside, a promising vitamin B3 nutritional supplement. Food Nutr. Res. 2019, 63, 3419. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, G.A.; Sarett, H.P.; Register, U.D.; Gibbens, J. Studies of niacin requirement in man. I. Experimental pellagra in subjects on corn diets low in niacin and tryptophan. J. Clin. Investig. 1952, 31, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogan, K.L.; Brenner, C. Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knip, M.; Douek, I.F.; Moore, W.P.T.; Gillmor, H.A.; McLean, A.E.M.; Bingley, P.J.; Gale, E.A.M. Safety of high-dose nicotinamide: A review. Diabetologia 2000, 43, 1337–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Health (NIH) Office of Dietary Supplement (ODS), Bethesda, MD 20817, USA. Available online: https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/ (accessed on 25 May 2022).
- Winter, S.L.; Boyer, J.L. Hepatic Toxicity from Large Doses of Vitamin B3(Nicotinamide). N. Engl. J. Med. 1973, 289, 1180–1182. [Google Scholar] [CrossRef]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Nagana Gowda, G.A.; Raftery, D. Whole blood metabolomics by 1H NMR spectroscopy provides a new opportunity to evaluate coenzymes of redox reactions, coenzymes of energy and antioxidants. Anal. Chem. 2017, 89, 4620–4627. [Google Scholar] [CrossRef]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Gowda, G.A.N.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. [Google Scholar] [CrossRef]
- Vercoulen, J.H.M.M.; Swanink, C.M.A.; Fennis, J.F.M.; Galama, J.M.D.; van der Meer, J.W.M.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef] [Green Version]
- This Clinical Trial Is Registered at ClinicalTrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04483011 (accessed on 25 May 2022).
- Zerez, C.R.; Tanaka, K.R. Impaired erythrocyte nad synthesis: A metabolic abnormality in thalassemia. Am. J. Hematol. 1989, 32, 1–7. [Google Scholar] [CrossRef]
- Maharjan, A.; Singhvi, M.; Kim, B.S. Biosynthesis of a Therapeutically Important Nicotinamide Mononucleotide through a Phosphoribosyl Pyrophosphate Synthetase 1 and 2 Engineered Strain of Escherichia coli. ACS Synth. Biol. 2021, 10, 3055–3065. [Google Scholar] [CrossRef]
- Seifert, J.G.; Subudhi, A.; Fu, M.-X.; Riska, K.L.; John, J.C.; Shecterle, L.M.; Cyr, J.A.S. The Role of Ribose on Oxidative Stress During Hypoxic Exercise: A Pilot Study. J. Med. Food 2009, 12, 690–693. [Google Scholar] [CrossRef]
- Vida, C.; Gonzalez, E.; Fuente, M. Increase of Oxidation and Inflammation in Nervous and Immune Systems with Aging and Anxiety. Curr. Pharm. Des. 2014, 20, 4656–4678. [Google Scholar] [CrossRef]
- Pescarmona, G.P.; Bracone, A.; David, O.; Sartori, M.L.; Bosia, A. Regulation of NAD and NADP synthesis in human red cell. Acta Biol. Med. Ger. 1977, 36, 759–763. [Google Scholar]
- Zhong, O.; Wang, J.; Tan, Y.; Lei, X.; Tang, Z. Effects of NAD+ precursor supplementation on glucose and lipid metabolism in humans: A meta-analysis. Nutr. Metab. 2022, 19, 20. [Google Scholar] [CrossRef]
- Dollerup, O.L.; Christensen, B.; Svart, M.; Schmidt, M.; Sulek, K.; Ringgaard, S.; Stødkilde-Jørgensen, H.; Moller, N.; Brenner, C.; Treebak, J.T.; et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018, 108, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.-I.; et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021, 372, 1224–1229. [Google Scholar] [CrossRef]
- Seifert, J.; Frelich, A.; Shecterle, L.; Cyr, J.S. Assessment of Hematological and Biochemical parameters with extended D-Ribose ingestion. J. Int. Soc. Sports Nutr. 2008, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.S.; Bowker, S.S.; Deschoolmeester, J.; Gerhardt, S.; Hargreaves, D.; Kilgour, E.; Lloyd, A.; Mayers, R.M.; McCoull, W.; Newcombe, N.J.; et al. Discovery of a potent, selective, and orally bioavailable acidic 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitor: Discovery of 2-[(3S)-1-[5-(cyclohexylcarbamoyl)-6-propylsulfanylpyridin-2-yl]-3-piperidyl]acetic acid (AZD4017). J. Med. Chem. 2012, 55, 5951. [Google Scholar] [CrossRef]
- Beehan-Quirk, C.; Jarman, L.; Maharaj, S.; Simpson, A.; Nassif, N.; Lal, S. Investigating the effects of fatigue on blood glucose levels—Implications for diabetes. Transl. Metab. Syndr. Res. 2020, 3, 17–20. [Google Scholar] [CrossRef]
- Conze, D.; Brenner, C.; Kruger, C.L. Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults. Sci. Rep. 2019, 9, 9772. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Song, S.B. Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment. Biomolecules 2020, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.jinfiniti.com/intracellular-nad-test/ (accessed on 25 May 2022).


| Parameter | Placebo—RiaGev Mean ± SD (n) Median (Min–Max) | RiaGev—Placebo Mean ± SD (n) Median (Min–Max) | Between Groups p-Value |
|---|---|---|---|
| Age (years) | 53.3 ± 7.3 (9) | 50.1 ± 8.6 (9) | 0.405 |
| 53 (39 to 64) | 53 (38 to 62) | ||
| Gender | 1.000 | ||
| Female [n (%)] | 6 (66.7%) | 5 (55.6%) | |
| Male [n (%)] | 3 (33.3%) | 4 (44.4%) | |
| Weight (kg) | 79.1 ± 20.0 (9) | 75.2 ± 14.6 (9) | 0.642 |
| 68.8 (57.8 to 114.4) | 70.3 (59.4 to 102.2) | ||
| Height (cm) | 172.7 ± 14.2 (9) | 175.2 ± 8.1 (9) | 0.645 |
| 166.0 (157.0 to 200.0) | 172.0 (165.0 to 189.0) | ||
| BMI (kg/m2) | 26.1 ± 2.8 (9) | 24.3 ± 3.0 (9) | 0.193 |
| 27.2 (21.0 to 28.9) | 23.7 (20.3 to 28.8) | ||
| Heart Rate (bpm) | 66.3 ± 9.1 (9) | 64.1 ± 19.6 (9) | 0.243 |
| 63 (55 to 80) | 60 (49 to 112) | ||
| Hemoglobin A1c (%) | 5.24 ± 0.37 | 5.50 ± 0.25 | 0.108 |
| 5.2 (4.8 to 6.0) | 5.4 (5.2 to 6.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Shamp, T.; Nagana Gowda, G.A.; Crabtree, M.; Bagchi, D.; Raftery, D. A Combination of Nicotinamide and D-Ribose (RiaGev) Is Safe and Effective to Increase NAD+ Metabolome in Healthy Middle-Aged Adults: A Randomized, Triple-Blind, Placebo-Controlled, Cross-Over Pilot Clinical Trial. Nutrients 2022, 14, 2219. https://doi.org/10.3390/nu14112219
Xue Y, Shamp T, Nagana Gowda GA, Crabtree M, Bagchi D, Raftery D. A Combination of Nicotinamide and D-Ribose (RiaGev) Is Safe and Effective to Increase NAD+ Metabolome in Healthy Middle-Aged Adults: A Randomized, Triple-Blind, Placebo-Controlled, Cross-Over Pilot Clinical Trial. Nutrients. 2022; 14(11):2219. https://doi.org/10.3390/nu14112219
Chicago/Turabian StyleXue, Yongquan, Trisha Shamp, G. A. Nagana Gowda, Michael Crabtree, Debasis Bagchi, and Daniel Raftery. 2022. "A Combination of Nicotinamide and D-Ribose (RiaGev) Is Safe and Effective to Increase NAD+ Metabolome in Healthy Middle-Aged Adults: A Randomized, Triple-Blind, Placebo-Controlled, Cross-Over Pilot Clinical Trial" Nutrients 14, no. 11: 2219. https://doi.org/10.3390/nu14112219
APA StyleXue, Y., Shamp, T., Nagana Gowda, G. A., Crabtree, M., Bagchi, D., & Raftery, D. (2022). A Combination of Nicotinamide and D-Ribose (RiaGev) Is Safe and Effective to Increase NAD+ Metabolome in Healthy Middle-Aged Adults: A Randomized, Triple-Blind, Placebo-Controlled, Cross-Over Pilot Clinical Trial. Nutrients, 14(11), 2219. https://doi.org/10.3390/nu14112219








