Association between Vitamin Intake and Chronic Kidney Disease According to a Variant Located Upstream of the PTGS1 Gene: A Cross-Sectional Analysis of Shika Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Genotyping of rs883484
2.3. Assessment of Chronic Kidney Disease
2.4. Vitamin Intake Assessment
2.5. Other Variables
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics According to CKD Status
3.2. Participant Characteristics According to rs883484 Genotypes and CKD Status
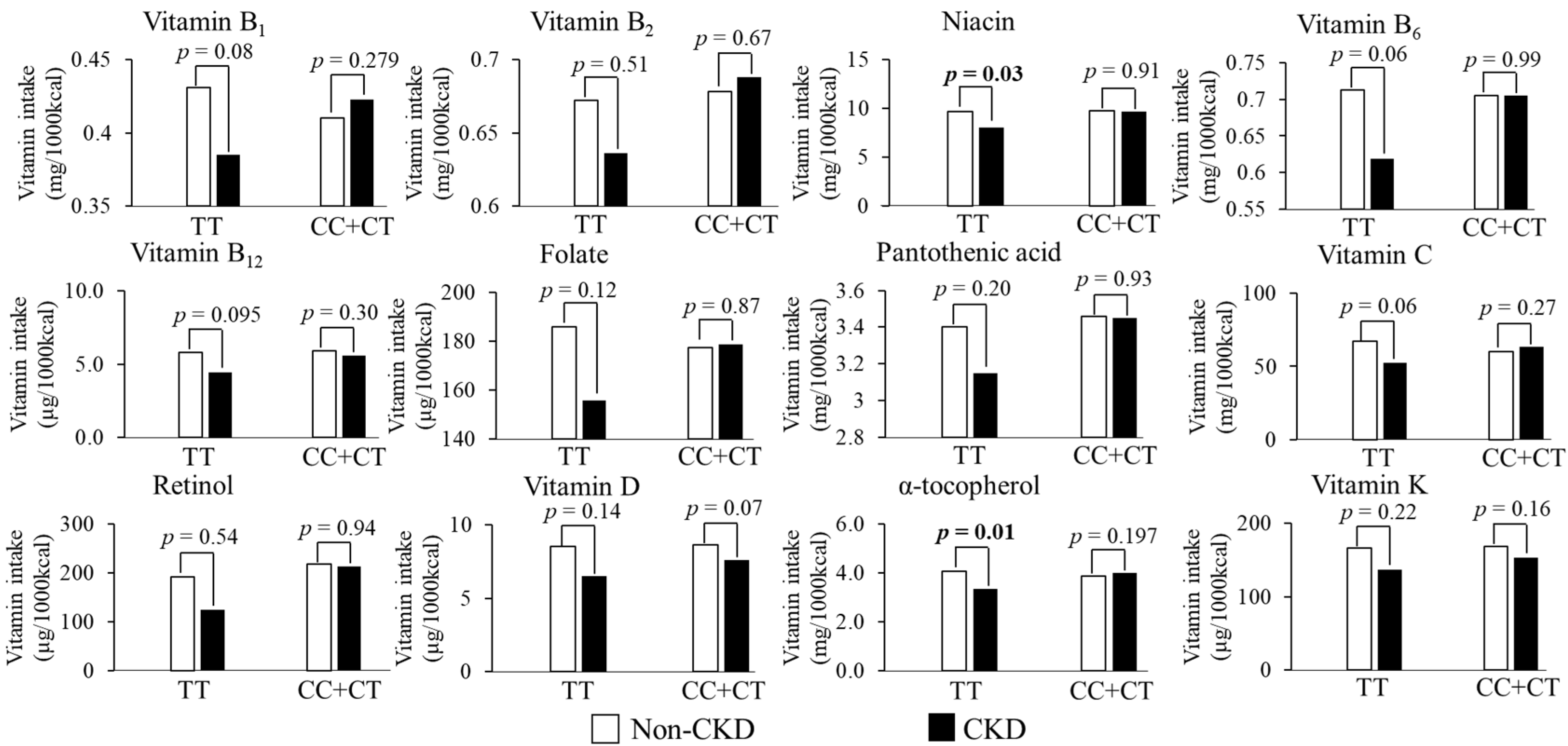
3.3. Relationship between Antioxidant Vitamins Intake and CKD According to Gene Variants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic Kidney Disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef]
- Mullins, V.A.; Bresette, W.; Johnstone, L.; Hallmark, B.; Chilton, F.H. Genomics in Personalized Nutrition: Can You “Eat for Your Genes”? Nutrients 2020, 12, 3118. [Google Scholar] [CrossRef]
- Peña-Romero, A.C.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. The Future of Nutrition: Nutrigenomics and Nutrigenetics in Obesity and Cardiovascular Diseases. Crit. Rev. Food. Sci. Nutr. 2018, 58, 3030–3041. [Google Scholar] [CrossRef]
- Wuttke, M.; Li, Y.; Li, M.; Sieber, K.B.; Feitosa, M.F.; Gorski, M.; Tin, A.; Wang, L.; Chu, A.Y.; Hoppmann, A.; et al. A Catalog of Genetic Loci Associated with Kidney Function from Analyses of a Million Individuals. Nat. Genet. 2019, 51, 957–972. [Google Scholar] [CrossRef]
- Shi, S.; Xue, F. Current Antioxidant Treatments in Organ Transplantation. Oxid. Med. Cell. Longev. 2016, 2016, 8678510. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y.H.; Lee, C. Te Transcriptome Analysis in Rat Kidneys: Importance of Genes Involved in Programmed Hypertension. Int. J. Mol. Sci. 2015, 16, 4744–4758. [Google Scholar] [CrossRef] [PubMed]
- Helmersson, J.; Ärnlöv, J.; Axelsson, T.; Basu, S. A Polymorphism in the Cyclooxygenase 1 Gene Is Associated with Decreased Inflammatory Prostaglandin F2α Formation and Lower Risk of Cardiovascular Disease. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; North, K.E.; Bray, M.S.; Couper, D.J.; Heiss, G.; Zeldin, D.C. Cyclooxygenase Polymorphisms and Risk of Cardiovascular Events: The Atherosclerosis Risk in Communities (ARIC) Study. Clin. Pharmacol. Ther 2008, 83, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kato, K.; Fujimaki, T.; Yokoi, K.; Oguri, M.; Watanabe, S.; Metoki, N.; Yoshida, H.; Satoh, K.; Aoyagi, Y.; et al. Association of a Polymorphism of the Apolipoprotein E Gene with Chronic Kidney Disease in Japanese Individuals with Metabolic Syndrome. Genomics 2009, 93, 221–226. [Google Scholar] [CrossRef]
- Yoshida, T.; Kato, K.; Yokoi, K.; Watanabe, S.; Metoki, N.; Satoh, K.; Aoyagi, Y.; Nishigaki, Y.; Nozawa, Y.; Yamada, Y. Association of Candidate Gene Polymorphisms with Chronic Kidney Disease in Japanese Individuals with Hypertension. Hypertens. Res. 2009, 32, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kato, K.; Fujimaki, T.; Yokoi, K.; Oguri, M.; Watanabe, S.; Metoki, N.; Yoshida, H.; Satoh, K.; Aoyagi, Y.; et al. Association of Genetic Variants with Chronic Kidney Disease in Japanese Individuals. Clin. J. Am. Soc. Nephrol. 2009, 4, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Qian, Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Borran, M.; Dashti-Khavidaki, S.; Alamdari, A.; Naderi, N. Vitamin C and Kidney Transplantation: Nutritional Status, Potential Efficacy, Safety, and Interactions. Clin. Nutr. ESPEN 2021, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Raimann, J.G.; Levin, N.W.; Craig, R.G.; Sirover, W.; Kotanko, P.; Handelman, G. Is Vitamin C Intake Too Low in Dialysis Patients? Semin. Dial. 2013, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rutkowski, B.; Dębska-Ślizień, A. Vitamins and Microelement Bioavailability in Different Stages of Chronic Kidney Disease. Nutrients 2017, 9, 282. [Google Scholar] [CrossRef]
- MacCubbin, D.; Tipping, D.; Kuznetsova, O.; Hanlon, W.A.; Bostom, A.G. Hypophosphatemic Effect of Niacin in Patients without Renal Failure: A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2010, 5, 582–589. [Google Scholar] [CrossRef]
- Kawai, Y.; Mimori, T.; Kojima, K.; Nariai, N.; Danjoh, I.; Saito, R.; Yasuda, J.; Yamamoto, M.; Nagasaki, M. Japonica Array: Improved Genotype Imputation by Designing a Population-Specific SNP Array with 1070 Japanese Individuals. J. Hum. Genet. 2015, 60, 581–587. [Google Scholar] [CrossRef]
- Nomura, A.; Sato, T.; Tada, H.; Kannon, T.; Hosomichi, K.; Tsujiguchi, H.; Nakamura, H.; Takamura, M.; Tajima, A.; Kawashiri, M. Polygenic Risk Scores for Low-Density Lipoprotein Cholesterol and Familial Hypercholesterolemia. J. Hum. Genet. 2021, 66, 1079–1087. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Levin, A.; Bilous, R.; Coresh, J. Chapter 1: Definition and Classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar]
- Kobayashi, S.; Yuan, X.; Sasaki, S.; Osawa, Y.; Hirata, T.; Abe, Y.; Takayama, M.; Arai, Y.; Masui, Y.; Ishizaki, T. Relative Validity of Brief-Type Self-Administered Diet History Questionnaire among Very Old Japanese Aged 80 Years or Older. Public Health Nutr. 2019, 22, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef] [PubMed]
- Society, T.J.; All, H.; Observational, S.R. Chapter 4. Lifestyle Modifications. Hypertens. Res. 2014, 37, 286–290. [Google Scholar] [CrossRef][Green Version]
- Hara, A.; Tsujiguchi, H.; Suzuki, K.; Suzuki, F.; Kasahara, T.; Oanh, P.K.; Miyagi, S.; Kannon, T.; Tajima, A.; Wada, T.; et al. Gender Difference in the Association of Dietary Intake of Antioxidant Vitamins with Kidney Function in Middle-Aged and Elderly Japanese. J. Nutr. Sci. 2021, 10, 1–7. [Google Scholar] [CrossRef]
- Ahmed, M.H. Niacin as Potential Treatment for Dyslipidemia and Hyperphosphatemia Associated with Chronic Renal Failure: The Need for Clinical Trials. Ren. Fail. 2010, 32, 642–646. [Google Scholar] [CrossRef]
- Cho, K.; Kim, H.; Kamanna, V.S.; Vaziri, N.D. Niacin Improves Renal Lipid Metabolism and Slows Progression in Chronic Kidney Disease. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 6–15. [Google Scholar] [CrossRef]
- Ginsberg, C.; Lx, J.H. Nicotinamide and Phosphate Homeostasis in Chronic Kidney Disease. Cur. Opin. Nephrol. Hypertens. 2016, 25, 285–291. [Google Scholar] [CrossRef]
- Streja, E.; Kovesdy, C.P.; Streja, D.A.; Moradi, H.; Kalantar-Zadeh, K.; Kashyap, M.L. Niacin and Progression of CKD. Am. J. Kidney Dis. 2015, 65, 785–798. [Google Scholar] [CrossRef]
- Dennis, J.M.; Witting, P.K. Protective Role for Antioxidants in Acute Kidney Disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Thrasher, K.D.; Gundy, P.D.; Hughson, M.D.; Manning, R.D. Antioxidant Treatment Prevents Renal Damage and Dysfunction and Reduces Arterial Pressure in Salt-Sensitive Hypertension. Hypertension 2005, 45, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef] [PubMed]
- ElAttar, T.M.A.; Lin, H.S. Effect of Vitamin C and Vitamin E on Prostaglandin Synthesis by Fibroblasts and Squamous Carcinoma Cells. Prostaglandins Leukot. Essent. Fat. Acids 1992, 47, 253–257. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Cano, M.P.; De Ancos, B.; Plaza, L.; Olmedilla, B.; Granado, F.; Martín, A. Consumption of High-Pressurized Vegetable Soup Increases Plasma Vitamin C and Decreases Oxidative Stress and Inflammatory Biomarkers in Healthy Humans. J. Nutr. 2004, 134, 3021–3025. [Google Scholar] [CrossRef]
- Fujimori, K.; Amano, F. Niacin Promotes Adipogenesis by Reducing Production of Anti-Adipogenic PGF2α through Suppression of C/EBPβ-Activated COX-2 Expression. Prostaglandins Other Lipid Mediat. 2011, 94, 96–103. [Google Scholar] [CrossRef]
- Sugita, K.; Ikenouchi-Sugita, A.; Nakayama, Y.; Yoshioka, H.; Nomura, T.; Sakabe, J.I.; Nakahigashi, K.; Kuroda, E.; Uematsu, S.; Nakamura, J.; et al. Prostaglandin e 2 is Critical for the Development of Niacin-Deficiency- Induced Photosensitivity via ROS Production. Sci. Rep. 2013, 3, 2973. [Google Scholar] [CrossRef]
- Song, W.L.; Stubbe, J.; Ricciotti, E.; Alamuddin, N.; Ibrahim, S.; Crichton, I.; Prempeh, M.; Lawson, J.A.; Wilensky, R.L.; Rasmussen, L.M.; et al. Niacin and Biosynthesis of PGD 2 by Platelet COX-1 in Mice and Humans. J. Clin. Investig. 2012, 122, 1459–1468. [Google Scholar] [CrossRef]
| Total (n = 684) | non-CKD (n = 570) | CKD (n = 114) | p-Value *6 | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Characteristics | ||||
| Age (years) | 62.1 (10.8) | 60.7 (10.5) | 68.9 (9.6) | 0.000 |
| Female, n (%) | 375 (54.8) | 324 (56.8) | 51 (44.7) | 0.018 |
| BMI *1 (kg/m2) | 23.2 (3.2) | 23.1 (3.1) | 23.9 (3.8) | 0.023 |
| SBP *2 (mmHg) | 138.7 (19.4) | 137.5 (19.1) | 144.4 (20.0) | 0.001 |
| DBP *3 (mmHg) | 80.1 (11.4) | 80.1 (11.2) | 80.2 (12.2) | 0.900 |
| Hypertension, n (%) | 320 (46.8) | 254 (44.6) | 66 (57.9) | 0.009 |
| HbA1c *4 (%) | 5.9 (0.7) | 5.9 (0.7) | 6.0 (0.5) | 0.406 |
| FPG *5 (mg/dL) | 96.5 (18.0) | 96.3 (18.2) | 97.6 (16.9) | 0.526 |
| Diabetes, n (%) | 95 (13.9) | 76 (13.3) | 19 (16.7) | 0.347 |
| Triglyceride (mg/dL) | 118.6 (85.9) | 117.4 (87.1) | 124.6 (79.9) | 0.411 |
| Total cholesterol (mg/dL) | 215.0 (34.5) | 215.7 (34.9) | 211.5 (32.1) | 0.240 |
| Lifestyle habit | ||||
| Current smoker, n (%) | 130 (19.0) | 120 (21.1) | 10 (8.8) | 0.002 |
| Current drinker, n (%) | 331 (48.4) | 279 (48.9) | 52 (45.6) | 0.516 |
| Frequent exercise, n (%) | 407 (40.5) | 331 (58.1) | 76 (66.7) | 0.088 |
| Genotypes of rs883484 | 0.669 | |||
| TT, n (%) | 105 (15.4) | 89 (15.6) | 16 (14.0) | |
| CC + CT, n (%) | 579 (84.6) | 481 (84.4) | 98 (86.0) | |
| Nutrition intake | ||||
| Vitamin B1 (mg/1000 kcal) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.352 |
| Vitamin B2 (mg/1000 kcal) | 0.7 (0.2) | 0.7 (0.2) | 0.7 (0.2) | 0.251 |
| Niacin (mg/1000 kcal) | 9.7 (2.8) | 9.7 (2.8) | 9.4 (3.2) | 0.285 |
| Vitamin B6 (mg/1000 kcal) | 0.7 (0.2) | 0.7 (0.2) | 0.7 (0.2) | 0.674 |
| Vitamin B12 (μg/1000 kcal) | 5.8 (3.1) | 5.9 (3.1) | 5.7 (3.1) | 0.693 |
| Folate (μg/1000 kcal) | 178.2 (75.7) | 176.9 (76.3) | 184.7 (72.8) | 0.313 |
| Pantothenic acid (mg/1000 kcal) | 3.4 (0.8) | 3.4 (0.8) | 3.5 (0.8) | 0.569 |
| Vitamin C (mg/1000 kcal) | 61.1 (31.6) | 59.9 (30.7) | 67.4 (35.6) | 0.037 |
| Retinol (μg/1000 kcal) | 210.9 (400.4) | 213.4 (434.2) | 198.8 (139.5) | 0.722 |
| Vitamin D (μg/1000 kcal) | 8.4 (5.1) | 8.5 (5.1) | 8.2 (5.3) | 0.649 |
| α-tocopherol (mg/1000 kcal) | 3.9 (1.1) | 3.9 (1.1) | 4.0 (1.2) | 0.361 |
| Vitamin K (μg/1000 kcal) | 164.8 (92.8) | 165.1 (93.5) | 163.2 (89.9) | 0.838 |
| Daily salt intake (g/day) | 9.5 (2.4) | 9.7 (2.4) | 8.8 (2.4) | 0.001 |
| CC + CT (n = 579) | TT (n = 105) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| non-CKD (n = 481) | CKD (n = 98) | p-Value * | non-CKD (n = 89) | CKD (n = 16) | p-Value | |||||
| Mean (n) | SD (%) | Mean (n) | SD (%) | Mean (n) | SD (%) | Mean (n) | SD (%) | |||
| Characteristics | ||||||||||
| Age (years) | 60.6 | 10.4 | 69.0 | 9.8 | 0.000 | 61.3 | 10.9 | 68.0 | 8.3 | 0.020 |
| Sex, n (%) Female | 271 | 56.3 | 41 | 41.8 | 0.009 | 53 | 59.6 | 10 | 62.5 | 0.825 |
| BMI (kg/m2) | 23.1 | 3.1 | 24.2 | 3.6 | 0.002 | 22.8 | 2.9 | 22.3 | 4.5 | 0.637 |
| SBP (mmHg) | 137.7 | 18.7 | 145.3 | 19.8 | 0.000 | 136.6 | 21.2 | 138.6 | 21.3 | 0.722 |
| DBP (mmHg) | 80.2 | 11.3 | 80.1 | 12.4 | 0.985 | 79.6 | 10.9 | 80.7 | 11.6 | 0.708 |
| Hypertension | 217 | 45.1 | 59 | 60.2 | 0.006 | 37.0 | 41.6 | 7.0 | 43.8 | 0.871 |
| HbA1c (%) | 5.9 | 0.7 | 6.0 | 0.6 | 0.471 | 5.9 | 0.6 | 5.9 | 0.4 | 0.685 |
| FPG (mg/dL) | 96.5 | 18.3 | 98.2 | 17.3 | 0.415 | 95.8 | 17.8 | 93.0 | 13.5 | 0.611 |
| Diabetes | 65 | 13.5 | 16 | 16.3 | 0.464 | 11 | 12.4 | 3 | 18.8 | 0.489 |
| Triglyceride (mg/dL) | 117.7 | 91.1 | 125.6 | 83.3 | 0.425 | 115.7 | 61.6 | 118.6 | 56.4 | 0.863 |
| Total cholesterol (mg/dL) | 215.7 | 35.3 | 211.9 | 33.5 | 0.339 | 215.6 | 32.7 | 208.8 | 22.1 | 0.421 |
| Lifestyle habit | ||||||||||
| Current smoker, n (%) | 105.0 | 21.8 | 6.0 | 6.1 | 0.000 | 15.0 | 16.9 | 4.0 | 21.1 | 0.436 |
| Current drinker, n (%) | 236.0 | 49.1 | 47.0 | 48.0 | 0.842 | 43.0 | 48.3 | 5.0 | 4.8 | 0.207 |
| Frequent exercise, n (%) | 280.0 | 58.2 | 67.0 | 68.4 | 0.062 | 51.0 | 42.7 | 9.0 | 56.3 | 0.938 |
| Nutrition intake | ||||||||||
| Vitamin B1 (mg/1000 kcal) | 0.4 | 0.1 | 0.4 | 0.1 | 0.124 | 0.4 | 0.1 | 0.4 | 0.1 | 0.255 |
| Vitamin B2 (mg/1000 kcal) | 0.7 | 0.2 | 0.7 | 0.2 | 0.207 | 0.7 | 0.2 | 0.7 | 0.3 | 0.902 |
| Niacin (mg/1000 kcal) | 9.7 | 2.8 | 9.6 | 3.2 | 0.750 | 9.7 | 2.6 | 8.1 | 2.8 | 0.027 |
| Vitamin B6 (mg/1000 kcal) | 0.7 | 0.2 | 0.7 | 0.2 | 0.308 | 0.7 | 0.2 | 0.6 | 0.2 | 0.191 |
| Vitamin B12 (μg/1000 kcal) | 5.9 | 3.1 | 5.9 | 3.2 | 0.935 | 5.8 | 3.0 | 4.7 | 2.3 | 0.179 |
| Folate (μg/1000 kcal) | 175.2 | 77.6 | 187.6 | 74.9 | 0.147 | 186.0 | 68.5 | 167.1 | 56.8 | 0.302 |
| Pantothenic acid (mg/1000 kcal) | 3.4 | 0.8 | 3.5 | 0.8 | 0.379 | 3.4 | 0.8 | 3.3 | 0.9 | 0.499 |
| Vitamin C (mg/1000 kcal) | 58.5 | 30.2 | 68.7 | 37.3 | 0.012 | 67.1 | 32.4 | 58.9 | 21.6 | 0.214 |
| Retinol (μg/1000 kcal) | 217.3 | 468.8 | 209.6 | 145.4 | 0.872 | 192.2 | 140.7 | 132.3 | 66.4 | 0.099 |
| Vitamin D (μg/1000 kcal) | 8.5 | 5.1 | 8.4 | 5.4 | 0.923 | 8.5 | 5.2 | 7.1 | 4.8 | 0.332 |
| α-tocopherol (mg/1000 kcal) | 3.8 | 1.1 | 4.1 | 1.2 | 0.067 | 4.1 | 1.1 | 3.5 | 1.4 | <0.05 |
| Vitamin K (μg/1000 kcal) | 165.0 | 95.0 | 165.5 | 92.4 | 0.966 | 165.7 | 85.6 | 149.2 | 73.4 | 0.471 |
| Daily salt intake (g/day) | 9.6 | 2.5 | 8.8 | 2.5 | 0.003 | 9.9 | 2.1 | 8.8 | 2.1 | 0.060 |
| p-Value for CKD Status * | p-Value for Genotypes * | p-Value for Interaction * | |
|---|---|---|---|
| Vitamin B1 | 0.238 | 0.545 | 0.041 |
| Vitamin B2 | 0.658 | 0.326 | 0.435 |
| Niacin | 0.039 | 0.037 | 0.046 |
| Vitamin B6 | 0.092 | 0.152 | 0.085 |
| Vitamin B12 | 0.054 | 0.166 | 0.256 |
| Folate | 0.174 | 0.486 | 0.133 |
| Pantothenic acid | 0.229 | 0.103 | 0.252 |
| Vitamin C | 0.192 | 0.620 | 0.028 |
| Retinol | 0.551 | 0.338 | 0.591 |
| Vitamin D | 0.038 | 0.413 | 0.504 |
| α-tocopherol | 0.073 | 0.133 | 0.005 |
| Vitamin K | 0.097 | 0.475 | 0.569 |
| CC + CT | p-Value | TT | p-Value | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||||
| Model 1 | Water-soluble vitamins | ||||||
| Vitamin B1 | 7.668 | 0.743–79.106 | 0.087 | 0.005 | 0.000–1.384 | 0.065 | |
| Vitamin B2 | 1.522 | 0.509–4.547 | 0.452 | 0.081 | 0.003–2.497 | 0.151 | |
| Niacin | 1.008 | 0.930–1.093 | 0.840 | 0.744 | 0.582–0.951 | 0.018 | |
| Vitamin B6 | 1.344 | 0.404–4.476 | 0.630 | 0.037 | 0.002–0.845 | 0.039 | |
| Vitamin B12 | 0.972 | 0.899–1.052 | 0.488 | 0.807 | 0.641–1.017 | 0.069 | |
| Folate | 1.001 | 0.998–1.004 | 0.613 | 0.988 | 0.975–1.000 | 0.046 | |
| Pantothenic acid | 1.073 | 0.789–1.459 | 0.652 | 0.418 | 0.174–1.003 | 0.051 | |
| Vitamin C | 1.006 | 0.998–1.013 | 0.134 | 0.975 | 0.975–0.951 | 0.042 | |
| Fat-soluble vitamins | |||||||
| Retinol | 1.000 | 0.999–1.001 | 0.955 | 0.991 | 0.982–1.000 | 0.059 | |
| Vitamin D | 0.97 | 0.924–1.018 | 0.215 | 0.906 | 0.789–1.040 | 0.159 | |
| α-tocopherol | 1.22 | 0.983–1.514 | 0.071 | 0.495 | 0.272–0.900 | 0.021 | |
| Vitamin K | 0.999 | 0.999–0.996 | 0.372 | 0.993 | 0.985–1.001 | 0.098 | |
| Model 2 | Water-soluble vitamins | ||||||
| Vitamin B1 | 5.557 | 0.493–62.609 | 0.165 | 0.006 | 0.000–2.213 | 0.089 | |
| Vitamin B2 | 1.447 | 0.458–4.573 | 0.529 | 0.092 | 0.003–2.917 | 0.176 | |
| Niacin | 1.014 | 0.934–1.101 | 0.737 | 0.764 | 0.592–0.986 | 0.039 | |
| Vitamin B6 | 1.265 | 0.368–4.351 | 0.709 | 0.055 | 0.002–1.373 | 0.077 | |
| Vitamin B12 | 0.98 | 0.905–1.062 | 0.621 | 0.831 | 0.831–1.059 | 0.135 | |
| Folate | 1.000 | 0.997–1.004 | 0.834 | 0.988 | 0.976–1.001 | 0.073 | |
| Pantothenic acid | 1.032 | 0.748–1.423 | 0.850 | 0.435 | 0.176–1.079 | 0.073 | |
| Vitamin C | 1.004 | 0.996–1.012 | 0.323 | 0.973 | 0.947–0.999 | 0.046 | |
| Fat-soluble vitamins | |||||||
| Retinol | 1.000 | 0.999–1.001 | 0.911 | 0.992 | 0.983–1.001 | 0.097 | |
| Vitamin D | 0.971 | 0.925–1.019 | 0.231 | 0.925 | 0.803–1.066 | 0.283 | |
| α-tocopherol | 1.179 | 0.946–1.470 | 0.144 | 0.512 | 0.276–0.952 | 0.034 | |
| Vitamin K | 0.998 | 0.996–1.001 | 0.258 | 0.994 | 0.985–1.002 | 0.147 | |
| Model 3 | Water-soluble vitamins | ||||||
| Vitamin B1 | 4.445 | 0.378–52.317 | 0.236 | 0.003 | 0.000–1.773 | 0.075 | |
| Vitamin B2 | 1.187 | 0.369–3.824 | 0.773 | 0.067 | 0.002–2.582 | 0.147 | |
| Niacin | 1.006 | 0.926–1.094 | 0.880 | 0.739 | 0.569–0.961 | 0.024 | |
| Vitamin B6 | 1.199 | 0.343–4.186 | 0.777 | 0.037 | 0.001–1.131 | 0.059 | |
| Vitamin B12 | 0.978 | 0.902–1.060 | 0.589 | 0.823 | 0.633–1.069 | 0.144 | |
| Folate | 1.000 | 0.974–1.001 | 0.960 | 0.987 | 0.997–1.003 | 0.063 | |
| Pantothenic acid | 0.981 | 0.707–1.361 | 0.909 | 0.407 | 0.153–1.078 | 0.070 | |
| Vitamin C | 1.003 | 0.995–1.011 | 0.428 | 0.971 | 0.945–0.998 | 0.037 | |
| Fat-soluble vitamins | |||||||
| Retinol | 1.000 | 0.999–1.001 | 0.880 | 0.992 | 0.982–1.002 | 0.126 | |
| Vitamin D | 0.972 | 0.925–1.021 | 0.253 | 0.924 | 0.792–1.079 | 0.317 | |
| α-tocopherol | 1.164 | 0.929–1.458 | 0.187 | 0.492 | 0.256–0.947 | 0.034 | |
| Vitamin K | 0.999 | 0.996–1.001 | 0.271 | 0.994 | 0.985–1.003 | 0.181 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, K.-O.; Hara, A.; Tsujiguchi, H.; Suzuki, K.; Suzuki, F.; Miyagi, S.; Kannon, T.; Sato, T.; Hosomichi, K.; Tsuboi, H.; et al. Association between Vitamin Intake and Chronic Kidney Disease According to a Variant Located Upstream of the PTGS1 Gene: A Cross-Sectional Analysis of Shika Study. Nutrients 2022, 14, 2082. https://doi.org/10.3390/nu14102082
Pham K-O, Hara A, Tsujiguchi H, Suzuki K, Suzuki F, Miyagi S, Kannon T, Sato T, Hosomichi K, Tsuboi H, et al. Association between Vitamin Intake and Chronic Kidney Disease According to a Variant Located Upstream of the PTGS1 Gene: A Cross-Sectional Analysis of Shika Study. Nutrients. 2022; 14(10):2082. https://doi.org/10.3390/nu14102082
Chicago/Turabian StylePham, Kim-Oanh, Akinori Hara, Hiromasa Tsujiguchi, Keita Suzuki, Fumihiko Suzuki, Sakae Miyagi, Takayuki Kannon, Takehiro Sato, Kazuyoshi Hosomichi, Hirohito Tsuboi, and et al. 2022. "Association between Vitamin Intake and Chronic Kidney Disease According to a Variant Located Upstream of the PTGS1 Gene: A Cross-Sectional Analysis of Shika Study" Nutrients 14, no. 10: 2082. https://doi.org/10.3390/nu14102082
APA StylePham, K.-O., Hara, A., Tsujiguchi, H., Suzuki, K., Suzuki, F., Miyagi, S., Kannon, T., Sato, T., Hosomichi, K., Tsuboi, H., Nguyen, T. T. T., Shimizu, Y., Kambayashi, Y., Nakamura, M., Takazawa, C., Nakamura, H., Hamagishi, T., Shibata, A., Konoshita, T., ... Nakamura, H. (2022). Association between Vitamin Intake and Chronic Kidney Disease According to a Variant Located Upstream of the PTGS1 Gene: A Cross-Sectional Analysis of Shika Study. Nutrients, 14(10), 2082. https://doi.org/10.3390/nu14102082







