β-Carotene Status Is Associated with Inflammation and Two Components of Metabolic Syndrome in Patients with and without Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Characteristics and Anthropometric Assessments
2.3. Blood Collection and Biochemical Measurements
2.4. β-Carotene Status Measurement
2.5. Metabolic Syndrome and Inflammation Status
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Subjects
3.2. β-Carotene Status
3.3. Correlations between β-Carotene Status and Metabolic Syndrome and Inflammation
3.4. Effect of β-Carotene and Inflammatory Status on Metabolic Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Coggon, D.; Reading, I.; Croft, P.; McLaren, M.; Barrett, D.; Cooper, C. Knee osteoarthritis and obesity. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 622–627. [Google Scholar] [CrossRef]
- Hussain, S.M.; Wang, Y.; Shaw, J.E.; Wluka, A.E.; Graves, S.; Gambhir, M.; Cicuttini, F.M. Relationship of weight and obesity with the risk of knee and hip arthroplasty for osteoarthritis across different levels of physical performance: A prospective cohort study. Scand. J. Rheumatol. 2019, 48, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.J. Prevalence of knee osteoarthritis, risk factors, and quality of life: The Fifth Korean National Health and Nutrition Examination Survey. Int. J. Rheum. Dis. 2017, 20, 809–817. [Google Scholar] [CrossRef]
- Dickson, B.M.; Roelofs, A.J.; Rochford, J.J.; Wilson, H.M.; De Bari, C. The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res. Ther. 2019, 21, 289. [Google Scholar] [CrossRef]
- Kluzek, S.; Newton, J.L.; Arden, N.K. Is osteoarthritis a metabolic disorder? Br. Med. Bull. 2015, 115, 111–121. [Google Scholar] [CrossRef]
- Chen, S.J.; Yen, C.H.; Huang, Y.C.; Lee, B.J.; Hsia, S.; Lin, P.T. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS ONE 2012, 7, e45693. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Shete, V.; Quadro, L. Mammalian metabolism of β-carotene: Gaps in knowledge. Nutrients 2013, 5, 4849–4868. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef]
- Marcelino, G.; Machate, D.J.; Freitas, K.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Suantawee, T.; Tantavisut, S.; Adisakwattana, S.; Tanavalee, A.; Yuktanandana, P.; Anomasiri, W.; Deepaisarnsakul, B.; Honsawek, S. Oxidative stress, vitamin E, and antioxidant capacity in knee osteoarthritis. J. Clin. Diagn. Res. 2013, 7, 1855–1859. [Google Scholar] [CrossRef]
- Surapaneni, K.M.; Venkataramana, G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J. Med. Sci. 2007, 61, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Okorodudu, D.O.; Jumean, M.F.; Montori, V.M.; Romero-Corral, A.; Somers, V.K.; Erwin, P.J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 791–799. [Google Scholar] [CrossRef]
- Kand’ár, R.; Novotná, P.; Drábková, P. Determination of Retinol, Tocopherol, Lycopene, and-Carotene in Human Plasma Using HPLC with UV-Vis Detection: Application to a Clinical Study. J. Chem. 2013, 2013, 460242. [Google Scholar] [CrossRef]
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan. The Diagnostic Criteria of Metabolic Syndrome in Adults. 2007. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=639&pid=1219 (accessed on 5 March 2021).
- Dhingra, R.; Gona, P.; Nam, B.H.; D’Agostino, R.B., Sr.; Wilson, P.W.; Benjamin, E.J.; O’Donnell, C.J. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am. J. Med. 2007, 120, 1054–1062. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.O.; Kim, M.H.; Kim, H. β-Carotene Inhibits Activation of NF-κB, Activator Protein-1, and STAT3 and Regulates Abnormal Expression of Some Adipokines in 3T3-L1 Adipocytes. J. Cancer Prev. 2018, 23, 37–43. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2020, 79, 544–573. [Google Scholar] [CrossRef]
- Seki, T.; Hasegawa, Y.; Yamaguchi, J.; Kanoh, T.; Ishiguro, N.; Tsuboi, M.; Ito, Y.; Hamajima, N.; Suzuki, K. Association of serum carotenoids, retinol, and tocopherols with radiographic knee osteoarthritis: Possible risk factors in rural Japanese inhabitants. J. Orthop. Sci. 2010, 15, 477–484. [Google Scholar] [CrossRef]
- Bliddal, H.; Leeds, A.R.; Christensen, R. Osteoarthritis, obesity and weight loss: Evidence, hypotheses and horizons—A scoping review. Obes. Rev. 2014, 15, 578–586. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Puenpatom, R.A.; Victor, T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad. Med. 2009, 121, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Connell, J.M. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Yan, S.; Guo, Y.; Wang, H.; Li, X.; Wang, L.; Hu, W.; Li, B.; Cui, W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta-analysis. Food Funct. 2021, 12, 4768–4782. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. Medscape Gen. Med. 2006, 8, 59. [Google Scholar]
- Suzuki, K.; Ito, Y.; Ochiai, J.; Kusuhara, Y.; Hashimoto, S.; Tokudome, S.; Kojima, M.; Wakai, K.; Toyoshima, H.; Tamakoshi, K.; et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac. J. Cancer Prev. 2003, 4, 259–266. [Google Scholar] [PubMed]
- Suzuki, K.; Inoue, T.; Hioki, R.; Ochiai, J.; Kusuhara, Y.; Ichino, N.; Osakabe, K.; Hamajima, N.; Ito, Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin. Nutr. 2006, 25, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Gouranton, E.; van Helden, Y.G.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Lobo, G.P.; Amengual, J.; Li, H.N.; Golczak, M.; Bonet, M.L.; Palczewski, K.; von Lintig, J. β,β-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a β,β-carotene oxygenase 1-dependent manner. J. Biol. Chem. 2010, 285, 27891–27899. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, G.; Gu, H.; Nguyen, B.N. Role of PPAR in the nutritional and pharmacological actions of carotenoids. Res. Rep. Biochem. 2016, 6, 13–24. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Chen, J.; Zhang, D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007–2014. J. Hypertens. 2019, 37, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Xavier, A.A.; Perez-Galvez, A. Carotenoids as a source of antioxidants in the diet. In Carotenoids in Nature; Stange, C., Ed.; Subcellular Biochemistry; Springer: Cham, Switzerland, 2016; Volume 79, pp. 359–375. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Saito, I.; Maruyama, K.; Eguchi, E.; Mori, H.; Tanno, S.; Sakurai, S.; Kishida, T.; Nishida, W.; Osawa, H.; et al. Associations of serum β-carotene and retinol concentrations with insulin resistance: The Toon Health Study. Nutrition 2015, 31, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Berenbaum, F.; Sellam, J. The Phenotypic Approach to Osteoarthritis: A Look at Metabolic Syndrome-Associated Osteoarthritis. Jt. Bone Spine 2019, 86, 725–730. [Google Scholar] [CrossRef]
- Gába, A.; Přidalová, M. Diagnostic performance of body mass index to identify adiposity in women. Eur. J. Clin. Nutr. 2016, 70, 898–903. [Google Scholar] [CrossRef]
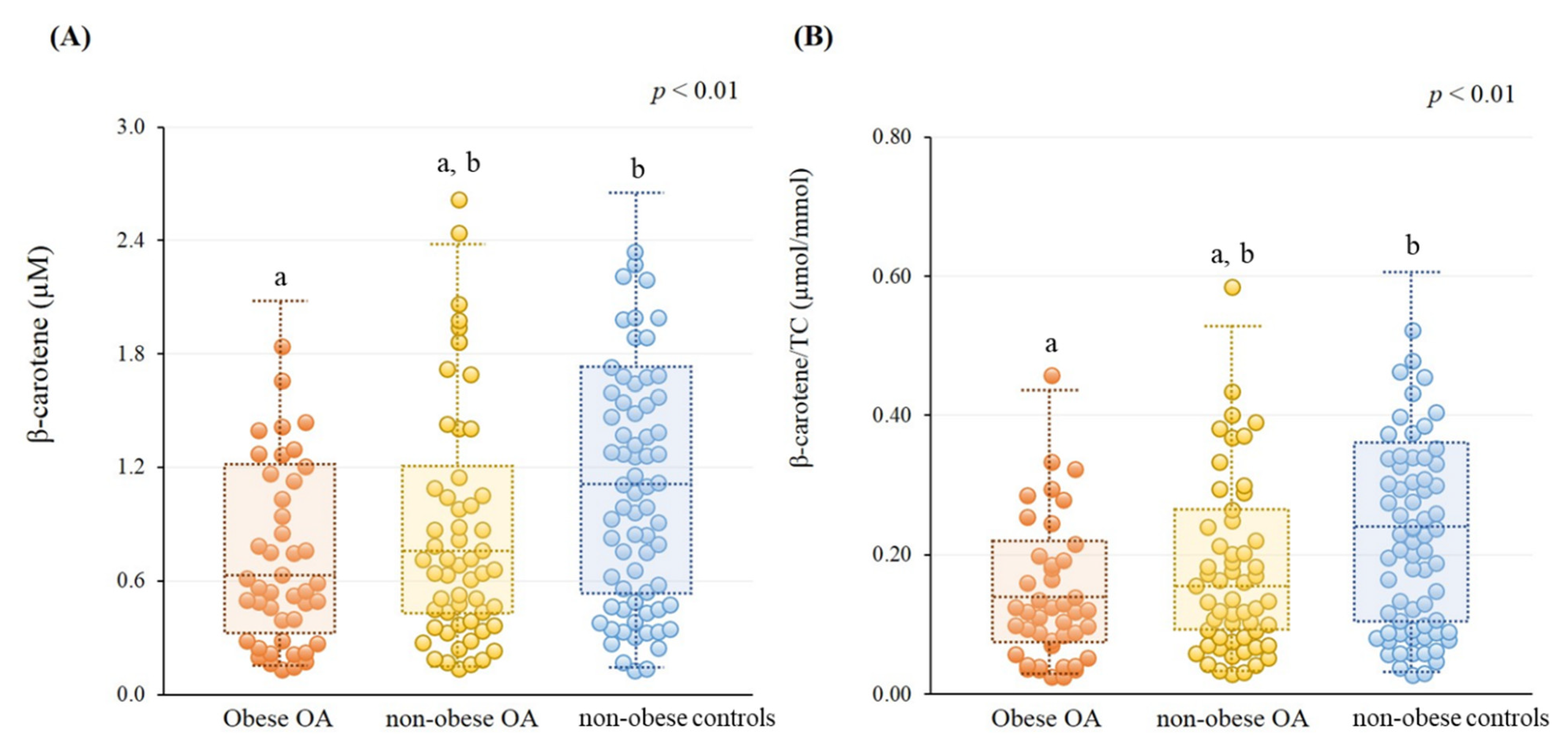
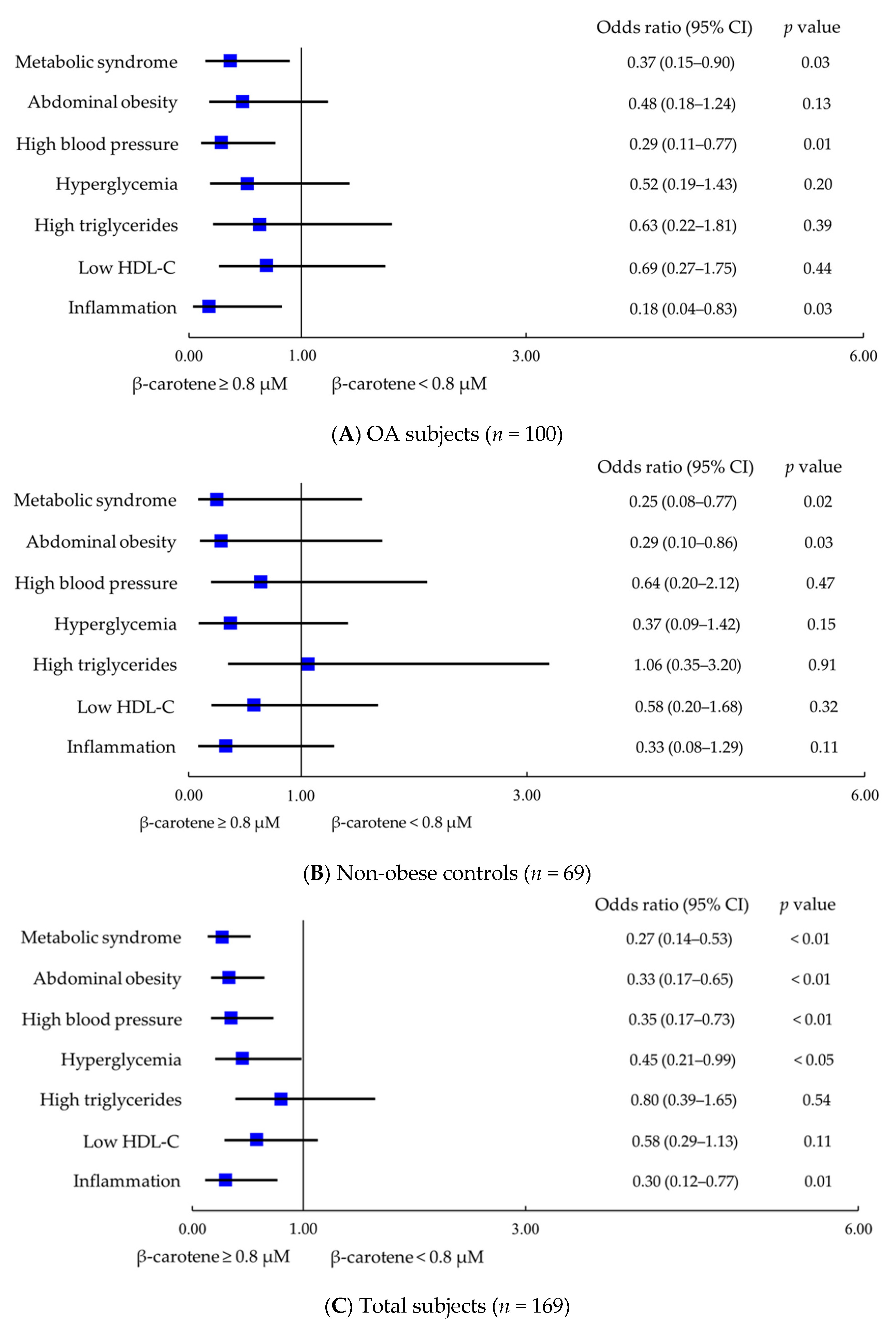
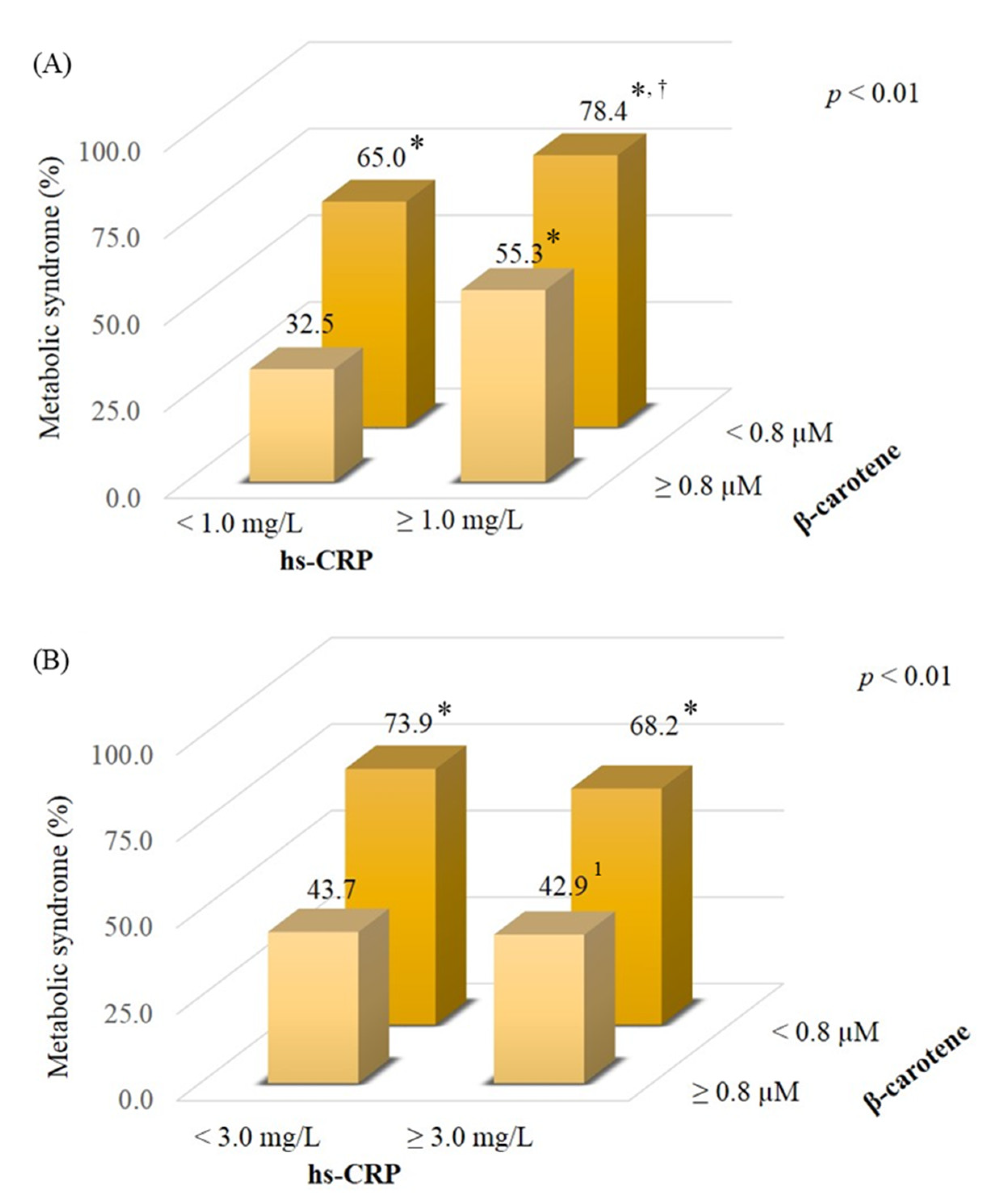
| Characteristics | Obese OA 2 (n = 44) | Non-Obese OA (n = 56) | Non-Obese Controls (n = 69) | p Value |
|---|---|---|---|---|
| Age (years) | 72.0 ± 8.7 (73.0) 1,a | 67.5 ± 9.5 (68.0) b | 60.4 ± 9.6 (60.0) c | <0.01 |
| Female (n, %) | 38 (86.4%) a | 35 (62.5%) b | 33 (47.8%) b | <0.01 |
| BMI (kg/m2) | 26.6 ± 3.3 (26.9) a | 24.3 ± 3.8 (24.0) b | 23.8 ± 4.2 (23.7) b | <0.01 |
| Waist (cm) | 90.2 ± 7.9 (90.9) a | 86.1 ± 11.2 (86.2) a,b | 83.7 ± 11.7 (82.8) b | <0.01 |
| Visceral fat mass (g) | 607.8 ± 159.2 (621.0) a | 414.9 ± 192.8 (412.5) b | 394.4 ± 185.6 (380.5) b | <0.01 |
| Whole body muscle mass (kg) | 39.4 ± 6.4 (38.7) a | 43.8 ± 10.1 (42.2) a,b | 45.3 ± 11.6 (44.3) b | 0.02 |
| Trunk muscle mass (kg) | 20.1 ± 3.6 (19.4) a | 22.4 ± 5.0 (22.1) b | 22.7 ± 5.6 (21.9) b | 0.02 |
| WSMI (kg/m2) | 16.5 ± 2.2 (16.4) | 17.3 ± 2.9 (17.3) | 17.4 ± 3.1 (17.3) | 0.30 |
| ASMI (kg/m2) | 6.8 ± 1.0 (6.9) | 7.2 ± 1.4 (7.1) | 7.4 ± 1.6 (7.4) | 0.11 |
| SBP (mmHg) | 139.3 ± 19.5 (137.0) a | 133.6 ± 19.2 (133.0) a,b | 130.6 ± 16.1 (129.0) b | <0.05 |
| DBP (mmHg) | 80.3 ± 12.5 (78.5) | 80.8 ± 10.2 (80.0) | 82.9 ± 13.0 (84.0) | 0.46 |
| Fasting glucose (mmol/L) | 6.5 ± 1.2 (6.1) | 6.4 ± 1.2 (6.2) | 6.2 ± 1.3 (5.8) | 0.33 |
| Triglycerides (mmol/L) | 1.4 ± 0.7 (1.2) | 1.2 ± 0.7 (1.0) | 1.5 ± 0.8 (1.3) | 0.11 |
| HDL-C (mmol/L) | 1.4 ± 0.3 (1.3) | 1.4 ± 0.4 (1.3) | 1.3 ± 0.4 (1.2) | 0.43 |
| LDL-C (mmol/L) | 3.2 ± 1.0 (3.0) | 2.9 ± 0.7 (2.9) | 3.0 ± 0.7 (2.9) | 0.28 |
| Total cholesterol (mmol/L) | 5.2 ± 1.1 (4.9) | 4.9 ± 0.8 (5.0) | 5.0 ± 0.9 (4.9) | 0.71 |
| Hs-CRP (mg/L) | 1.8 ± 1.7 (1.4) | 3.0 ± 9.5 (1.1) | 1.8 ± 3.5 (0.9) | 0.09 |
| Metabolic syndrome (%) 3 | 34 (77.3%) a | 29 (51.8%) b | 37 (53.6%) b | 0.02 |
| Abdominal obesity (%) 4 | 38 (86.4%) a | 34 (60.7%) b | 32 (46.4%) b | <0.01 |
| High blood pressure (%) 5 | 37 (84.1%) | 39 (69.6%) | 49 (71.0%) | 0.20 |
| Hyperglycemia (%) 6 | 38 (86.4%) | 42 (75.0%) | 54 (78.3%) | 0.37 |
| High triglycerides (%) 7 | 10 (22.7%) | 12 (21.4%) | 22 (31.9%) | 0.35 |
| Low HDL-C (%) 8 | 20 (45.5%) | 15 (26.8%) | 26 (37.7%) | 0.15 |
| Inflammation (%) 9 | 6 (13.6%) | 11 (19.6%) | 12 (17.4%) | 0.73 |
| Lifestyle | ||||
| Smokes cigarettes | 0.17 | |||
| Current | 0 (0.0%) | 1 (1.8%) | 3 (4.3%) | |
| Ever | 5 (11.4%) | 7 (12.5%) | 16 (23.2%) | |
| Never | 39 (88.6%) | 48 (85.7%) | 50 (72.5%) | |
| Alcohol use | 0.09 | |||
| Current | 0 (0.0%) | 5 (8.9%) | 7 (10.1%) | |
| Ever | 1 (2.3%) | 3 (5.4%) | 7 (10.1%) | |
| Never | 43 (97.7%) | 48 (85.7%) | 55 (79.7%) | |
| Exercise | 22 (50.0%) | 30 (53.6%) | 39 (56.5%) | 0.79 |
| Parameters | β-Carotene (µM) | β-Carotene/TC (µmol/mmol) |
|---|---|---|
| r 1 (p value) | ||
| OA subjects (n = 100) | ||
| Metabolic syndrome (%) | −0.21 (0.03) | −0.21 (0.03) |
| Abdominal obesity (%) | −0.24 (0.02) | −0.22 (0.03) |
| High blood pressure (%) | −0.24 (0.02) | −0.28 (<0.01) |
| Hyperglycemia (%) | −0.16 (0.12) | −0.12 (0.23) |
| High triglycerides (%) | −0.07 (0.50) | −0.13 (0.20) |
| Low HDL-C (%) | −0.09 (0.40) | −0.04 (0.70) |
| Inflammation (%) | −0.23 (0.02) | −0.18 (0.07) |
| Total body fat obesity (%) | −0.10 (0.31) | −0.14 (0.18) |
| Non-obese controls (n = 69) | ||
| Metabolic syndrome (%) | −0.35 (<0.01) | −0.36 (<0.01) |
| Abdominal obesity (%) | −0.22 (0.07) | −0.27 (0.03) |
| High blood pressure (%) | −0.12 (0.32) | −0.11 (0.36) |
| Hyperglycemia (%) | −0.14 (0.25) | −0.10 (0.41) |
| High triglycerides (%) | −0.08 (0.53) | −0.16 (0.20) |
| Low HDL-C (%) | −0.08 (0.52) | −0.04 (0.75) |
| Inflammation (%) | −0.22 (0.08) | −0.22 (0.07) |
| Total body fat obesity (%) 2 | No analysis 2 | No analysis 2 |
| Total subjects (n = 169) | ||
| Metabolic syndrome (%) | −0.30 (<0.01) | −0.29 (<0.01) |
| Abdominal obesity (%) | −0.29 (<0.01) | −0.29 (<0.01) |
| High blood pressure (%) | −0.20 (<0.01) | −0.21 (<0.01) |
| Hyperglycemia (%) | −0.16 (0.04) | −0.12 (0.11) |
| High triglycerides (%) | −0.05 (0.52) | −0.10 (0.20) |
| Low HDL-C (%) | −0.09 (0.24) | −0.04 (0.58) |
| Inflammation (%) | −0.22 (<0.01) | −0.20 (0.01) |
| Total body fat obesity (%) | −0.18 (0.02) | −0.20 (<0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-H.; Chang, P.-S.; Chiu, C.-J.; Huang, Y.-Y.; Lin, P.-T. β-Carotene Status Is Associated with Inflammation and Two Components of Metabolic Syndrome in Patients with and without Osteoarthritis. Nutrients 2021, 13, 2280. https://doi.org/10.3390/nu13072280
Yen C-H, Chang P-S, Chiu C-J, Huang Y-Y, Lin P-T. β-Carotene Status Is Associated with Inflammation and Two Components of Metabolic Syndrome in Patients with and without Osteoarthritis. Nutrients. 2021; 13(7):2280. https://doi.org/10.3390/nu13072280
Chicago/Turabian StyleYen, Chi-Hua, Po-Sheng Chang, Ching-Ju Chiu, Yu-Yun Huang, and Ping-Ting Lin. 2021. "β-Carotene Status Is Associated with Inflammation and Two Components of Metabolic Syndrome in Patients with and without Osteoarthritis" Nutrients 13, no. 7: 2280. https://doi.org/10.3390/nu13072280
APA StyleYen, C.-H., Chang, P.-S., Chiu, C.-J., Huang, Y.-Y., & Lin, P.-T. (2021). β-Carotene Status Is Associated with Inflammation and Two Components of Metabolic Syndrome in Patients with and without Osteoarthritis. Nutrients, 13(7), 2280. https://doi.org/10.3390/nu13072280






