Applications of Ketogenic Diets in Patients with Headache: Clinical Recommendations
Abstract
:1. Introduction
2. Ketogenic Diet: Overview
- 1
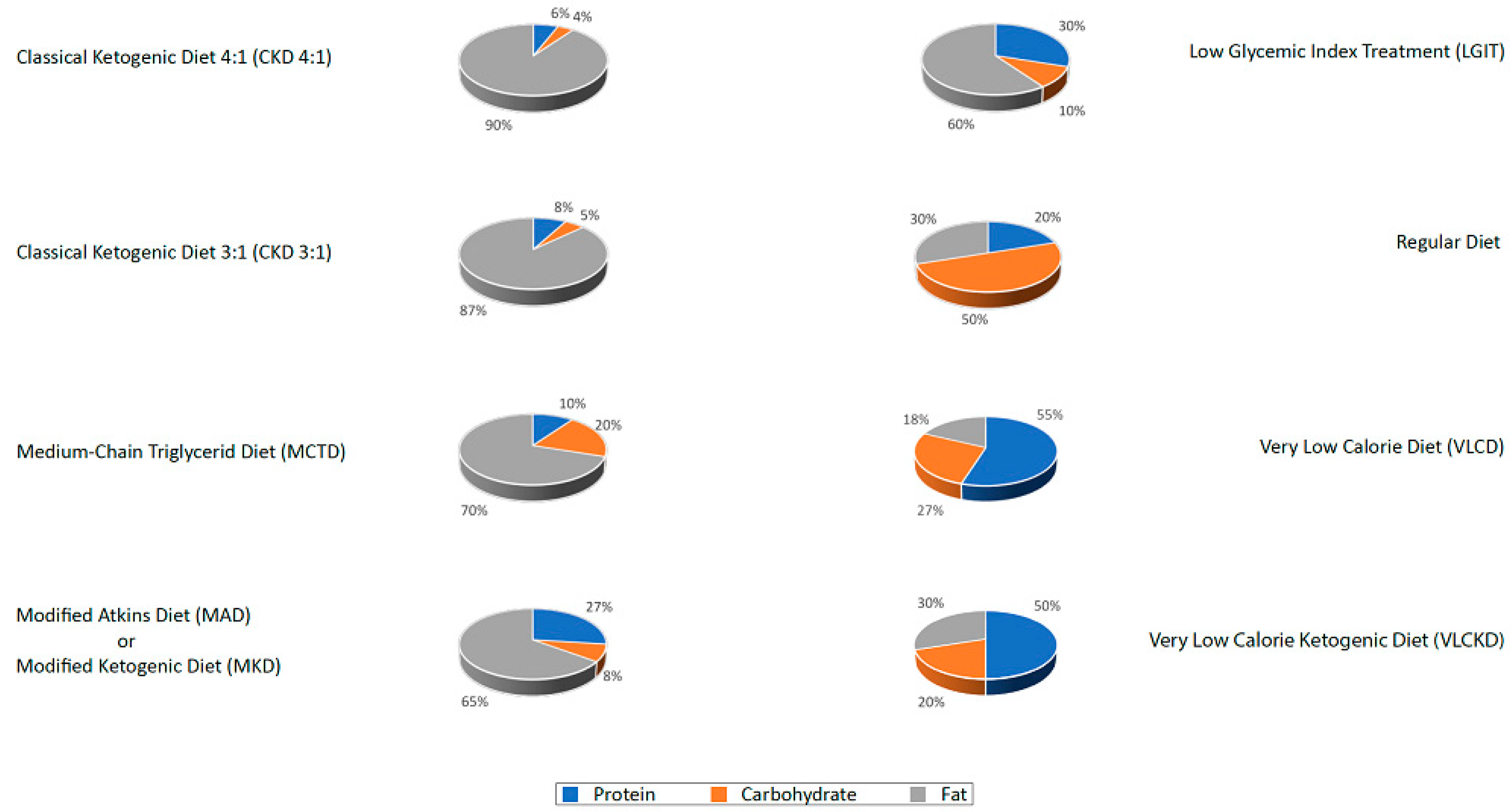
- The classic ketogenic diet (CKD), in which the ratio between fats and non-fats (carbohydrates + proteins) must be calculated; generally, this ratio is 3:1 or 4:1 (i.e., the intake in grams of fats is three or four times that of non-fats). This protocol is characterized by the higher content of fats compared to the protein portion (slightly reduced or normal) and carbohydrates (greatly reduced) [27];
- 2
- The supplementation of medium-chain triglycerides (MCT), in which about 60% of the caloric intake comes from MCT, whose metabolic fate can only be the production of energy; if taken in excess, acetyl-CoA will accumulate, resulting in turn in the biosynthesis of ketones [27];
- 3
- The modified Atkins diet (MAD), the most liberal in terms of protein intake and the least restrictive in terms of the need to weigh each food [27];
- 4
- The Very Low-Calorie Ketogenic Diet (VLCKD), an extremely restrictive nutritional protocol (600–800 kcal), limited in time (up to 12 weeks), characterized by a minimum protein content (≥75 g/day), a very limited carbohydrate content (30–50 g/day), a fixed amount of fat (20 g/day, mainly from olive oil and omega-3), and micronutrients to meet the Dietary Reference Intake (DRI), in accordance with the European Food Safety Authority (EFSA) [31,34].
3. Specific Evidence of the Effectiveness of KD in Headaches
3.1. Clinical Evidence in Migraine
3.2. Clinical Evidence in Cluster Headache
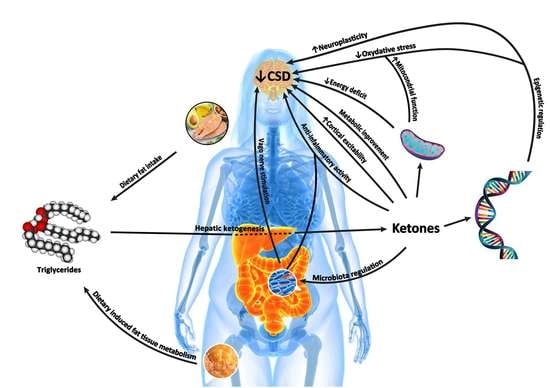
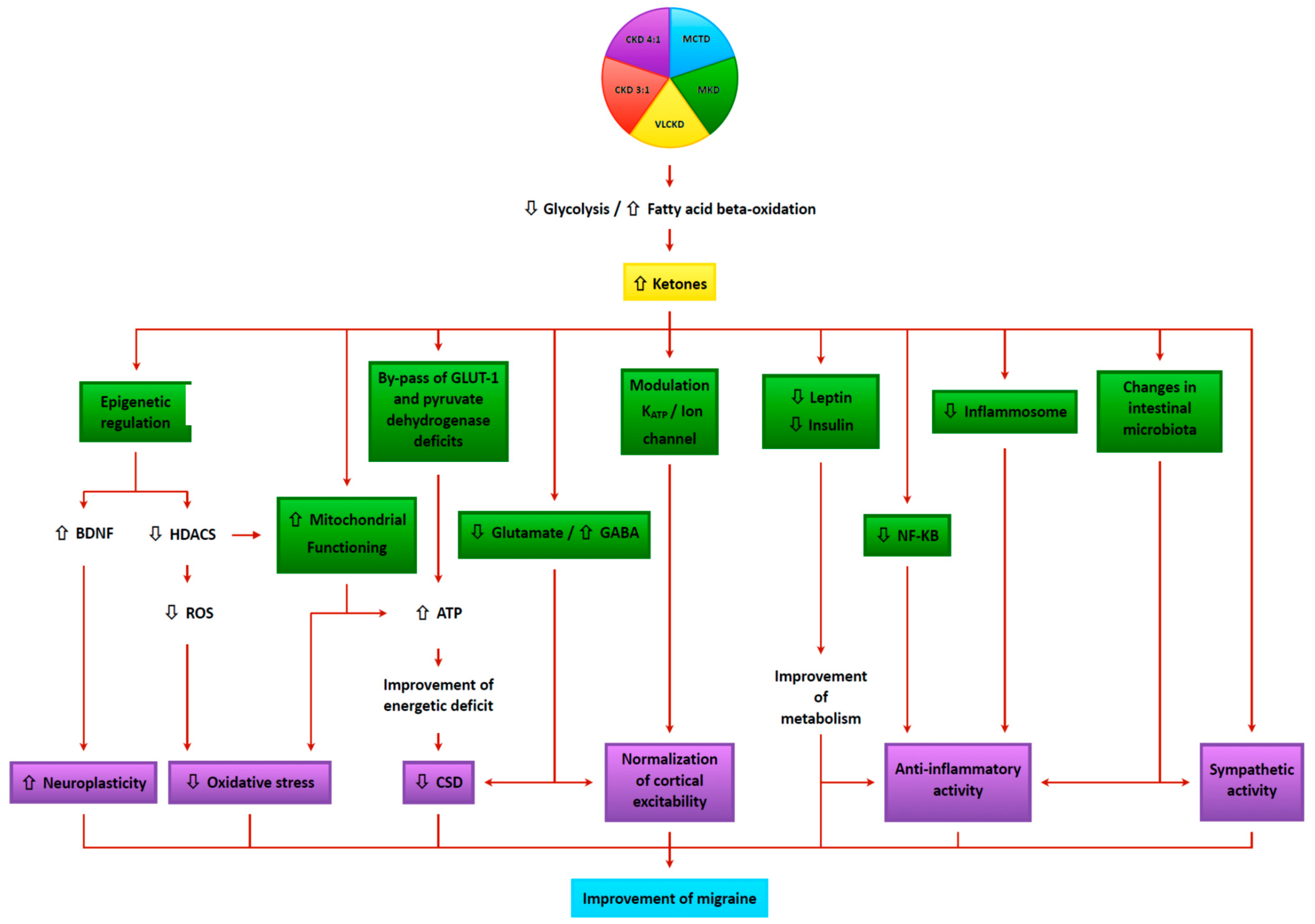
4. Mechanisms of Action of Kd in Treating Headache
4.1. Neurophysiologic Modulation
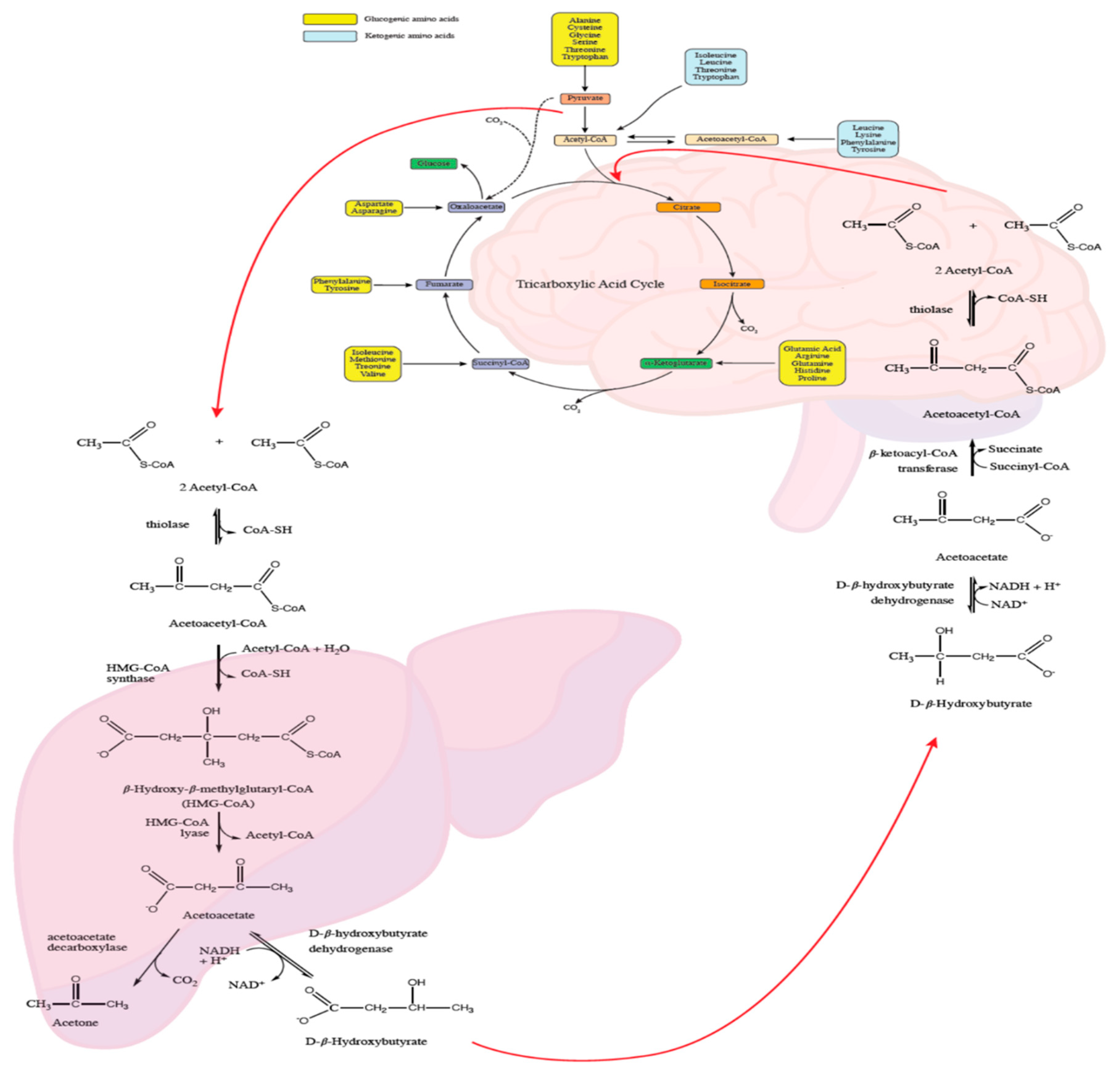
4.2. Cerebral Energy Metabolism
4.3. Alteration of Mitochondrial Dysfunction
4.4. Oxidative Stress
4.5. Anti-Inflammatory Mechanisms
4.6. Epigenetics
4.7. Cortical Spreading Depression (CSD)
4.8. Glutamate/GABA Balance
4.9. Ion Channels
4.10. Gut–Brain Axis
4.11. Intracerebral Glucose Metabolism
4.12. Migraine and Metabolic Syndrome
- -
- obesity, defined as abdominal circumference (≥95 cm man and ≥80 cm woman) plus at least 2 of the criteria below:
- -
- hypertension (blood pressure ≥130/85 mmHg or patient on antihypertensive therapy);
- -
- fasting blood glucose (≥110 mg/dL);
- -
- triglyceridemia (≥150 mg/dL);
- -
- low plasma HDL levels (<40 mg/dL man and <50 mg/dL woman).
5. Study Group Recommendations on the Management of Headache Patients Using a Ketogenic Diet
6. Recommendations
6.1. Patient Selection
6.2. Multidisciplinary Evaluation and Diet Therapy
- 1
- Unlike other neurological diseases, migraine can in some cases be triggered by specific foods; in general, excessive use of foods containing biogenic amines (aged cheeses and sausages), especially histamine (nuts), monosodium glutamate and processed foods should be avoided [154]. In addition, some patients have reported worsening headaches when consuming foods with gluten additives, excess fermentable oligo- and mono-saccharides and polyols (FODMAP) or using seed oil.
- 2
- In Italy, the VLCKD protocols are widely used for weight loss, generally not used in the neurological field as ketogenic therapies. However, these protocols have been used on numerous obese patients by all centers in our working group and their efficacy on migraine has been repeatedly reported in the literature [39,40,41,42]. The use of these diets should be limited to no more than 12 consecutive weeks [34], at the end of which the patient either exits the state of ketosis (even receiving the indication to follow a maintenance diet of LGIT or Mediterranean type without added sugars), or transit to a normo-caloric KD of longer duration.
- 3
- In the field of epilepsy, the use of 3:1 CKD is commonly used in children, as can be seen in the literature. On the contrary, since the centers involved in this board are mainly active in the treatment of adult subjects, few of their patients have been prescribed a CKD with a high ketogenic ratio (≥3:1) because of the difficulty of matching the caloric requirement to protein needs (for instance, in case of a protein need of 70 gr and considering a daily intake of 30 gr of carbohydrates, to fulfill the 3:1 ratio should include 300 gr of fats, for a total daily intake of 3100 kcal). Therefore, the most used diets were MKD (maintaining a ketogenic ratio around 2:1), MCTD, and VLCKD (for obese patients).
- 4
- Although exogenous ketone sources (salts or esters) are already commercially available and regarded as a promising therapy in both epilepsy [155] and migraine [21], no center of those involved in the working group has clinical experience with the use of these supplements. While waiting for data from clinical trials [156], some concerns were raised however about their use as it may only confer a partial effect compared to that of a KD. In fact, the therapeutic action of the diet is not only due to the role played by ketones, but also to the change in the macronutrients consumed (see LGIT diet) and the correction of insulin resistance typical of migraine sufferers. Therefore, the board expresses some skepticism and concluded that further investigations is warranted.
6.3. Contraindications
6.4. Patient Monitoring
6.5. Side Effects
6.6. Causes for Reduced Adherence, Compliance and Diet Cessation
6.7. Duration of the Diet
6.8. Pharmacological Management
- 1
- Topiramate may interfere with renal function by promoting nephrolithiasis and modifying the proper excretion of ketones through the urine, facilitating metabolic acidosis. In addition, it can cause weakness and electrolyte abnormalities related to diet. Its use should be carefully monitored, possibly limited and, where feasible, the dosage should be scaled back in subjects who were already taking it before starting a KD.
- 2
- Valproic acid can alter hepatic metabolism, potentially limiting ketone bodies’ production. The use of this drug should also be monitored or limited during KD.
- 3
- Beta-blockers and verapamil may result in bradycardia and weakness that could be exacerbated by KD, in which case drug dosages should be reduced.
- 4
- Corticosteroids, often used as salvage therapy in status migrainosus (a debilitating migraine attack lasting for more than 72 h), as a strategy to interrupt the medication overuse in MOH, and as prevention in CH, may interfere with ketogenesis because of its impact on hepatic functioning and produce hyperglycemia. Therefore, the use of steroids should be carefully evaluated, severely limited, and monitored during a KD.
- 5
- Flunarizine and amitriptyline, in addition to causing ECG alterations, may, along with valproic acid, result in increased appetite and weight. This could impede compliance with the diet and with progress in losing weight. These aspects should be considered in the case of co-administration.
- 6
- Although symptomatic drugs (non-steroidal anti-inflammatory drugs (NSAIDs), Triptans, Ergot-derivatives, combination drugs) are not contraindicated in KD, sometimes their overuse can nullify the diet’s preventive effect on migraine. From the experience of centers that have treated many patients with MOH using KD, even when patients do not halt medication overuse, it appears that in more than 50% of cases the diet is effective in blocking overuse of analgesics and headache chronicity. If this does not happen, a medication overuse withdrawal without corticosteroids can be combined without interrupting the diet, often with excellent results even in patients in whom previous attempts at medication overuse end have failed. In general, however, caution should be exercised with the overuse of NSAIDs and acetaminophen because of the impact these drugs may have on renal and hepatic metabolism.
6.9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Research Involving Human or Animals’ Participants
Abbreviations
| ATP | Adenosine Triphosphate |
| BDNF | Brain-Derived Neurotrophic Factor |
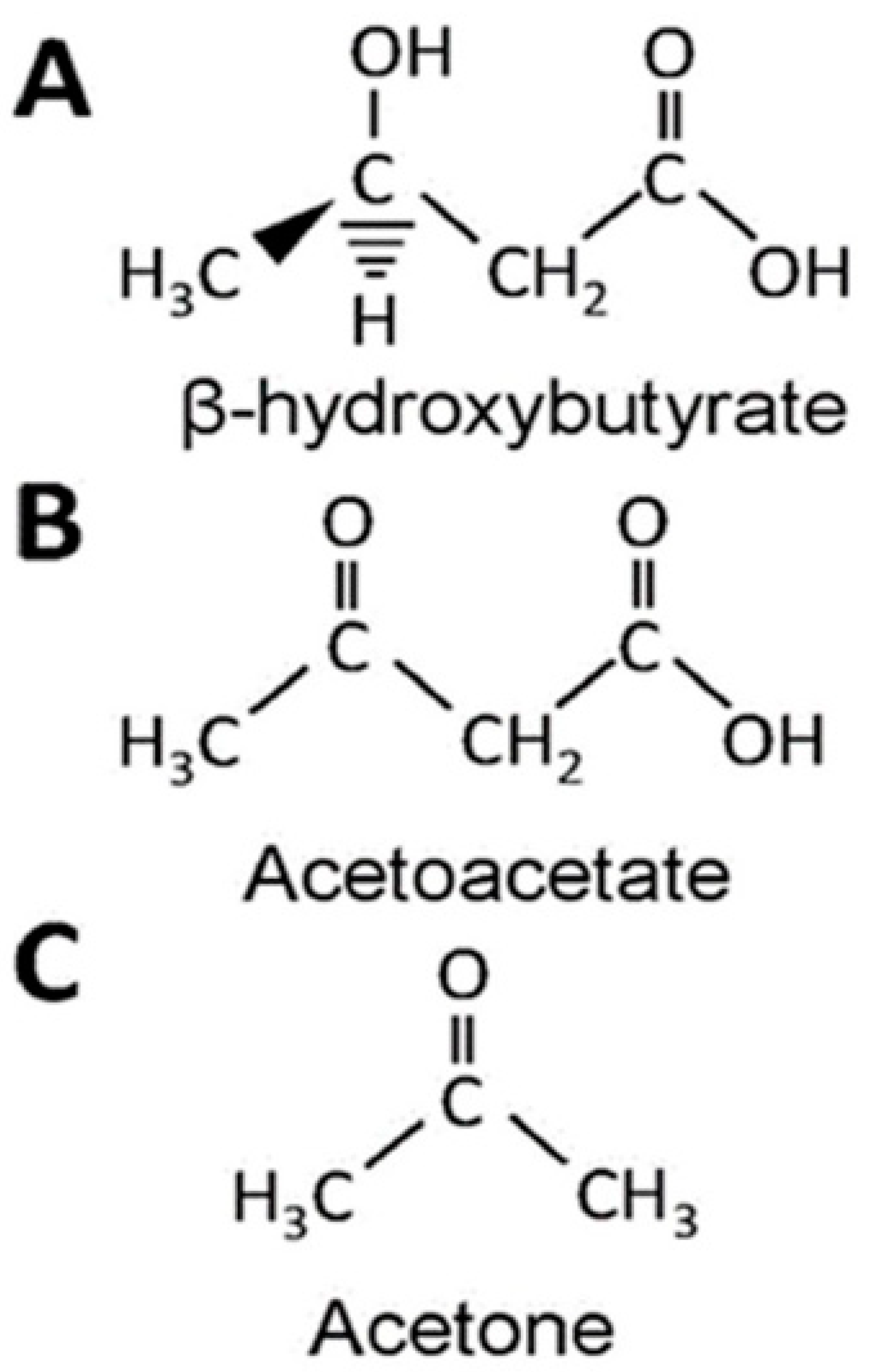
| BHB | β-Hydroxybutyrate |
| CCH | Chronic Cluster Headache |
| CGRP | Calcitonin-Gene Related Peptide |
| CH | Cluster Headache |
| CKD | Classic Ketogenic Diet |
| CoA-SH | Coenzyme A |
| CSD | Cortical Spreading Depression |
| EP | Evoked Potentials |
| DRI | Dietary Reference Intake |
| EFSA | European Food Safety Authority |
| GABA | Gamma-Amino Hydroxybutyric Acid |
| GLU | Glutamate |
| GLUT-1 | Glucose transporter protein 1 |
| HCA2 | Hydroxy-Carboxylic Acid Receptor 2 |
| HDAC | Histone Deacetylase |
| KATP | Adenosine triphosphate-sensitive potassium channels |
| KD | Ketogenic Diet |
| LCHF | Low-Carb High-Fat |
| LGIT | Low Glycemic Index Diet |
| MAD | Modified Atkins Diet |
| MCT | Medium-Chain Triglycerides |
| MCTD | Medium-Chain Triglycerides Diet |
| MetS | Metabolic Syndrome |
| miRNA | Micro RNA |
| MKD | Modified Ketogenic Diet |
| MOH | Medication Overuse Headache |
| Mn-SOD | Mitochondrial Superoxide Dismutase |
| NAD+ | Nicotinamide adenine dinucleotide (oxidized form) |
| NADH | Nicotinamide adenine dinucleotide (reduced form) |
| NF-KB | Nuclear Factor kappaB |
| NO | Nitric Oxide |
| ROS | Reactive Oxygen Species |
| OGTT | Oral glucose Tolerance Test |
| VLCKD | Very Low-Calorie Ketogenic Diet |
| VLCnKD | Very Low-Calorie non-Ketogenic Diet |
References
- WHO. Atlas of Headache Disorders and Resources in the World; WHO: Geneva, Switzerland, 2011; ISBN 9789241564212. [Google Scholar]
- Olesen, J. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [Green Version]
- Katsarava, Z.; Mania, M.; Lampl, C.; Herberhold, J.; Steiner, T.J. Poor medical care for people with migraine in Europe—Evidence from the Eurolight study. J. Headache Pain 2018, 19. [Google Scholar] [CrossRef]
- Piccinni, C.; Cevoli, S.; Ronconi, G.; Dondi, L.; Calabria, S.; Pedrini, A.; Esposito, I.; Favoni, V.; Pierangeli, G.; Cortelli, P.; et al. A real-world study on unmet medical needs in triptan-treated migraine: Prevalence, preventive therapies and triptan use modification from a large Italian population along two years. J. Headache Pain 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kadian, R. Headache, Migraine Prophylaxis; StatPearls Publishing: Treasure Island, FL, USA, 2018.
- Lipton, R.B.; Bigal, M.E.; Diamond, M.; Freitag, F.; Reed, M.L.; Stewart, W.F. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007, 68, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Whyte, C.A.; Tepper, S.J. Adverse effects of medications commonly used in the treatment of migraine. Exp. Rev. Neurother. 2009, 9, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Shah, P.V.; Balani, P.; Lopez, A.R.; Nobleza, C.M.N.; Khan, S. Comparing the efficacy, safety, and superiority of calcitonin gene-related peptide monoclonal antibodies and botox in preventing and treating migraines. Cureus 2021, 13. [Google Scholar] [CrossRef]
- How to Discuss Migraine Lifestyle Changes | First Contact. Available online: https://americanheadachesociety.org/topic/lifestyle-changes/ (accessed on 21 May 2021).
- Simshäuser, K.; Lüking, M.; Kaube, H.; Schultz, C.; Schmidt, S. Is mindfulness-based stress reduction a promising and feasible intervention for patients suffering from migraine? A randomized controlled pilot trial. Complement. Med. Res. 2020, 27, 19–30. [Google Scholar] [CrossRef]
- Fernando Prieto Peres, M.; Prieto Peres Mercante, J.; Belitardo de Oliveira, A. Non-pharmacological treatment for primary headaches prevention and lifestyle changes in a low-income community of Brazil: A randomized clinical trial. Headache 2019, 59, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, L.; Butler, N.; Seng, E.K. Health behaviors in episodic migraine: Why behavior change matters. Curr. Pain Headache Rep. 2018, 22. [Google Scholar] [CrossRef]
- Daniel, O.; Mauskop, A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr. Treat. Options Neurol. 2016, 18, 14. [Google Scholar] [CrossRef]
- Hindiyeh, N.A.; Zhang, N.; Farrar, M.; Banerjee, P.; Lombard, L.; Aurora, S.K. The role of diet and nutrition in migraine triggers and treatment: A systematic literature review. Headache 2020, 60, 1300–1316. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.; Vij, B. Diet and headache: Part 1. Headache 2016, 56, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. A bidirectional view of migraine and diet relationship. Neuropsychiatr. Dis. Treat. 2021, 17, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.; Vij, B. Diet and headache: Part 2. Headache 2016, 56, 1553–1562. [Google Scholar] [CrossRef]
- Gazerani, P. Migraine and diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Fofi, L.; Aurilia, C.; Egeo, G.; Caprio, M. Ketogenic diet in migraine: Rationale, findings and perspectives. Neurol. Sci. 2017, 38, 111–115. [Google Scholar] [CrossRef]
- Gross, E.C.; Klement, R.J.; Schoenen, J.; D’Agostino, D.P.; Fischer, D. Potential protective mechanisms of ketone bodies in migraine prevention. Nutrients 2019, 11, 811. [Google Scholar] [CrossRef] [Green Version]
- McDonald, T.J.W.; Cervenka, M.C. Ketogenic diets for adult neurological disorders. Neurotherapeutics 2018, 15, 1018–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temkin, O. The Falling Sickness: A History of Epilepsy from the Greeks to the Beginnings of Modern Neurology, The Johns Hopkins University Press, Revised ed.; Baltimore, MD, USA, 1994. [Google Scholar]
- Guelpa, G. La lutte contre l’épilepsie par la désintoxication et par la rééducation alimentaire. Rev. Ther. Med. Chir. 1911, 78, 8–13. [Google Scholar]
- Wilder, R. The effect of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307. [Google Scholar]
- Barborka, C.J. Ketogenic diet treatment of epilepsy in adults. JAMA 1928, 91, 73–78. [Google Scholar] [CrossRef]
- Kossoff, E.; Turnern, Z.; Doerrer, S.; Cervenka, M.; Henry, B. The Ketogenic and Modified Atkins Diet Treatments for Epilepsy and Other Disorders, 6th ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Cervenka, M.C.; Wood, S.; Bagary, M.; Balabanov, A.; Bercovici, E.; Brown, M.-G.; Devinsky, O.; Di Lorenzo, C.; Doherty, C.P.; Felton, E.; et al. International recommendations for the management of adults treated with ketogenic diet therapies. Neurol. Clin. Pract. 2020. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Invest. 2019, 42. [Google Scholar] [CrossRef]
- McNally, M.A.; Hartman, A.L. Ketone bodies in epilepsy. J. Neurochem. 2012, 121. [Google Scholar] [CrossRef] [Green Version]
- Cervenka, M.C.; Kossoff, E.H. Dietary treatment of intractable epilepsy. Contin. Lifelong Learn. Neurol. 2013, 19. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Product Nutrition and Allergies (NDA). Scientific opinion on the essential composition of total diet replacements for weight control. EFSA J. 2015, 13. [Google Scholar] [CrossRef]
- Koppel, S.J.; Swerdlow, R.H. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem. Int. 2018, 117. [Google Scholar] [CrossRef]
- Al-Mudallal, A.S.; LaManna, J.C.; Lust, W.D.; Harik, S.I. Diet-induced ketosis does not cause cerebral acidosis. Epilepsia 1996, 37. [Google Scholar] [CrossRef]
- Schnabel, T.G. An experience with a ketogenic dietary in migraine. Ann. Intern. Med. 1928, 2. [Google Scholar] [CrossRef]
- Barborka, C.J. Migraine: Results of treatment by ketogenic diet in fifty cases. J. Am. Med. Assoc. 1930, 95, 1825–1828. [Google Scholar] [CrossRef]
- Strahlman, R.S. Can ketosis help migraine sufferers? A case report. Headache J. Head Face Pain 2006, 46. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Currà, A.; Sirianni, G.; Coppola, G.; Bracaglia, M.; Cardillo, A.; De Nardis, L.; Pierelli, F. Diet transiently improves migraine in two twin sisters: Possible role of ketogenesis? Funct. Neurol. 2013, 28, 305–308. [Google Scholar] [PubMed]
- Di Lorenzo, C.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Bracaglia, M.; Di Lenola, D.; Siracusano, A.; Rossi, P.; Pierelli, F. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur. J. Neurol. 2015, 22. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Pinto, A.; Ienca, R.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Spagnoli, A.; Vestri, A.; et al. A randomized double-blind, cross-over trial of very low-calorie diet in overweight migraine patients: A possible role for ketones? Nutrients 2019, 11, 1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolkodoff, N.E.; Haase, G.M.; Firger, R.A. The effects of a unique medium chain triglyceride complex on migraine symptoms: A beta pilot study. World J. Adv. Res. Rev. 2020, 8. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Benedetto, C.; Corvisieri, S.; Del Favero, C.; Orlandi, F.; Allais, G.; Sinigaglia, S.; Fadda, M. Effectiveness of ketogenic diet in treatment of patients with refractory chronic migraine. Neurol. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Huffman, J.; Turner, Z.; Gladstein, J. Use of the modified Atkins diet for adolescents with chronic daily headache. Cephalalgia 2010, 30, 1014–1016. [Google Scholar] [CrossRef] [PubMed]
- Evcili, G.; Utku, U.; Oün, M.N.; Özdemir, G. Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri 2018, 30, 8–11. [Google Scholar] [CrossRef]
- Finsterer, J.; Frank, M. Low-glycemic-index diet relieving migraine but inducing muscle cramps. J. Neurosci. Rural Pract. 2019, 10, 552–554. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, C.; Coppola, G.; Di Lenola, D.; Evangelista, M.; Sirianni, G.; Rossi, P.; Di Lorenzo, G.; Serrao, M.; Pierelli, F. Efficacy of modified Atkins ketogenic diet in chronic cluster headache: An open-label, single-arm, clinical trial. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- OUCH (UK). Available online: https://ouchuk.org/forum/possible-cch-remission-ketoketogeniclchf-diet (accessed on 3 July 2021).
- ClusterHeadaches. Available online: http://www.clusterheadaches.com/cgi-bin/yabb2/YaBB.pl?num=1534559885 (accessed on 3 July 2021).
- OUCH. Italia ONLUS. Available online: https://www.grappolaiuto.it/forum/index.php?topic=10690.msg120329#msg120329 (accessed on 3 July 2021).
- ClusterBusters. Available online: https://clusterbusters.org/forums/topic/4655-ketogenic-diet/ (accessed on 3 July 2021).
- Coppola, G.; Di Lorenzo, C.; Schoenen, J.; Pierelli, F. Habituation and sensitization in primary headaches. J. Headache Pain 2013, 14. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Coppola, G.; Bracaglia, M.; Di Lenola, D.; Evangelista, M.; Sirianni, G.; Rossi, P.; Di Lorenzo, G.; Serrao, M.; Parisi, V.; et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: A multimodal evoked potentials study. J. Headache Pain 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, C.; Coppola, G.; Bracaglia, M.; Di Lenola, D.; Sirianni, G.; Rossi, P.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Cervenka, M.C.; et al. A ketogenic diet normalizes interictal cortical but not subcortical responsivity in migraineurs. BMC Neurol. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.H.; May, A. The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevoli, S.; Favoni, V.; Cortelli, P. Energy metabolism impairment in migraine. Curr. Med. Chem. 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.L.; Niddam, D.M. Brain metabolism and structure in chronic migraine. Curr. Pain Headache Rep. 2020, 24. [Google Scholar] [CrossRef] [PubMed]
- Salway, J. Metabolism at a Glance, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chowdhury, G.M.; Jiang, L.; Rothman, D.L.; Behar, K.L. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J. Cereb. Blood Flow Metab. 2014, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, G.F. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmond, J.; Robbins, R.A.; Bergstrom, J.D.; Cole, R.A.; de Vellis, J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J. Neurosci. Res. 1987, 18. [Google Scholar] [CrossRef]
- McKenna, M.C.; Tildon, J.T.; Stevenson, J.H.; Boatright, R.; Huang, S. Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: Differences revealed using aminooxyacetate. Dev. Neurosci. 1993, 15. [Google Scholar] [CrossRef]
- Srivastava, S.; Baxa, U.; Niu, G.; Chen, X.; Veech, R.L. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 2013, 65. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Geffroy, G.; Desquiret-Dumas, V.; Gueguen, N.; Bris, C.; Belal, S.; Amati-Bonneau, P.; Chevrollier, A.; Barth, M.; Henrion, D.; et al. The addition of ketone bodies alleviates mitochondrial dysfunction by restoring complex I assembly in a MELAS cellular model. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Martins-Oliveira, M.; Akerman, S.; Holland, P.R.; Hoffmann, J.R.; Tavares, I.; Goadsby, P.J. Neuroendocrine signaling modulates specific neural networks relevant to migraine. Neurobiol. Dis. 2017, 101. [Google Scholar] [CrossRef] [Green Version]
- Noebels, J.L.; Avoli, M.; Rogawski, M.; Olsen, R.; Delgado-Escueta, A.V. “Jasper’s basic mechanisms of the epilepsies” workshop. Epilepsia 2010, 51. [Google Scholar] [CrossRef]
- Kim, D.Y.; Simeone, K.A.; Simeone, T.A.; Pandya, J.D.; Wilke, J.C.; Ahn, Y.; Geddes, J.W.; Sullivan, P.G.; Rho, J.M. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 2015, 78. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, C.; Pierelli, F.; Coppola, G.; Grieco, G.S.; Rengo, C.; Ciccolella, M.; Magis, D.; Bolla, M.; Casali, C.; Santorelli, F.M.; et al. Mitochondrial DNA haplogroups influence the therapeutic response to riboflavin in migraineurs. Neurology 2009, 72, 1588–1594. [Google Scholar] [CrossRef]
- Gross, E.C.; Putananickal, N.; Orsini, A.L.; Vogt, D.R.; Sandor, P.S.; Schoenen, J.; Fischer, D. Mitochondrial function and oxidative stress markers in higher-frequency episodic migraine. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Lucchesi, C.; Baldacci, F.; Cafalli, M.; Chico, L.; Lo Gerfo, A.; Bonuccelli, U.; Siciliano, G.; Gori, S. Evidences of reduced antioxidant activity in patients with chronic migraine and medication-overuse headache. Headache J. Head Face Pain 2015, 55. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Migraine triggers and oxidative stress: A narrative review and synthesis. Headache 2016, 56, 12–35. [Google Scholar] [CrossRef]
- Goschorska, M.; Gutowska, I.; Baranowska-bosiacka, I.; Barczak, K.; Chlubek, D. The use of antioxidants in the treatment of migraine. Antioxidants 2020, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Davis, L.M.; Sullivan, P.G.; Maalouf, M.; Simeone, T.; Van Brederode, J.; Rho, J.M. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007, 101. [Google Scholar] [CrossRef]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.-C.; Yan, S.-D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Invest. 2003, 112. [Google Scholar] [CrossRef] [Green Version]
- Julio-Amilpas, A.; Montiel, T.; Soto-Tinoco, E.; Gerónimo-Olvera, C.; Massieu, L. Protection of hypoglycemia-induced neuronal death by β-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J. Cereb. Blood Flow Metab. 2015, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350. [Google Scholar] [CrossRef]
- Mejía-Toiber, J.; Montiel, T.; Massieu, L. d-β-hydroxybutyrate prevents glutamate-mediated lipoperoxidation and neuronal damage elicited during glycolysis inhibition in vivo. Neurochem. Res. 2006, 31. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339. [Google Scholar] [CrossRef] [Green Version]
- Neeb, L.; Reuter, U. Nitric oxide in migraine. CNS Neurol. Disord. Drug Targets 2008, 6, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Tassorelli, C.; Cappelletti, D.; Sandrini, G.; Nappi, G. Activation of the transcription factor NF-κB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology 2005, 26, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Zandi-Nejad, K.; Takakura, A.; Jurewicz, M.; Chandraker, A.K.; Offermanns, S.; Mount, D.; Abdi, R. The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J. 2013, 27, 4366–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taggart, A.K.P.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Müller-Fielitz, H.; Pokorná, B.; Vollbrandt, T.; Stölting, I.; Nadrowitz, R.; et al. The b-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the brain: One molecule, multiple mechanisms. Neurochem. Res. 2017, 42. [Google Scholar] [CrossRef]
- Schuh, E.; Lohse, P.; Ertl-Wagner, B.; Witt, M.; Krumbholz, M.; Frankenberger, M.; Gerdes, L.-A.; Hohlfeld, R.; Kümpfel, T. Expanding spectrum of neurologic manifestations in patients with NLRP3 low-penetrance mutations. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21. [Google Scholar] [CrossRef] [Green Version]
- Sekhavat, A.; Sun, J.-M.; Davie, J.R. Competitive inhibition of histone deacetylase activity by trichostatin A and butyrate. Biochem. Cell Biol. 2007, 85. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Terrazzino, S.; Cargnin, S.; Viana, M.; Sances, G.; Tassorelli, C. Brain-derived neurotrophic factor Val66Met gene polymorphism impacts on migraine susceptibility: A meta-analysis of case-control studies. Front. Neurol. 2017, 8, 159. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Di Lorenzo, G.; Sances, G.; Ghiotto, N.; Guaschino, E.; Grieco, G.S.; Santorelli, F.M.; Casali, C.; Troisi, A.; Siracusano, A.; et al. Drug consumption in medication overuse headache is influenced by brain-derived neurotrophic factor Val66Met polymorphism. J. Headache Pain 2009, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, C.; Di Lorenzo, G.; Daverio, A.; Pasqualetti, P.; Coppola, G.; Giannoudas, I.; Barone, Y.; Grieco, G.S.; Niolu, C.; Pascale, E.; et al. The Val66Met polymorphism of the BDNF gene influences trigeminal pain-related evoked responses. J. Pain 2012, 13. [Google Scholar] [CrossRef]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic diet and microRNAs linked to antioxidant biochemical homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Park, K.Y.; Fletcher, J.R.; Raddant, A.C.; Russo, A.F. Epigenetic regulation of the calcitonin gene-related peptide gene in trigeminal glia. Cephalalgia 2011, 31, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; He, X.; Luan, G.; Li, T. Role of DNA methylation and adenosine in ketogenic diet for pharmacoresistant epilepsy: Focus on epileptogenesis and associated comorbidities. Front. Neurol. 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.D.A.R.; Ataíde, T.D.R.; de Oliveira, S.L.; Lucena, A.L.D.M.; de Lira, C.E.P.R.; Soares, A.A.; de Almeida, C.B.S.; da Silva, A.X. Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci. Lett. 2008, 434. [Google Scholar] [CrossRef]
- Masino, S.; Rho, J. Mechanisms of ketogenic diet action. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J., Avoli, M., Rogawski, M., Olsen, R., Delgado-Escueta, A., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Zaletel, M.; Strucl, M.; Bajrovi, F.; Pogacnik, T. Coupling between visual evoked cerebral blood flow velocity responses and visual evoked potentials in migraneurs. Cephalalgia 2005, 25. [Google Scholar] [CrossRef]
- Montagna, P.; Cortelli, P.; Monari, L.; Pierangeli, G.; Parchi, P.; Lodi, R.; Iotti, S.; Frassineti, C.; Zaniol, P.; Lugaresi, E.; et al. 31P-magnetic resonance spectroscopy in migraine without aura. Neurology 1994, 44. [Google Scholar] [CrossRef] [PubMed]
- Lisicki, M.; D’Ostilio, K.; Coppola, G.; Scholtes, F.; Maertens de Noordhout, A.; Parisi, V.; Schoenen, J.; Magis, D. Evidence of an increased neuronal activation-to-resting glucose uptake ratio in the visual cortex of migraine patients: A study comparing 18FDG-PET and visual evoked potentials. J. Headache Pain 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, J. Deficient habituation of evoked cortical potentials in migraine: A link between brain biology, behavior and trigeminovascular activation? Biomed. Pharmacother. 1996, 50. [Google Scholar] [CrossRef]
- Charles, A.C.; Baca, S.M. Cortical spreading depression and migraine. Nat. Rev. Neurol. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D.; Odink, J.; Bos, K.D.; Malessy, M.J.A.; Bruyn, G.W. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology 1990, 40. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Cairns, B.E. Monosodium glutamate alters the response properties of rat trigeminovascular neurons through activation of peripheral NMDA receptors. Neuroscience 2016, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, J.; Charles, A. Glutamate and its receptors as therapeutic targets for migraine. Neurotherapeutics 2018, 15. [Google Scholar] [CrossRef] [Green Version]
- Bathel, A.; Schweizer, L.; Stude, P.; Glaubitz, B.; Wulms, N.; Delice, S.; Schmidt-Wilcke, T. Increased thalamic glutamate/glutamine levels in migraineurs. J. Headache Pain 2018, 19. [Google Scholar] [CrossRef]
- Ma, W.; Berg, J.; Yellen, G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J. Neurosci. 2007, 27, 3618–3625. [Google Scholar] [CrossRef] [Green Version]
- Al-Karagholi, M.A.M.; Hansen, J.M.; Severinsen, J.; Jansen-Olesen, I.; Ashina, M. The KATP channel in migraine pathophysiology: A novel therapeutic target for migraine. J. Headache Pain 2017, 18. [Google Scholar] [CrossRef]
- Potic, A.; Nmezi, B.; Padiath, Q.S. CAPOS syndrome and hemiplegic migraine in a novel pedigree with the specific ATP1A3 mutation. J. Neurol. Sci. 2015, 358, 453–456. [Google Scholar] [CrossRef]
- Anderson, G. Integrating pathophysiology in migraine: Role of the gut microbiome and melatonin. Curr. Pharm. Des. 2019, 25. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, S.; Shu, H.; Yanagisawa, L.; Tao, F. Gut microbiota dysbiosis enhances migraine-like pain via TNFα upregulation. Mol. Neurobiol. 2020, 57. [Google Scholar] [CrossRef]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68. [Google Scholar] [CrossRef]
- Forsythe, P.; Kunze, W.A.; Bienenstock, J. On communication between gut microbes and the brain. Curr. Opin. Gastroenterol. 2012, 28. [Google Scholar] [CrossRef]
- Miglis, M.G. Migraine and autonomic dysfunction: Which is the horse and which is the jockey? Curr. Pain Headache Rep. 2018, 22. [Google Scholar] [CrossRef]
- Mauskop, A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia 2005, 25, 82–86. [Google Scholar] [CrossRef]
- Cecchini, A.P.; Mea, E.; Tullo, V.; Curone, M.; Franzini, A.; Broggi, G.; Savino, M.; Bussone, G.; Leone, M. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: Preliminary data. Neurol. Sci. 2009, 30. [Google Scholar] [CrossRef]
- Sadler, R.; Purdy, R.; Rahey, S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia 2002, 22. [Google Scholar] [CrossRef] [PubMed]
- Basic, S.; Sporis, D.; Chudy, D.; Grahovac, G.; Nevajda, B. The effect of vagus nerve stimulation on migraine in patient with intractable epilepsy: Case report. Neurol. Sci. 2013, 34. [Google Scholar] [CrossRef] [PubMed]
- Mwamburi, M.; Liebler, E.J.; Tenaglia, A.T. Review of non-invasive vagus nerve stimulation (gammaCore): Efficacy, safety, potential impact on comorbidities, and economic burden for episodic and chronic cluster headache. Am. J. Manag. Care 2017, 23, S317–S325. [Google Scholar] [PubMed]
- Wheless, J.W.; Gienapp, A.J.; Ryvlin, P. Vagus nerve stimulation (VNS) therapy update. Epilepsy Behav. 2018, 88. [Google Scholar] [CrossRef]
- Won, Y.J.; Lu, V.B.; Puhl, H.L.; Ikeda, S.R. β- hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci. 2013, 33, 19314–19325. [Google Scholar] [CrossRef] [PubMed]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilm. Microbiomes 2019, 5. [Google Scholar] [CrossRef]
- De Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability–results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017, 71. [Google Scholar] [CrossRef]
- Martami, F.; Togha, M.; Seifishahpar, M.; Ghorbani, Z.; Ansari, H.; Karimi, T.; Jahromi, S.R. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhou, Q.; Qiu, C.Z.; Dai, W.K.; Wang, H.P.; Li, Y.H.; Liao, J.X.; Lu, X.G.; Lin, S.F.; Ye, J.H.; et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef]
- Weller, C.M.; Leen, W.G.; Neville, B.G.; Duncan, J.S.; de Vries, B.; Geilenkirchen, M.A.; Haan, J.; Kamsteeg, E.-J.; Ferrari, M.D.; van den Maagdenberg, A.M.; et al. A novel SLC2A1 mutation linking hemiplegic migraine with alternating hemiplegia of childhood. Cephalalgia 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- Urbizu, A.; Cuenca-León, E.; Raspall-Chaure, M.; Gratacòs, M.; Conill, J.; Redecillas, S.; Roig-Quilis, M.; Macaya, A. Paroxysmal exercise-induced dyskinesia, writer’s cramp, migraine with aura and absence epilepsy in twin brothers with a novel SLC2A1 missense mutation. J. Neurol. Sci. 2010, 295. [Google Scholar] [CrossRef]
- Mohammad, S.S.; Coman, D.; Calvert, S. Glucose transporter 1 deficiency syndrome and hemiplegic migraines as a dominant presenting clinical feature. J. Paediatr. Child Health 2014, 50, 1025–1026. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Andreeva, V.A.; Galan, P.; Julia, C.; Fezeu, L.; Hercberg, S.; Kesse-Guyot, E. A systematic literature review of observational studies of the bidirectional association between metabolic syndrome and migraine. Diabetes Metab. 2019, 45. [Google Scholar] [CrossRef]
- He, Z.; Dong, L.; Zhang, Y.; Kong, Q.; Tan, G.; Zhou, J. Metabolic syndrome in female migraine patients is associated with medication overuse headache: A clinic-based study in China. Eur. J. Neurol. 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- Streel, S.; Donneau, A.-F.; Dardenne, N.; Hoge, A.; Albert, A.; Schoenen, J.; Guillaume, M. Screening for the metabolic syndrome in subjects with migraine. Cephalalgia 2017, 37. [Google Scholar] [CrossRef]
- Rainero, I.; Limone, P.; Ferrero, M.; Valfrè, W.; Pelissetto, C.; Rubino, E.; Gentile, S.; Lo Giudice, R.; Pinessi, L. Insulin sensitivity is impaired in patients with migraine. Cephalalgia 2005, 25. [Google Scholar] [CrossRef]
- Cavestro, C.; Rosatello, A.; Micca, G.; Ravotto, M.; Pia Marino, M.; Asteggiano, G.; Beghi, E. Insulin metabolism is altered in migraineurs: A new pathogenic mechanism for migraine? Headache J. Head Face Pain 2007, 47. [Google Scholar] [CrossRef]
- Bigal, M.E. Migraine and cardiovascular disease. Arq. Neuropsiquiatr. 2011, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelaye, B.; Sacco, S.; Brown, W.J.; Nitchie, H.L.; Ornello, R.; Peterlin, B.L. Body composition status and the risk of migraine: A meta-analysis. Neurology 2017, 88, 1795–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossoni de Oliveira, V.; Camboim Rockett, F.; Castro, K.; da Silveira Perla, A.; Fagundes Chaves, M.L.; Schweigert Perry, I.D. Body mass index, abdominal obesity, body fat and migraine features in women. Nutr. Hosp. 2013, 28, 1115–1120. [Google Scholar]
- Jahromi, S.R.; Abolhasani, M.; Meysamie, A.; Togha, M. The effect of body fat mass and fat free mass on migraine headache. Iran. J. Neurol. 2013, 12, 23–27. [Google Scholar]
- Verrotti, A.; Carotenuto, M.; Altieri, L.; Parisi, P.; Tozzi, E.; Belcastro, V.; Esposito, M.; Guastaferro, N.; Ciuti, A.; Mohn, A.; et al. Migraine and obesity: Metabolic parameters and response to a weight loss programme. Pediatr. Obes. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Di Fonzo, A.; Penta, L.; Agostinelli, S.; Parisi, P. Obesity and headache/migraine: The importance of weight reduction through lifestyle modifications. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Romano, L.; Marchetti, M.; Gualtieri, P.; Belcastro, M.; De Santis, G.L.; Perrone, M.A.; De Lorenzo, A.; Di Renzo, L.; De Santis, L. Effects of a personalized VLCKD on body composition and resting energy expenditure in the reversal of diabetes to prevent complications. Nutrients 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Berilgen, M.; Bulut, S.; Gonen, M.; Tekatas, A.; Dag, E.; Mungen, B. Comparison of the effects of amitriptyline and flunarizine on weight gain and serum leptin, C peptide and insulin levels when used as migraine preventive treatment. Cephalalgia 2005, 25. [Google Scholar] [CrossRef]
- Strother, L.C.; Srikiatkhachorn, A.; Supronsinchai, W. Targeted orexin and hypothalamic neuropeptides for migraine. Neurotherapeutics 2018, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caproni, S.; Corbelli, I.; Pini, L.A.; Cupini, M.L.; Calabresi, P.; Sarchielli, P. Migraine preventive drug-induced weight gain may be mediated by effects on hypothalamic peptides: The results of a pilot study. Cephalalgia 2011, 31. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.R. Biology of neuropeptides: Orexinergic involvement in primary headache disorders. Headache J. Head Face Pain 2017, 57. [Google Scholar] [CrossRef] [PubMed]
- Rubino, E.; Vacca, A.; Govone, F.; Gai, A.; Boschi, S.; Zucca, M.; De Martino, P.; Gentile, S.; Pinessi, L.; Rainero, I. Investigating the role of adipokines in chronic migraine. Cephalalgia 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Granella, F.; Prudenzano, M.P.; Pini, L.A.; Guidetti, V.; Bono, G.; Pinessi, L.; Alessandri, M.; Antonaci, F.; Fanciullacci, M.; et al. Italian guidelines for primary headaches: 2012 revised version. J. Headache Pain 2012, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusticali, B.; Bussone, G.; Aguggia, M.; Allais, G.B.; Barbanti, P.; Cortelli, P.; De Simone, R.; Ferri, L.; Manzoni, G.C.; Merighi, L.; et al. Cefalea Nell’adulto: Linee Guida Nazionali di Riferimento per la Prevenzione e la Terapia; AGENAS: Rome, Italy, 2011.
- Raucci, U.; Boni, A.; Evangelisti, M.; Della Vecchia, N.; Velardi, M.; Ursitti, F.; Terrin, G.; Di Nardo, G.; Reale, A.; Villani, A.; et al. Lifestyle modifications to help prevent headache at a developmental age. Front. Neurol. 2021, 11. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Sani, M.A.; Dahri, M.; Ghalichi, F.; Ghavami, A.; Arjang, P.; Tarighat-Esfanjani, A. The role of nutrients in the pathogenesis and treatment of migraine headaches: Review. Biomed. Pharmacother. 2018, 102, 317–325. [Google Scholar] [CrossRef]
- Maghsoumi-Norouzabad, L.; Mansoori, A.; Abed, R.; Shishehbor, F. Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2018, 21. [Google Scholar] [CrossRef] [PubMed]
- Zaeem, Z.; Zhou, L.; Dilli, E. Headaches: A review of the role of dietary factors. Curr. Neurol. Neurosci. Rep. 2016, 16. [Google Scholar] [CrossRef]
- Poff, A.M.; Rho, J.M.; D’Agostino, D.P. Ketone administration for seizure disorders: History and rationale for ketone esters and metabolic alternatives. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, E.; Putananickal, N.; Orsini, A.-L.; Schmidt, S.; Vogt, D.R.; Cichon, S.; Sandor, P.; Fischer, D. Efficacy and safety of exogenous ketone bodies for preventive treatment of migraine: A study protocol for a single-centred, randomised, placebo-controlled, double-blind crossover trial. Trials 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Van der Louw, E.J.T.M.; Williams, T.J.; Henry-Barron, B.J.; Olieman, J.F.; Duvekot, J.J.; Vermeulen, M.J.; Bannink, N.; Williams, M.; Neuteboom, R.F.; Kossoff, E.H.; et al. Ketogenic diet therapy for epilepsy during pregnancy: A case series. Seizure 2017, 45. [Google Scholar] [CrossRef] [Green Version]
- Michaels, J.D.; Hoss, E.; DiCaudo, D.J.; Price, H. Prurigo pigmentosa after a strict ketogenic diet. Pediatr. Dermatol. 2015, 32. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhao, Y.; Zhang, X.; Li, B.; Cui, R. the effects of calorie restriction in depression and potential mechanisms. Curr. Neuropharmacol. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
| DIAGNOSIS |
|---|
| Migraine |
| MOH |
| CH |
| INDICATIONS |
| Overweight/Obese |
| Metabolic Syndrome |
| Drug resistance/non-tolerability |
| Patients’ specific request |
| AGE |
| Adulthood |
| Lack of data in childhood |
| TYPE OF DIET |
| CKD 4:1 |
| CKD 3:1 |
| MKD |
| MCTD |
| LGIT |
| VLCKD (only in case of overweight or obesity) |
| ABSOLUTE CONTRAINDICATIONS | RELATIVE CONTRAINDICATIONS |
|---|---|
| Carnitine deficiency (primary) Carnitine palmitoyltransferase (CPT) I or II deficiency Carnitine translocase deficiency β-oxidation dfects Medium-chain acyl dehydrogenase deficiency Long-chain acyl dehydrogenase deficiency Short-chain acyl dehydrogenase deficiency Long-chain 3-hydroxyacyl-CoA deficiency Medium-chain 3-hydroxyacyl-CoA deficiency Pyruvate carboxylase deficiency Porphyria | Pregnancy and breastfeeding Renal failure Severe nephrolithiasis Hepatic failure Pancreatitis Type1 Diabetes Mellitus Arrhythmias Angina Recent myocardial infarction Severe osteoporosis Alcoholism Eating disorder Poor compliance Inability to maintain adequate nutrition |
| MONITORING | ||||
|---|---|---|---|---|
| Before Starting | Every Day | Every 6 Months | Every 12 Months | In Case of Non-Response to KD |
| Blood Laboratory assessment Complete blood count Glucose * Basal insulin OGTT (Glucose level, Insulin level) Total cholesterol LDL, HDL, Tg Direct bilirubin Indirect bilirubin GOT e GPT Uric Acid/Nitrogen Creatinine Homocysteine Protein electrophoresis Na, K, Cl, Ca, P, Mg Folates Vitamin B12 Vitamin D Urinalysis EKG Blood pressure Weight | Headache diaries Eating diaries | Blood Laboratory assessment Complete blood count Glucose * Basal insulin Total cholesterol LDL, HDL, Tg Direct bilirubin Indirect bilirubin GOT e GPT Uric Acid/Nitrogen Creatinine Homocysteine Protein electrophoresis Na, K, Cl, Ca, P, Mg Folates Vitamin B12 Vitamin D Urinalysis EKG Blood pressure Weight | BMD/DEXA Abdominal ultrasonography | Evaluation of ketones (blood, urine or breath) |
| SIDE EFFECTS | ||
|---|---|---|
| COMMON | INFREQUENT | VERY INFREQUENT |
| Muscle cramps Fatigue Hypotension Constipation Undesired weight loss | Hiperlipidaemia Gallbladder stones Menstrual irregularity Alopecia Nail fragility | Nausea Vomiting Abdominal pain Diarrhoea Prurigo pigmentosa (keto rash) * Mood disorders * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, C.; Ballerini, G.; Barbanti, P.; Bernardini, A.; D’Arrigo, G.; Egeo, G.; Frediani, F.; Garbo, R.; Pierangeli, G.; Prudenzano, M.P.; et al. Applications of Ketogenic Diets in Patients with Headache: Clinical Recommendations. Nutrients 2021, 13, 2307. https://doi.org/10.3390/nu13072307
Di Lorenzo C, Ballerini G, Barbanti P, Bernardini A, D’Arrigo G, Egeo G, Frediani F, Garbo R, Pierangeli G, Prudenzano MP, et al. Applications of Ketogenic Diets in Patients with Headache: Clinical Recommendations. Nutrients. 2021; 13(7):2307. https://doi.org/10.3390/nu13072307
Chicago/Turabian StyleDi Lorenzo, Cherubino, Giovanna Ballerini, Piero Barbanti, Andrea Bernardini, Giacomo D’Arrigo, Gabriella Egeo, Fabio Frediani, Riccardo Garbo, Giulia Pierangeli, Maria Pia Prudenzano, and et al. 2021. "Applications of Ketogenic Diets in Patients with Headache: Clinical Recommendations" Nutrients 13, no. 7: 2307. https://doi.org/10.3390/nu13072307
APA StyleDi Lorenzo, C., Ballerini, G., Barbanti, P., Bernardini, A., D’Arrigo, G., Egeo, G., Frediani, F., Garbo, R., Pierangeli, G., Prudenzano, M. P., Rebaudengo, N., Semeraro, G., Sirianni, G., Valente, M., Coppola, G., Cervenka, M. C., & Spera, G. (2021). Applications of Ketogenic Diets in Patients with Headache: Clinical Recommendations. Nutrients, 13(7), 2307. https://doi.org/10.3390/nu13072307