Human Milk Endocannabinoid Levels as a Function of Obesity and Diurnal Rhythm
Abstract
:1. Introduction
2. Materials and Methods
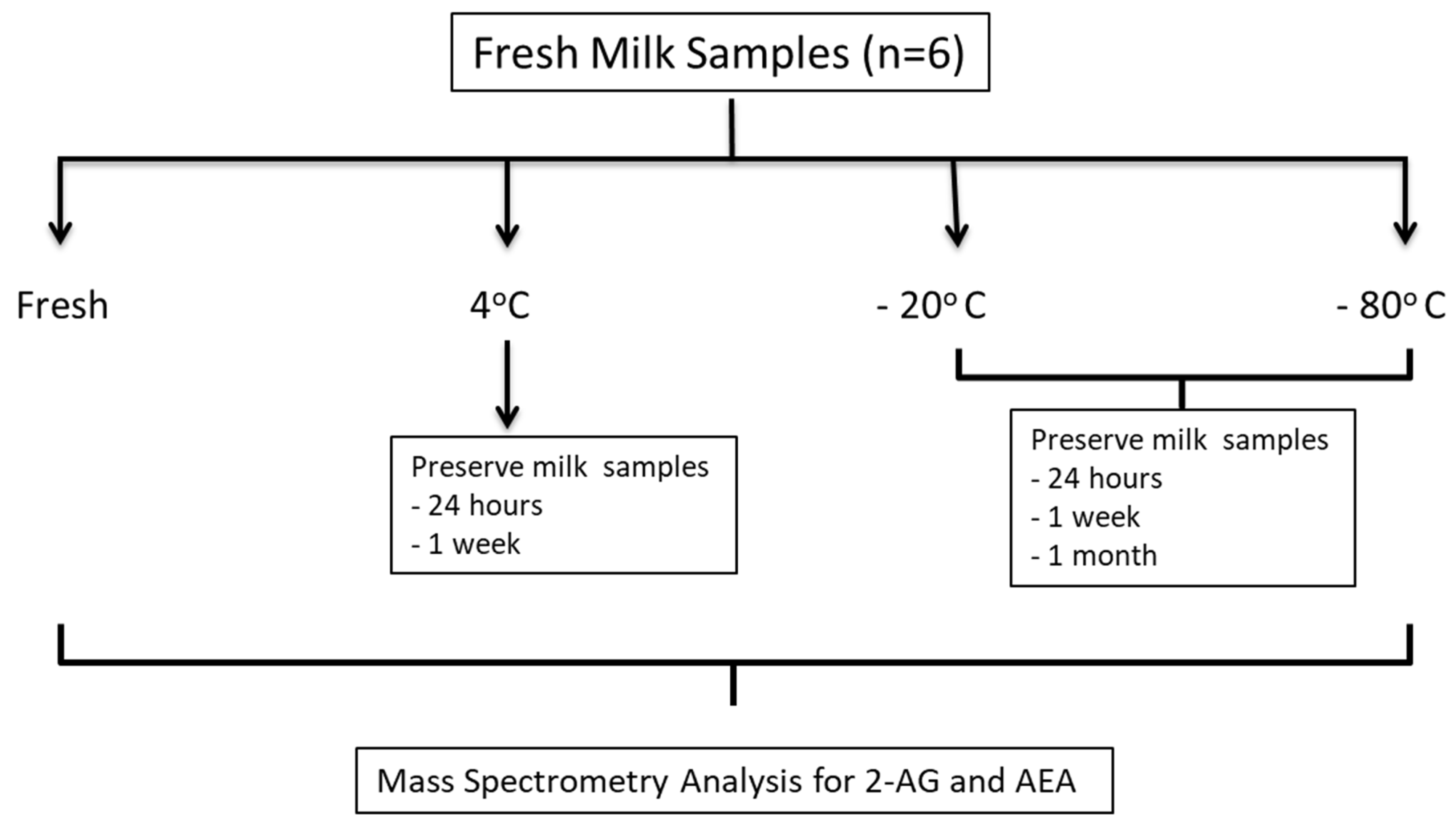
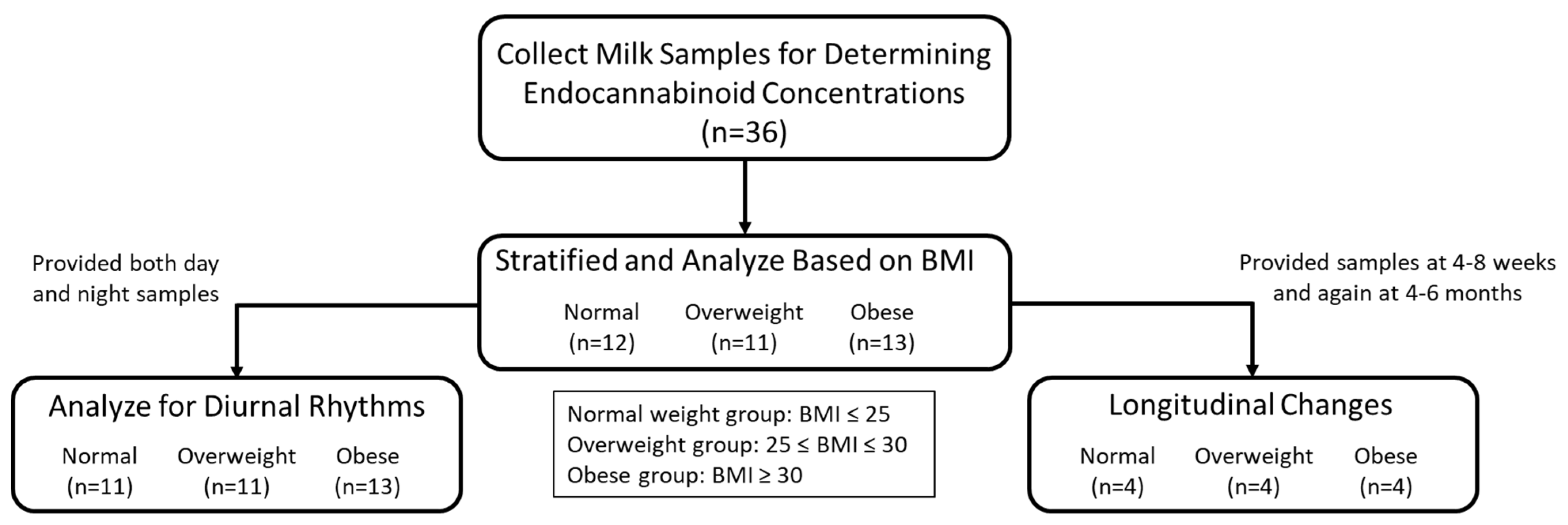
2.1. Study Design
2.2. Study Participants
2.3. Subject Recruitment and Collection
2.4. Quantitation Approach and Sample Analysis
2.5. Statistical Analysis
3. Results
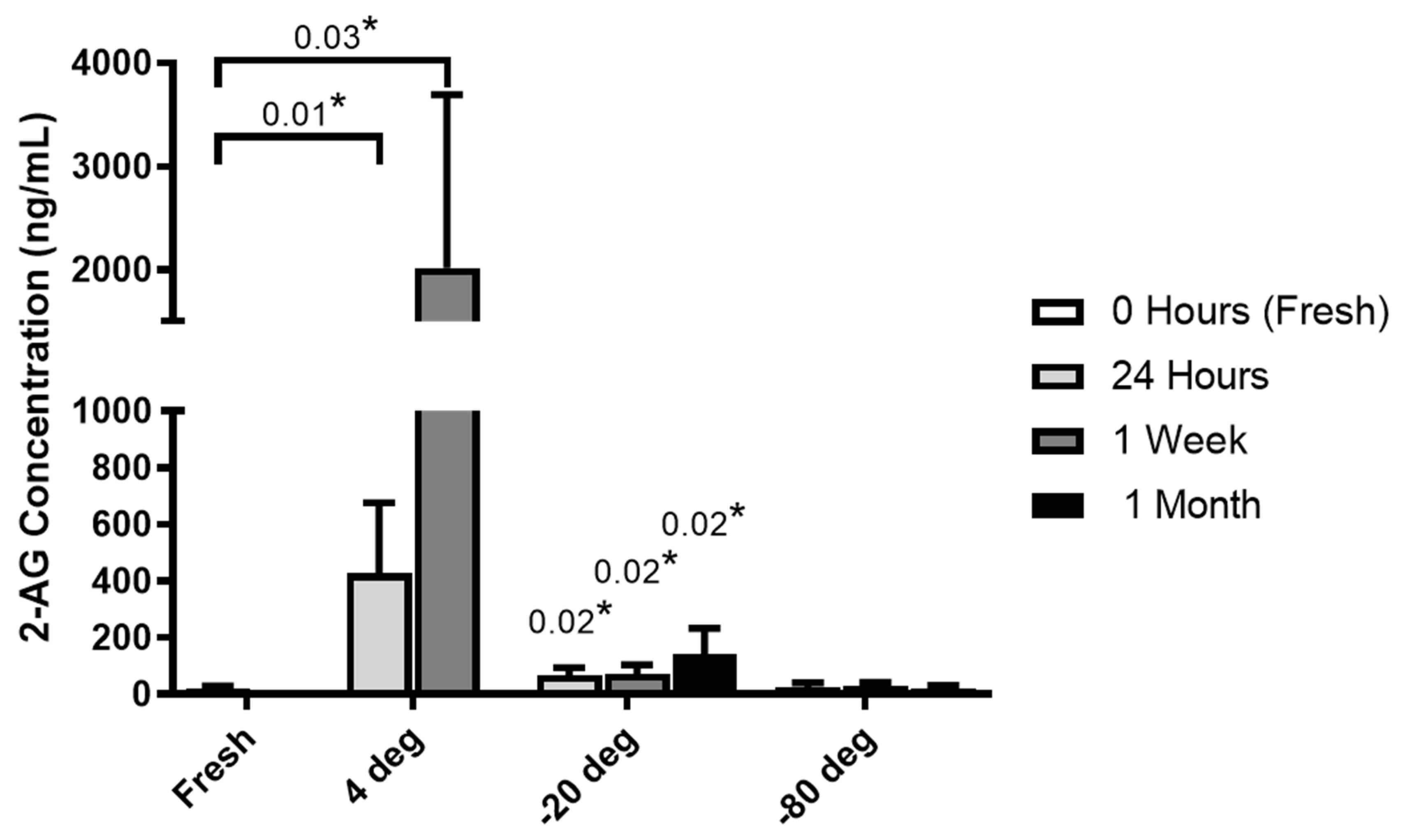
3.1. Milk Collection and Storage Conditions Affect Endocannabinoid Levels
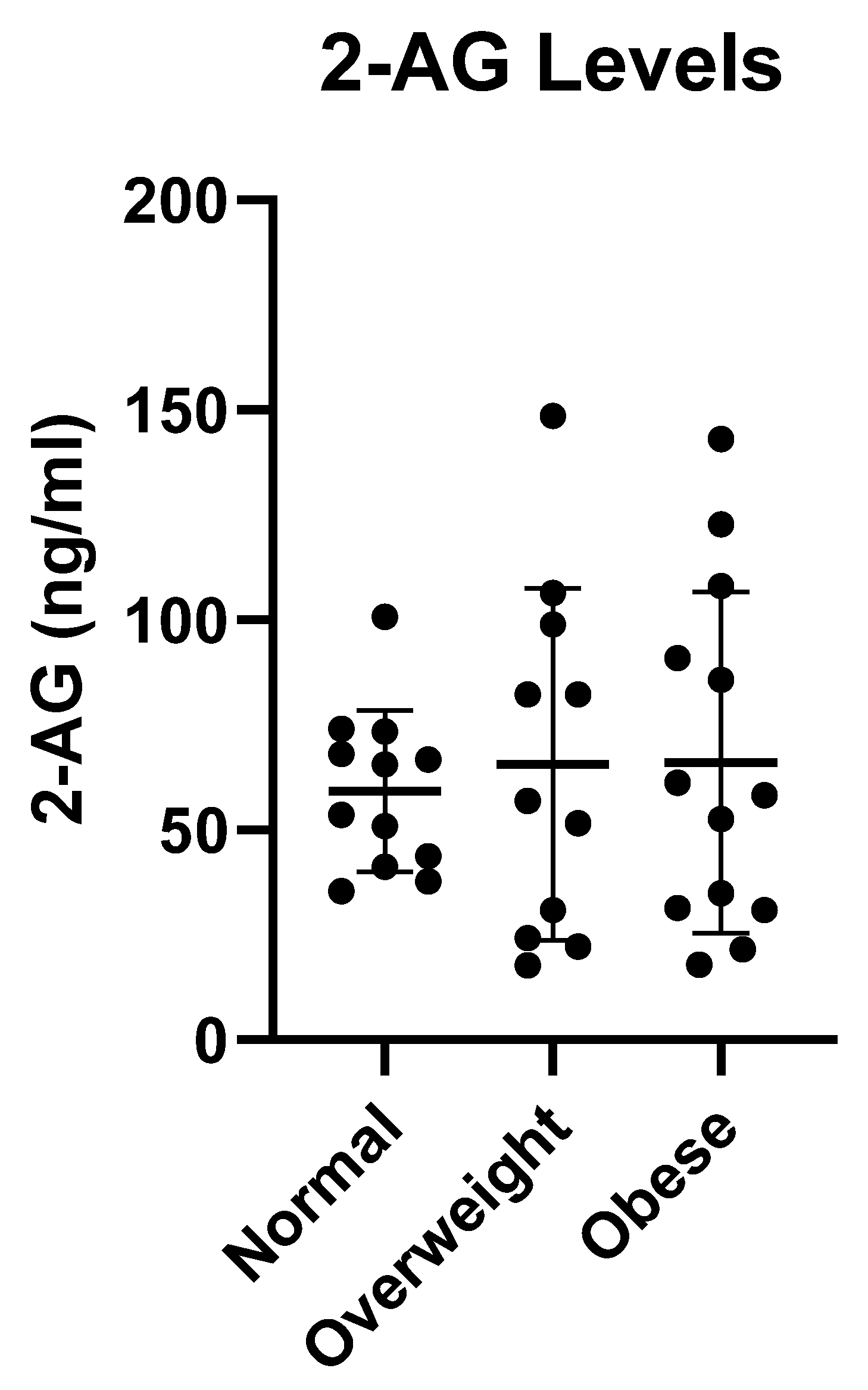
3.2. Comparison of Milk Endocannabinoid Levels for Normal versus Obese Women
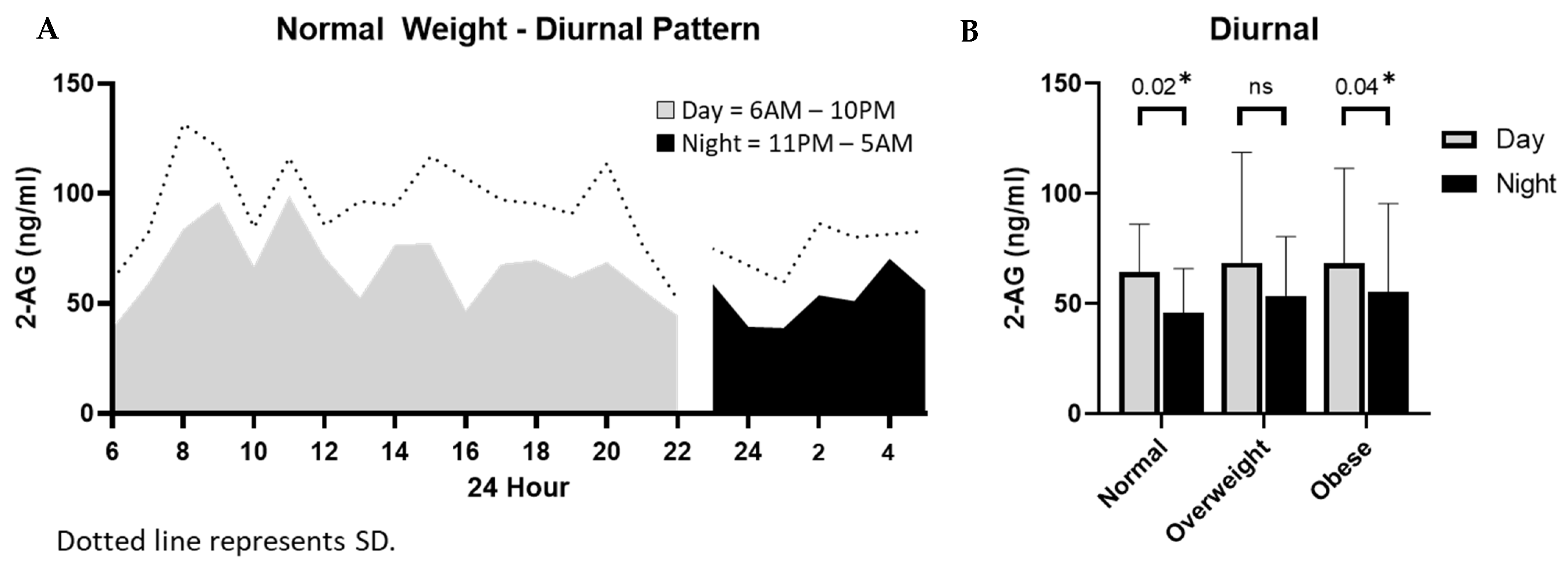
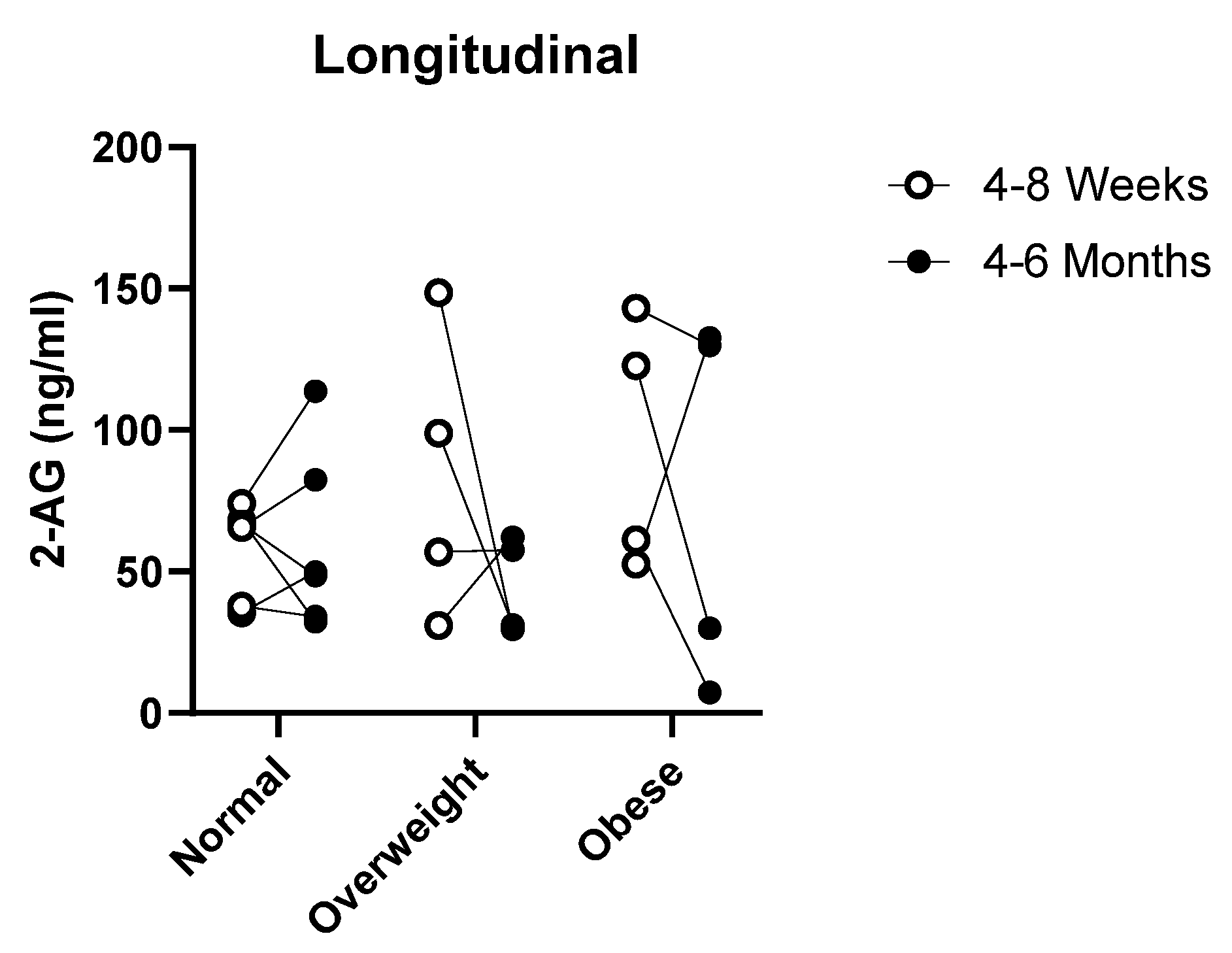
3.3. Diurnal and Longitudinal Changes in 2-AG Levels in Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N. The essentiality of arachidonic acid in infant development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Salem, N., Jr.; Van Dael, P. Arachidonic acid in human milk. Nutrients 2020, 12, 626. [Google Scholar] [CrossRef] [Green Version]
- Battista, N.; Bari, M.; Rapino, C.; Trasatti, F.; D’Agostino, A.; Maccarrone, M. Regulation of female fertility by the endocannabinoid system. Hum. Fertil. 2007, 10, 207–216. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Guo, Y.; Zhang, H.; Takahashi, T.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; Cravatt, B.F.; Dey, S.K. Fatty acid amide hydrolase deficiency limits early pregnancy events. J. Clin. Investig. 2006, 116, 2122–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fride, E. The endocannabinoid-CB(1) receptor system in pre- and postnatal life. Eur. J. Pharmacol. 2004, 500, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Aad, G.; Abbott, B.; Abdallah, J.; Khalek, S.A.; Aben, R.; Abi, B.; Abolins, M.; AbouZeid, O.S.; Abramowicz, H.; Abreu, H.; et al. Light-quark and gluon jet discrimination in [Formula: See text] collisions at [Formula: See text] with the ATLAS detector. Eur. Phys. J. C Part Fields 2014, 74, 3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The endogenous cannabinoid system: A budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2017, 22, 105–110. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Harvey-White, J.; Osei-Hyiaman, D.; Razdan, R.; Gong, Q.; Chan, A.C.; Zhou, Z.; Huang, B.X.; Kim, H.Y.; et al. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. USA 2006, 103, 13345–13350. [Google Scholar] [CrossRef] [Green Version]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Fride, E. The endocannabinoid-CB receptor system: Importance for development and in pediatric disease. Neuro. Endocrinol. Lett 2004, 25, 24–30. [Google Scholar]
- Fride, E.; Gobshtis, N.; Dahan, H.; Weller, A.; Giuffrida, A.; Ben-Shabat, S. The endocannabinoid system during development: Emphasis on perinatal events and delayed effects. Vitam. Horm. 2009, 81, 139–158. [Google Scholar] [PubMed]
- Wu, J.; Gouveia-Figueira, S.; Domellöf, M.; Zivkovic, A.M.; Nording, M.L. Oxylipins, endocannabinoids, and related compounds in human milk: Levels and effects of storage conditions. Prostaglandins Other Lipid Mediat. 2016, 122, 28–36. [Google Scholar] [CrossRef] [PubMed]
- CDC: Center for Disease Control and Prevention, Division of Nutrition. Defining Adult Overweight and Obesity. Available online: https://www.cdc.gov/obesity/adult/defining.html (accessed on 3 March 2021).
- Fride, E.; Ginzburg, Y.; Breuer, A.; Bisogno, T.; Di Marzo, V.; Mechoulam, R. Critical role of the endogenous cannabinoid system in mouse pup suckling and growth. Eur. J. Pharmacol. 2001, 419, 207–214. [Google Scholar] [CrossRef]
- Di Marzo, V.; Sepe, N.; De Petrocellis, L.; Berger, A.; Crozier, G.; Fride, E. Trick or treat from food endocannabinoids? Nature 1998, 396, 636–637. [Google Scholar] [CrossRef] [Green Version]
- Fride, E.; Foox, A.; Rosenberg, E.; Faigenboim, M.; Cohen, V.; Barda, L.; Blau, H.; Mechoulam, R. Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: Evidence for a “CB3” receptor. Eur. J. Pharmacol. 2003, 461, 27–34. [Google Scholar] [CrossRef]
- Gaitán, A.V.; Wood, J.T.; Zhang, F.; Makriyannis, A.; Lammi-Keefe, C.J. Endocannabinoid metabolome characterization of transitional and mature human milk. Nutrients 2018, 10, 1294. [Google Scholar] [CrossRef] [Green Version]
- Gaitán, A.V.; Wood, J.T.; Solomons, N.W.; Donohue, J.A.; Ji, L.; Liu, Y.; Nikas, S.P.; Zhang, F.; Allen, L.H.; Makriyannis, A.; et al. Endocannabinoid metabolome characterization of milk from Guatemalan women living in the western highlands. Curr. Dev. Nutr. 2019, 3, nzz018. [Google Scholar] [CrossRef] [PubMed]
- Nessel, I.; Khashu, M.; Dyall, S.C. The effects of storage conditions on long-chain polyunsaturated fatty acids, lipid mediators, and antioxidants in donor human milk—A review. Prostaglandins Leukot Essent Fat. Acids 2019, 146, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S. Dysregulation of the endocannabinoid system in obesity. J. Neuroendocrinol. 2008, 20, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, E.C.; Tasali, E.; Leproult, R.; Stuhr, K.L.; Doncheck, E.; De Wit, H.; Hillard, C.J.; Van Cauter, E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 2016, 39, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Hahn-Holbrook, J.; Saxbe, D.; Bixby, C.; Steele, C.; Glynn, L. Human milk as “chrononutrition”: Implications for child health and development. Pediatr. Res. 2019, 85, 936–942. [Google Scholar] [CrossRef]
- Boss, M.; Gardner, H.; Hartmann, P. Normal human lactation: Closing the gap. F1000Res 2018, 7, F1000 Faculty Rev-801. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Mean ± SD or % (Frequency) or Range | |||
|---|---|---|---|---|
| Maternal | Normal | Overweight | Obese | p-Value |
| (N = 12) | (N = 11) | (N = 13) | ||
| Age (yrs) | 29.1 ± 5 | 30.6 ± 1.2 | 32 ± 4 | 0.238 |
| Race (% (n/N)) | 0.030 * | |||
| White (non-hispanic) | 50 (6/12) | 90.09 (10/11) | 92.3 (12/13) | |
| Hispanic | 50 (6/12) | 9.09 (1/11) | 7.6 (1/13) | |
| BMI (kg/m2) (Mean ± SD) | 22.4 ± 1.6 | 27.0 ± 1.2 | 36.8 ± 6.2 | 0.000 ** |
| Post Pregnancy (at the time of study) | ||||
| Total number of births (% (n/N)) | 0.007 ** | |||
| Primiparous | 58.3 (7/12) | 9.1 (1/11) | 7.6 (1/13) | |
| Multiparous | 41.7 (5/12) | 90.9 (10/11) | 46.1 (12/13) | |
| Previous breastfeeding experience (% (n/N)) | 0.007 ** | |||
| Yes | 33.3 (5/12) | 90.9 (10/11) | 92.3 (12/13) | |
| No | 58.3 (7/12) | 9.1 (1/11) | 7.7 (1/13) | |
| Primary mode of feeding (% (n/N)) | 0.603 | |||
| Breast only | 66.6 (8/12) | 81.8 (9/11) | 84.6 (11/13) | |
| Breast and Pump | 33.4 (4/12) | 18.2 (2/11) | 15.4 (2/13) | |
| Exercise | ||||
| Yes (% (n/N)) | 41.6 (5/12) | 36.3 (4/11) | 38.4 (5/13) | 1.000 |
| Times/week | 3–7 | 3–4 | 2–3 | |
| Hours/week | 1–3 | 2–5 | 1–5 | |
| Infant | ||||
| Sex (% (n/N)) | 0.839 | |||
| Male | 58.3 (7/12) | 45.4 (5/11) | 46.3 (6/13) | |
| Female | 41.6 (5/12) | 54.5 (6/11) | 53.8 (7/13) | |
| Weight (lbs) (Mean ± SD) | ||||
| At birth | 6.9 ± 0.7 | 7.6 ± 1 | 8.1 ± 1.2 | 0.019 * |
| At 4–8 weeks | 9.6 ± 1.5 | 10.2 ± 1.6 | 9.9 ± 1.8 | 0.697 |
| Feeds per 24 h | 9 ± 1.6 | 9.2 ± 2.3 | 9.8 ± 1.8 | 0.550 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datta, P.; Melkus, M.W.; Rewers-Felkins, K.; Patel, D.; Bateman, T.; Baker, T.; Hale, T.W. Human Milk Endocannabinoid Levels as a Function of Obesity and Diurnal Rhythm. Nutrients 2021, 13, 2297. https://doi.org/10.3390/nu13072297
Datta P, Melkus MW, Rewers-Felkins K, Patel D, Bateman T, Baker T, Hale TW. Human Milk Endocannabinoid Levels as a Function of Obesity and Diurnal Rhythm. Nutrients. 2021; 13(7):2297. https://doi.org/10.3390/nu13072297
Chicago/Turabian StyleDatta, Palika, Michael W. Melkus, Kathleen Rewers-Felkins, Dhavalkumar Patel, Tiffany Bateman, Teresa Baker, and Thomas W. Hale. 2021. "Human Milk Endocannabinoid Levels as a Function of Obesity and Diurnal Rhythm" Nutrients 13, no. 7: 2297. https://doi.org/10.3390/nu13072297
APA StyleDatta, P., Melkus, M. W., Rewers-Felkins, K., Patel, D., Bateman, T., Baker, T., & Hale, T. W. (2021). Human Milk Endocannabinoid Levels as a Function of Obesity and Diurnal Rhythm. Nutrients, 13(7), 2297. https://doi.org/10.3390/nu13072297







